Abstract
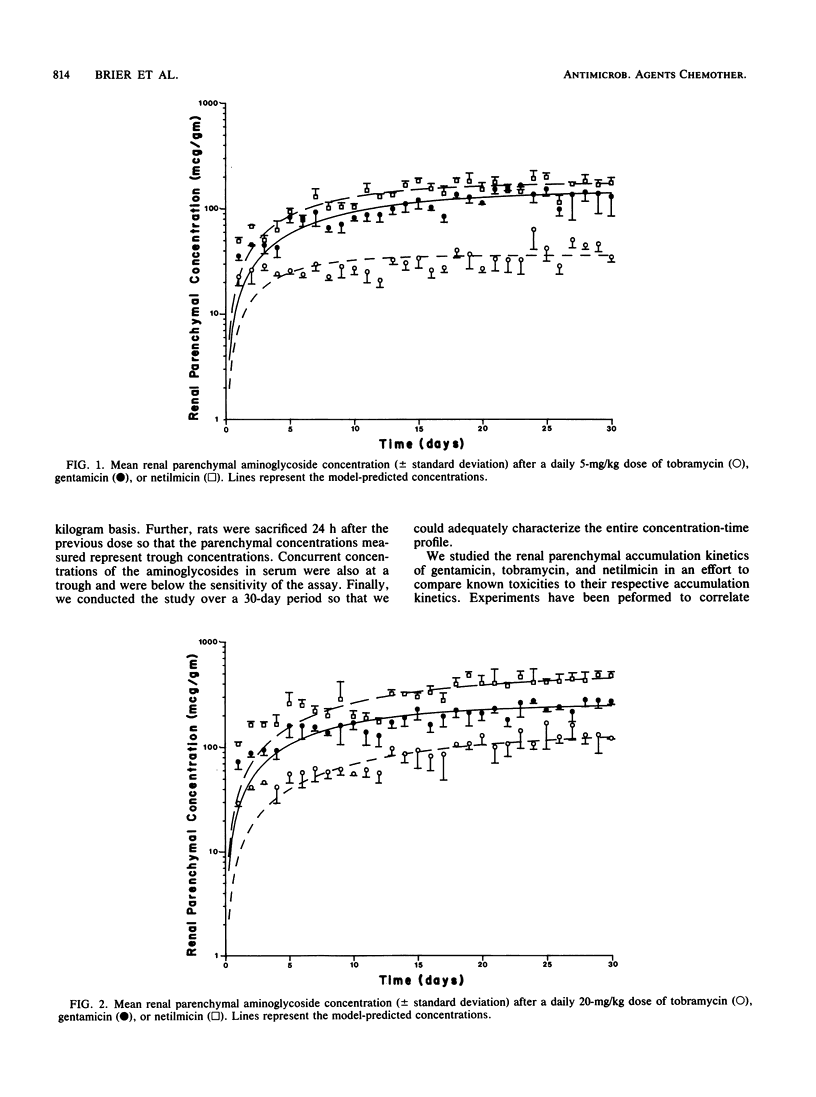
Gentamicin, tobramycin, and netilmicin were given to rats in daily doses of either 5 or 20 mg/kg for 30 days to determine the renal accumulation kinetics of the compounds and to correlate steady-state renal parenchymal concentrations with nephrotoxicity. Four rats from each group were sacrificed daily and renal parenchymal tissue concentrations were determined microbiologically. Nephrotoxicity was assessed by changes in creatinine values in serum, renal creatinine clearances, and pathological scores. There was no indication of aminoglycoside-induced nephrotoxicity in any tests performed. The following steady-state levels resulted: 36, 148, and 176 micrograms/g after 5 mg/kg per day and 148, 260, and 510 micrograms/g after 20 mg/kg per day for tobramycin, gentamicin, and netilmicin, respectively. We conclude that aminoglycoside parenchymal accumulation in rats follows this order: tobramycin less than gentamicin less than netilmicin. Therefore, differences in the relative toxicities of gentamicin, tobramycin, and netilmicin do not correlate with the renal parenchymal accumulation of these agents and may be more dependent on intrinsic toxicity to the renal proximal tubule than to the concentration of the aminoglycoside in the kidney.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronoff G. R., Pottratz S. T., Brier M. E., Walker N. E., Fineberg N. S., Glant M. D., Luft F. C. Aminoglycoside accumulation kinetics in rat renal parenchyma. Antimicrob Agents Chemother. 1983 Jan;23(1):74–78. doi: 10.1128/aac.23.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett W. M., Plamp C. E., Gilbert D. N., Parker R. A., Porter G. A. The influence of dosage regimen on experimental gentamicin nephrotoxicity: dissociation of peak serum levels from renal failure. J Infect Dis. 1979 Oct;140(4):576–580. doi: 10.1093/infdis/140.4.576. [DOI] [PubMed] [Google Scholar]
- Brion N., Barge J., Godefroy I., Dromer F., Dubois C., Contrepois A., Carbon C. Gentamicin, netilmicin, dibekacin, and amikacin nephrotoxicity and its relationship to tubular reabsorption in rabbits. Antimicrob Agents Chemother. 1984 Feb;25(2):168–172. doi: 10.1128/aac.25.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier V. U., Lietman P. S., Mitch W. E. Evidence for luminal uptake of gentamicin in the perfused rat kidney. J Pharmacol Exp Ther. 1979 Aug;210(2):247–251. [PubMed] [Google Scholar]
- Edwards D. J., Mangione A., Cumbo T. J., Schentag J. J. Predicted tissue accumulation of netilmicin in patients. Antimicrob Agents Chemother. 1981 Dec;20(6):714–717. doi: 10.1128/aac.20.6.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert D. N., Plamp C., Starr P., Bennet W. M., Houghton D. C., Porter G. Comparative nephrotoxicity of gentamicin and tobramycin in rats. Antimicrob Agents Chemother. 1978 Jan;13(1):34–40. doi: 10.1128/aac.13.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josepovitz C., Pastoriza-Munoz E., Timmerman D., Scott M., Feldman S., Kaloyanides G. J. Inhibition of gentamicin uptake in rat renal cortex in vivo by aminoglycosides and organic polycations. J Pharmacol Exp Ther. 1982 Nov;223(2):314–321. [PubMed] [Google Scholar]
- Luft F. C., Bloch R., Sloan R. S., Yum M. N., Costello R., Maxwell D. R. Comparative nephrotoxicity of aminoglycoside antibiotics in rats. J Infect Dis. 1978 Oct;138(4):541–545. doi: 10.1093/infdis/138.4.541. [DOI] [PubMed] [Google Scholar]
- Luft F. C., Kleit S. A. Renal parenchymal accumulation of aminoglycoside antibiotics in rats. J Infect Dis. 1974 Dec;130(6):656–659. doi: 10.1093/infdis/130.6.656. [DOI] [PubMed] [Google Scholar]
- Luft F. C. The nephrotoxic potential of netilmicin as determined in a rat model. Scand J Infect Dis Suppl. 1980;Suppl 23:82–90. [PubMed] [Google Scholar]
- Pastoriza-Munoz E., Bowman R. L., Kaloyanides G. J. Renal tubular transport of gentamicin in the rat. Kidney Int. 1979 Oct;16(4):440–450. doi: 10.1038/ki.1979.149. [DOI] [PubMed] [Google Scholar]
- Pastoriza-Munoz E., Timmerman D., Kaloyanides G. J. Renal transport of netilmicin in the rat. J Pharmacol Exp Ther. 1984 Jan;228(1):65–72. [PubMed] [Google Scholar]
- Reiner N. E., Bloxham D. D., Thompson W. L. Nephrotoxicity of gentamicin and tobramycin given once daily or continuously in dogs. J Antimicrob Chemother. 1978 May;4 (Suppl A):85–101. doi: 10.1093/jac/4.suppl_a.85. [DOI] [PubMed] [Google Scholar]
- Sastrasinh M., Knauss T. C., Weinberg J. M., Humes H. D. Identification of the aminoglycoside binding site in rat renal brush border membranes. J Pharmacol Exp Ther. 1982 Aug;222(2):350–358. [PubMed] [Google Scholar]
- Schentag J. J., Jusko W. J. Renal clearance and tissue accumulation of gentamicin. Clin Pharmacol Ther. 1977 Sep;22(3):364–370. doi: 10.1002/cpt1977223364. [DOI] [PubMed] [Google Scholar]
- Schentag J. J., Jusko W. J., Vance J. W., Cumbo T. J., Abrutyn E., DeLattre M., Gerbracht L. M. Gentamicin disposition and tissue accumulation on multiple dosing. J Pharmacokinet Biopharm. 1977 Dec;5(6):559–577. doi: 10.1007/BF01059684. [DOI] [PubMed] [Google Scholar]
- Schentag J. J., Lasezkay G., Cumbo T. J., Plaut M. E., Jusko W. J. Accumulation pharmacokinetics of tobramycin. Antimicrob Agents Chemother. 1978 Apr;13(4):649–656. doi: 10.1128/aac.13.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soberon L., Bowman R. L., Pastoriza-Munoz E., Kaloyanides G. J. Comparative nephrotoxicities of gentamicin, netilmicin and tobramycin in the rat. J Pharmacol Exp Ther. 1979 Sep;210(3):334–343. [PubMed] [Google Scholar]


