Abstract
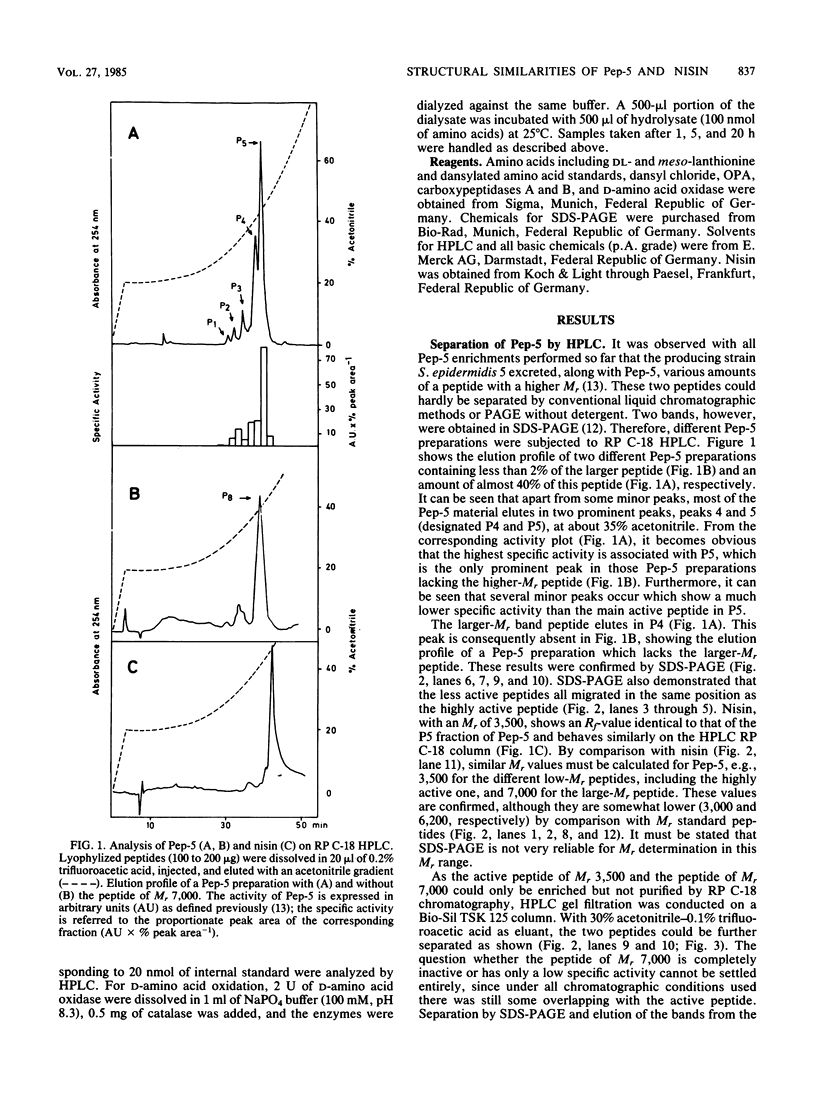
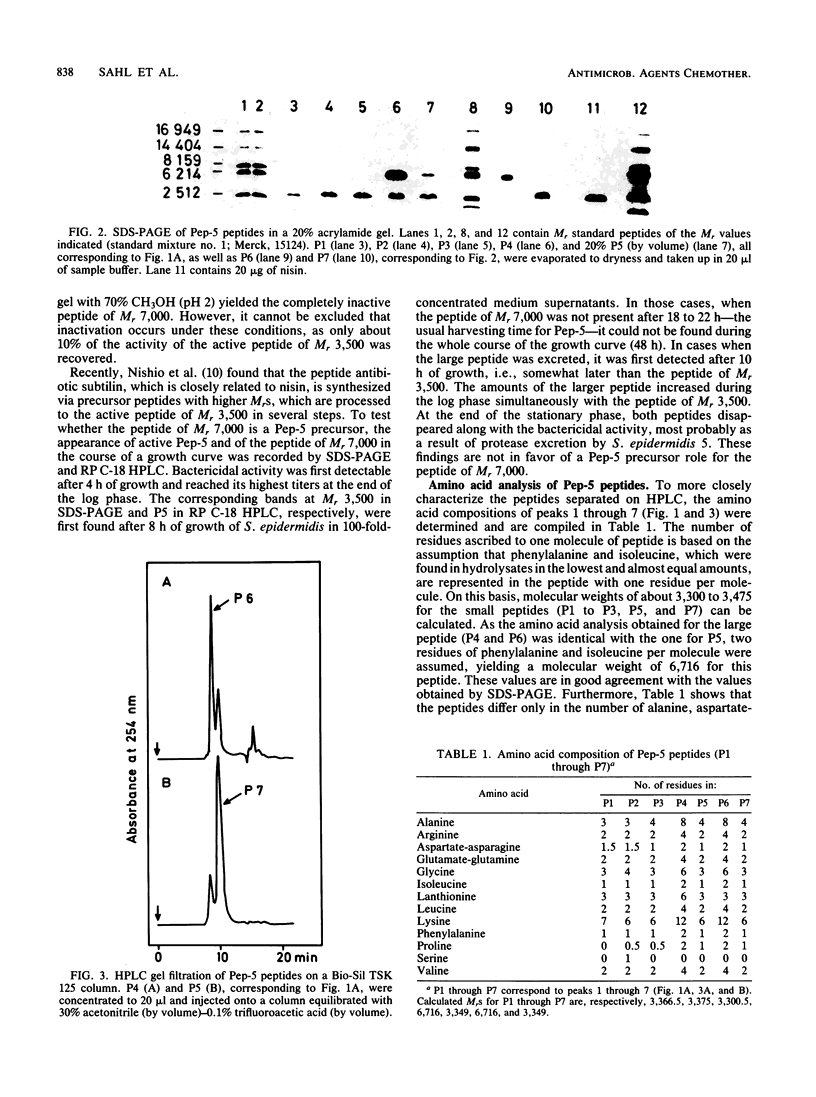
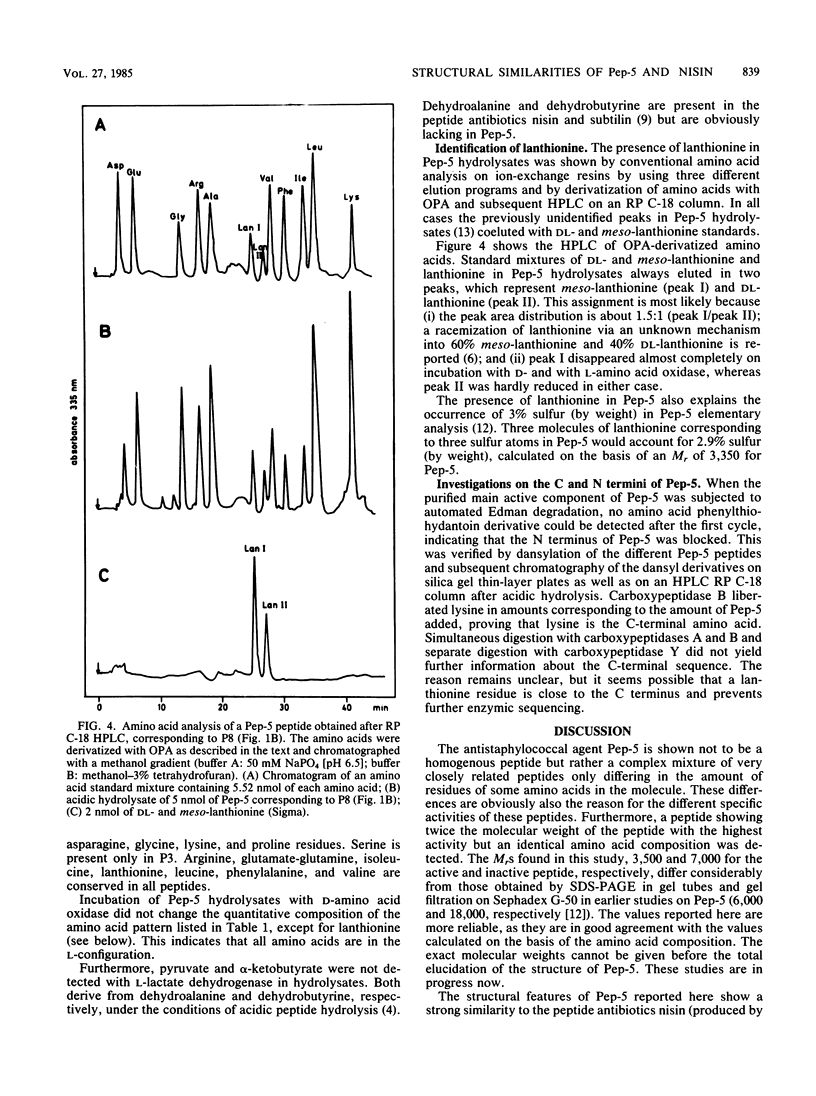
The staphylococcin-like peptide Pep-5 was shown to be a complex mixture of closely related and strongly basic peptides. Five peptides were purified by high-pressure liquid chromatography on reversed-phase and gel filtration columns and further characterized by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and amino acid analysis. Four peptides have molecular weights of ca. 3,500, whereas one is of double size. All contain the thioether amino acid lanthionine and a large number of lysine residues per molecule. The amino terminus of the main active peptide is blocked; the carboxy-terminal end is formed by a lysine residue. The data obtained for Pep-5 suggest striking structural similarities to the peptide antibiotics nisin and subtilin.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gross E., Kiltz H. H., Nebelin E. Subtilin, VI. Die Struktur des Subtilins. Hoppe Seylers Z Physiol Chem. 1973 Jul;354(7):810–812. [PubMed] [Google Scholar]
- Gross E., Kiltz H. H. The number and nature of , -unsaturated amino acids in subtilin. Biochem Biophys Res Commun. 1973 Jan 23;50(2):559–565. doi: 10.1016/0006-291x(73)90876-0. [DOI] [PubMed] [Google Scholar]
- Gross E., Morell J. L. Nisin. The assignment of sulfide bridges of beta-methyllanthionine to a novel bicyclic structure of identical ring size. J Am Chem Soc. 1970 May 6;92(9):2919–2920. doi: 10.1021/ja00712a055. [DOI] [PubMed] [Google Scholar]
- Gross E., Morell J. L. The presence of dehydroalanine in the antibiotic nisin and its relationship to activity. J Am Chem Soc. 1967 May 24;89(11):2791–2792. doi: 10.1021/ja00987a084. [DOI] [PubMed] [Google Scholar]
- Gross E., Morell J. L. The structure of nisin. J Am Chem Soc. 1971 Sep 8;93(18):4634–4635. doi: 10.1021/ja00747a073. [DOI] [PubMed] [Google Scholar]
- Ingram L. A ribosomal mechanism for synthesis of peptides related to nisin. Biochim Biophys Acta. 1970 Nov 12;224(1):263–265. doi: 10.1016/0005-2787(70)90642-8. [DOI] [PubMed] [Google Scholar]
- Kaneda N., Sato M., Yagi K. Analysis of dansyl amino acids by reverse-phase high-performance liquid chromatography. Anal Biochem. 1982 Nov 15;127(1):49–54. doi: 10.1016/0003-2697(82)90142-7. [DOI] [PubMed] [Google Scholar]
- Nishio C., Komura S., Kurahashi K. Peptide antibiotic subtilin is synthesized via precursor proteins. Biochem Biophys Res Commun. 1983 Oct 31;116(2):751–758. doi: 10.1016/0006-291x(83)90588-0. [DOI] [PubMed] [Google Scholar]
- Ruhr E., Sahl H. G. Mode of action of the peptide antibiotic nisin and influence on the membrane potential of whole cells and on cytoplasmic and artificial membrane vesicles. Antimicrob Agents Chemother. 1985 May;27(5):841–845. doi: 10.1128/aac.27.5.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahl H. G., Brandis H. Mode of action of the staphylococcin-like peptide Pep 5 and culture conditions effecting its activity. Zentralbl Bakteriol Mikrobiol Hyg A. 1982 Jun;252(2):166–175. [PubMed] [Google Scholar]
- Sahl H. G., Brandis H. Production, purification and chemical properties of an antistaphylococcal agent produced by Staphylococcus epidermidis. J Gen Microbiol. 1981 Dec;127(2):377–384. doi: 10.1099/00221287-127-2-377. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]



