Abstract
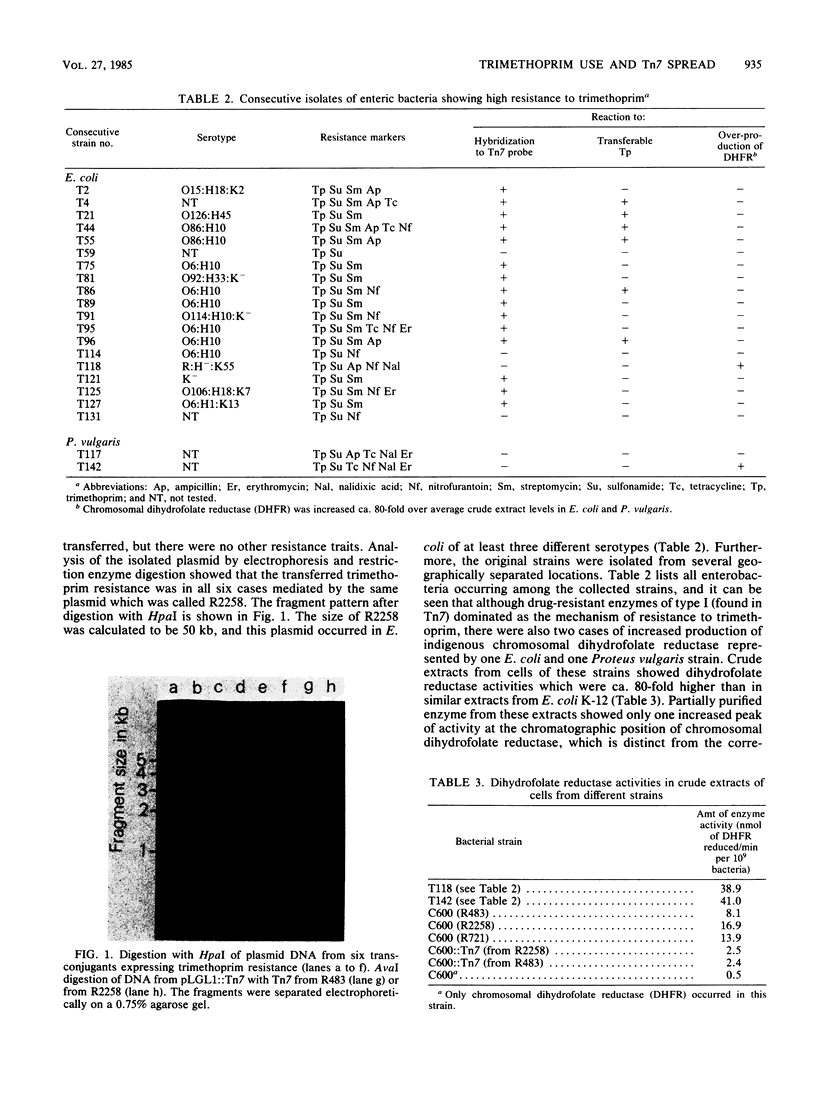
The folic acid analog trimethoprim has been in clinical use for more than 10 years. The use of it in Sweden has doubled in the last 6 to 7 years, and from the distribution statistics it can be calculated that during 1 year 4 to 5% of the population in Sweden are given this drug. The bacterial resistance mechanisms to be found in response to such a selection pressure were investigated in a relatively isolated population in northern Sweden (the county of Jämtland), in which one centrally located bacteriological laboratory serves the area. Trimethoprim-resistant strains were collected during an 8-month period from consecutive specimens of bacteria from the urinary tracts of patients. Among the highly resistant strains of enteric bacteria, trimethoprim resistance mediated by transposon-borne dihydrofolate reductase of type I was found to dominate. The corresponding Tn7-like transposon was found to be localized both on the chromosome of isolated Escherichia coli strains and also on a 50-kilobase IncI transferable plasmid which was found in several different serotypes of E. coli. In two enterobacterial strains, resistance to more than 10(3) micrograms of trimethoprim per ml was furthermore found to be caused by a ca. 80-fold increase in the formation of chromosomal dihydrofolate reductase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amyes S. G., Smith J. T. R-factor trimethoprim resistance mechanism: an insusceptible target site. Biochem Biophys Res Commun. 1974 May 20;58(2):412–418. doi: 10.1016/0006-291x(74)90380-5. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth P. T., Datta N., Hedges R. W., Grinter N. J. Transposition of a deoxyribonucleic acid sequence encoding trimethoprim and streptomycin resistances from R483 to other replicons. J Bacteriol. 1976 Mar;125(3):800–810. doi: 10.1128/jb.125.3.800-810.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth P. T., Datta N. Two naturally occurring transposons indistinguishable from Tn7. J Gen Microbiol. 1977 Sep;102(1):129–134. doi: 10.1099/00221287-102-1-129. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Datta N., Barth P. T. Compatibility properties of R483, a member of the I plasmid complex. J Bacteriol. 1976 Mar;125(3):796–799. doi: 10.1128/jb.125.3.796-799.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Dacey S., Hughes V., Knight S., Richards H., Williams G., Casewell M., Shannon K. P. Distribution of genes for trimethoprim and gentamicin resistance in bacteria and their plasmids in a general hospital. J Gen Microbiol. 1980 Jun;118(2):495–508. doi: 10.1099/00221287-118-2-495. [DOI] [PubMed] [Google Scholar]
- Flensburg J., Sköld O. Regulatory changes in the formation of chromosomal dihydrofolate reductase causing resistance to trimethoprim. J Bacteriol. 1984 Jul;159(1):184–190. doi: 10.1128/jb.159.1.184-190.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling M. E., Walton L., Elwell L. P. Monitoring of plasmid-encoded, trimethoprim-resistant dihydrofolate reductase genes: detection of a new resistant enzyme. Antimicrob Agents Chemother. 1982 Nov;22(5):882–888. doi: 10.1128/aac.22.5.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosti-Testu F., Norris V., Brevet J. Restriction map of Tn7. Plasmid. 1983 Jul;10(1):96–99. doi: 10.1016/0147-619x(83)90061-6. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J. B., Olsen R. H. Isolation of large bacterial plasmids and characterization of the P2 incompatibility group plasmids pMG1 and pMG5. J Bacteriol. 1978 Jul;135(1):227–238. doi: 10.1128/jb.135.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W., Datta N., Fleming M. P. R factors conferring resistance to trimethoprim but not sulphonamides. J Gen Microbiol. 1972 Dec;73(3):573–575. doi: 10.1099/00221287-73-3-573. [DOI] [PubMed] [Google Scholar]
- Koch A. L. Evolution of antibiotic resistance gene function. Microbiol Rev. 1981 Jun;45(2):355–378. doi: 10.1128/mr.45.2.355-378.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein C., Brenner S. Site-specific properties of Tn7 transposition into the E. coli chromosome. Mol Gen Genet. 1981;183(2):380–387. doi: 10.1007/BF00270644. [DOI] [PubMed] [Google Scholar]
- Lichtenstein C., Brenner S. Unique insertion site of Tn7 in the E. coli chromosome. Nature. 1982 Jun 17;297(5867):601–603. doi: 10.1038/297601a0. [DOI] [PubMed] [Google Scholar]
- Meynell E., Datta N. Mutant drug resistant factors of high transmissibility. Nature. 1967 May 27;214(5091):885–887. doi: 10.1038/214885a0. [DOI] [PubMed] [Google Scholar]
- Pulkkinen L., Huovinen P., Vuorio E., Toivanen P. Characterization of trimethoprim resistance by use of probes specific for transposon Tn7. Antimicrob Agents Chemother. 1984 Jul;26(1):82–86. doi: 10.1128/aac.26.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sköld O. R-factor-mediated resistance to sulfonamides by a plasmid-borne, drug-resistant dihydropteroate synthase. Antimicrob Agents Chemother. 1976 Jan;9(1):49–54. doi: 10.1128/aac.9.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sköld O., Widh A. A new dihydrofolate reductase with low trimethoprim sensitivity induced by an R factor mediating high resistance to trimethoprim. J Biol Chem. 1974 Jul 10;249(13):4324–4325. [PubMed] [Google Scholar]
- Tennhammar-Ekman B., Sköld O. Trimethoprim resistance plasmids of different origin encode different drug-resistant dihydrofolate reductases. Plasmid. 1979 Jul;2(3):334–346. doi: 10.1016/0147-619x(79)90017-9. [DOI] [PubMed] [Google Scholar]
- Tietze E., Prager R., Tschäpe H. Characterization of the transposons Tn1822 (Tc) and Tn1824 (TpSm) and the light they throw on the natural spread of resistance genes. Plasmid. 1982 Nov;8(3):253–260. doi: 10.1016/0147-619x(82)90063-4. [DOI] [PubMed] [Google Scholar]
- Warner H. R., Lewis N. The synthesis of deoxycytidylate deaminase and dihydrofolate reductase and its control in Escherichia coli infected with bacteriophage T4 and T-4 amber mutants. Virology. 1966 May;29(1):172–175. doi: 10.1016/0042-6822(66)90208-x. [DOI] [PubMed] [Google Scholar]