Abstract
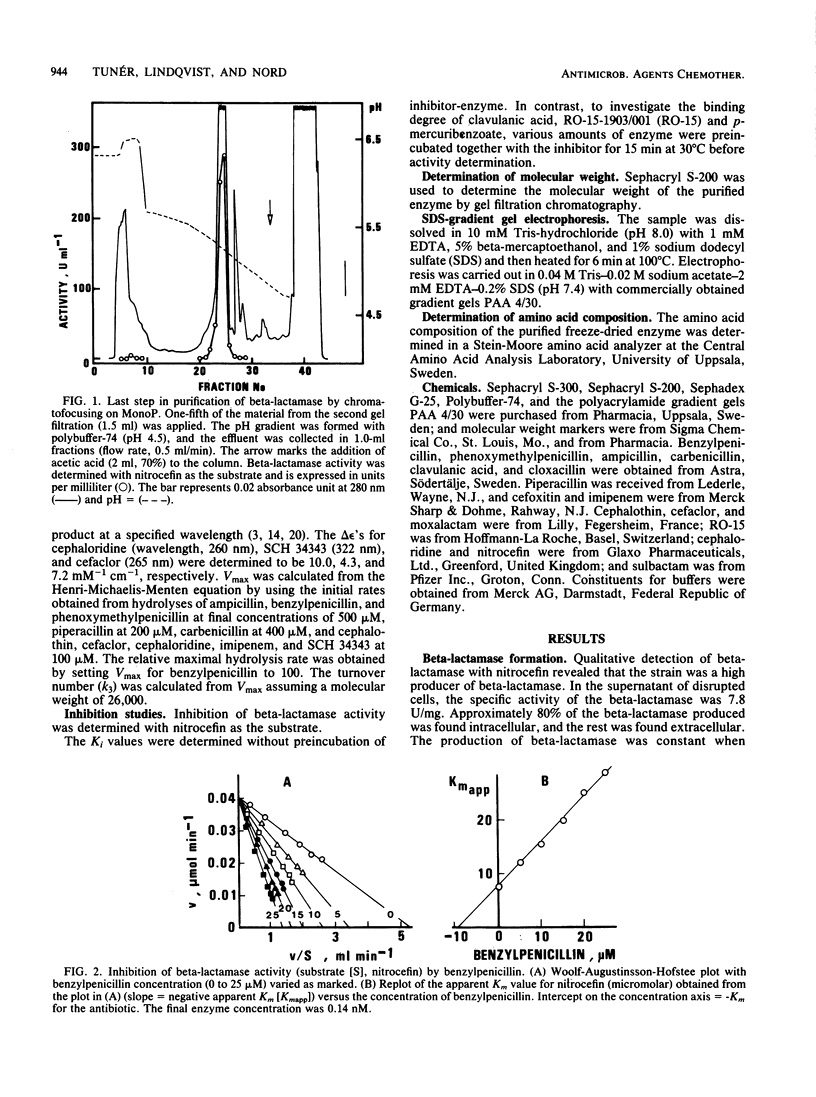
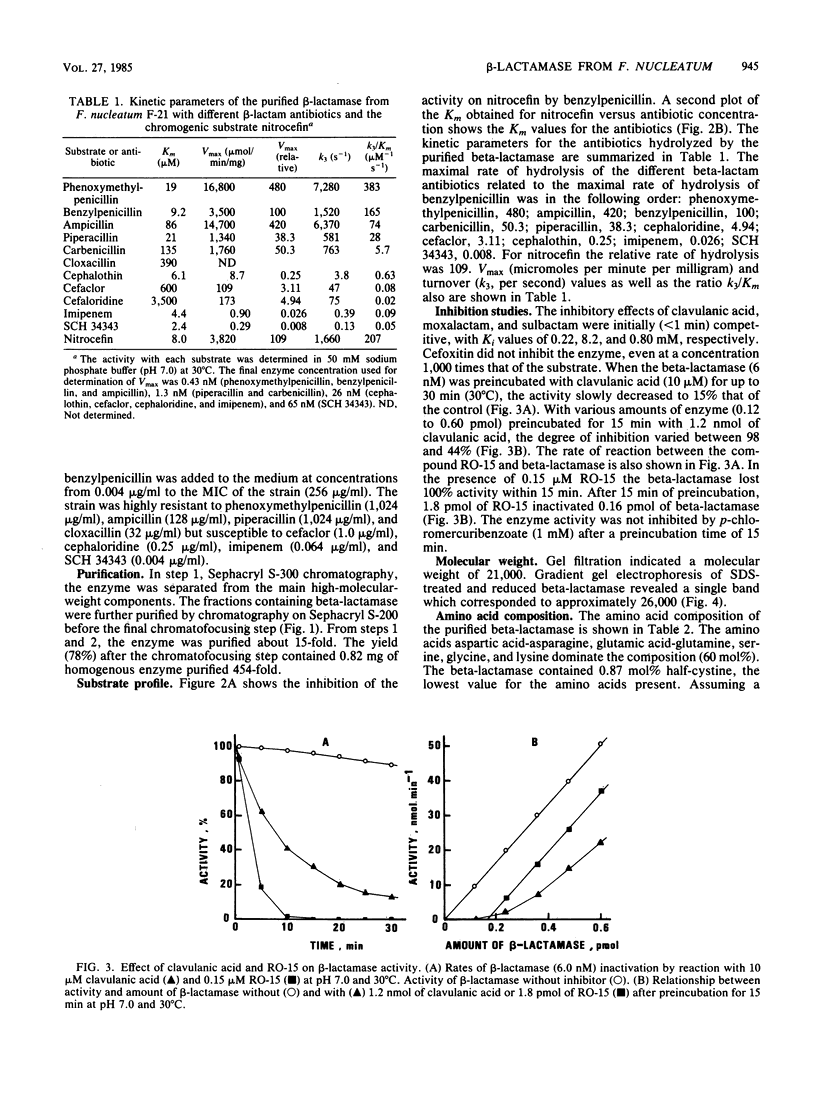
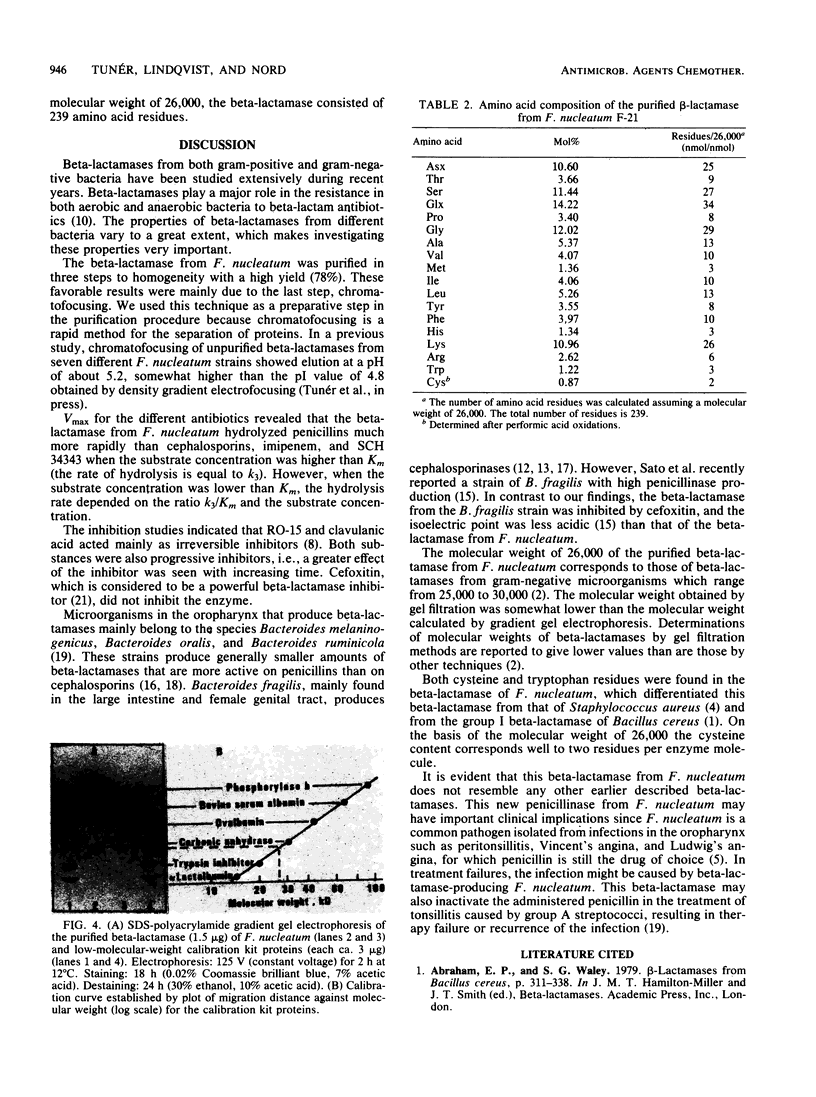
A strain of Fusobacterium nucleatum which produced high levels of beta-lactamase was isolated. The specific activity of the unpurified beta-lactamase was 7.8 U/mg of protein. By Sephacryl S-300 and S-200 column passage and chromatofocusing, the enzyme was purified 450-fold. Sodium dodecyl sulfate-gradient gel electrophoresis revealed a single band. The enzyme hydrolyzed phenoxymethylpenicillin, benzylpenicillin, and ampicillin more rapidly than carbenicillin and piperacillin. Cephaloridine, cefaclor, cephalothin, imipenem, and SCH 34343 had hydrolysis rates of less than or equal to 1% that of phenoxymethylpenicillin. The enzyme was inhibited by clavulanic acid and RO-15-1903/001 but not by p-mercuribenzoate or cefoxitin. Molecular weight by gel filtration was determined to be 21,000 and by sodium dodecyl sulfate-gradient gel electrophoresis was determined to be 26,000. The amino acids aspartic acid-asparagine, glutamic acid-glutamine, serine, glycine, and lysine dominated the amino acid composition.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Kammer R. B., Preston D. A., Turner J. R., Hawley L. C. Rapid detection of ampicillin-resistant Haemophilus influenzae and their susceptibility to sixteen antibiotics. Antimicrob Agents Chemother. 1975 Jul;8(1):91–94. doi: 10.1128/aac.8.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson-Liljequist B., Dornbusch K., Nord C. E. Characterization of three different beta-lactamases from the Bacteroides fragilis group. Antimicrob Agents Chemother. 1980 Aug;18(2):220–225. doi: 10.1128/aac.18.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson B., Nord C. E., Wadström T. Formation of beta-lactamase in Bacteroides fragilis: cell-bound and extracellular activity. Antimicrob Agents Chemother. 1976 May;9(5):727–735. doi: 10.1128/aac.9.5.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers A. A., Wong J., Wilkins T. D. Beta-Lactamase activity in strains of Bacteroides melaninogenicus and Bacteroides oralis. Antimicrob Agents Chemother. 1977 Jan;11(1):142–146. doi: 10.1128/aac.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuni A. A direct spectrophotometric assay and determination of Michaelis constants for the beta-lactamase reaction. Anal Biochem. 1975 Jan;63(1):17–26. doi: 10.1016/0003-2697(75)90185-2. [DOI] [PubMed] [Google Scholar]
- Sato K., Matsuura Y., Inoue M., Mitsuhashi S. Properties of a new penicillinase type produced by Bacteroides fragilis. Antimicrob Agents Chemother. 1982 Oct;22(4):579–584. doi: 10.1128/aac.22.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tally F. P., O'Keefe J. P., Sullivan N. M., Gorbach S. L. Inactivation of cephalosporins by Bacteroides. Antimicrob Agents Chemother. 1979 Nov;16(5):565–571. doi: 10.1128/aac.16.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timewell R., Taylor E., Phillips I. The beta-lactamases of Bacteroides species. J Antimicrob Chemother. 1981 Feb;7(2):137–146. doi: 10.1093/jac/7.2.137. [DOI] [PubMed] [Google Scholar]
- Tunér K., Nord C. E. Betalactamase-producing microorganisms in recurrent tonsillitis. Scand J Infect Dis Suppl. 1983;39:83–85. [PubMed] [Google Scholar]
- Waley S. G. A spectrophotometric assay of beta-lactamase action on penicillins. Biochem J. 1974 Jun;139(3):789–790. doi: 10.1042/bj1390789. [DOI] [PMC free article] [PubMed] [Google Scholar]




