Abstract
The diurnal variation in the incidence of myocardial infarction and stroke may reflect an influence of the molecular clock and/or the time dependence of exposure to environmental stress. The circadian variation in blood pressure and heart rate is disrupted in mice, Bmal1−/−, Clockmut, and Npas2mut, in which core clock genes are deleted or mutated. Although Bmal1 deletion abolishes the 24-h frequency in cardiovascular rhythms, a shorter ultradian rhythm remains. Sympathoadrenal function is disrupted in these mice, which reflects control of enzymes relevant to both synthesis (phenylethanolamine N-methyl transferase) and disposition (monoamine oxidase B and catechol-O-methyl transferase) of catecholamines by the clock. Both timing and disruption or mutation of clock genes modulate the magnitude of both the sympathoadrenal and pressor but not the adrenocortical response to stress. Despite diurnal variation of catecholamines and corticosteroids, they are regulated differentially by the molecular clock. Furthermore, the clock may influence the time-dependent incidence of cardiovascular events by controlling the integration of selective asynchronous stress responses with an underlying circadian rhythm in cardiovascular function.
Keywords: catecholamine, clock
The clinical onset of both myocardial infarction and stroke occurs more frequently in the early morning than at other times of day (1); there may also be interseasonal variation in the incidence of these conditions (2). Although the circadian variation in vascular events corresponds to that in blood pressure (BP) and to oscillation of genes relevant to hemostasis (3), it is unknown whether this reflects an important role for the molecular clock or merely the physical and emotional stress associated with arousal and initial mobilization. A third potential variable is the time dependence of access to diagnostic procedures (4). Given the importance of BP in the determination of clinical cardiovascular outcomes, including both myocardial infarction and stroke and the coincidence of circadian rhythms in BP and sympathoadrenal function, as reflected by plasma catecholamines, we chose to define the importance of the molecular clock in this system.
The molecular clock in mammals is located centrally in the suprachiasmatic nucleus (SCN) (5). Although this is the master regulator, it is now appreciated that there are molecular clocks in most peripheral tissues, themselves capable of entrained and autonomous functions (6). The exception, so far, is the testis, in which core clock genes are expressed at a constant high level (7). Clocks of potential relevance to cardiovascular function have been identified in aorta (8), liver, kidney, and heart (9, 10); there is also evidence to suggest diversity among clock components within the cardiovascular system (11). Although the entrainment signals that link the SCN with the periphery are presently poorly understood, they are likely to include circulating hormones (8).
The molecular clock comprises interlocking positive and negative transcriptional and translational feedback loops that drive circadian gene expression (12), further refined by posttranslational modifications of their corresponding proteins. Heterodimers of the basic helix–loop–helix Per Arnt Sim transcription factors, CLOCK and BMAL1, comprise the positive limb of an autoregulatory feedback loop (13, 14). CLOCK:BMAL1 heterodimers drive expression of per and cry, which themselves then dimerize and comprise the negative limb of the feedback loop by repressing their own transcription (12, 15).
Results
BMAL1 and CLOCK Are Indispensable for the Circadian Rhythm in BP.
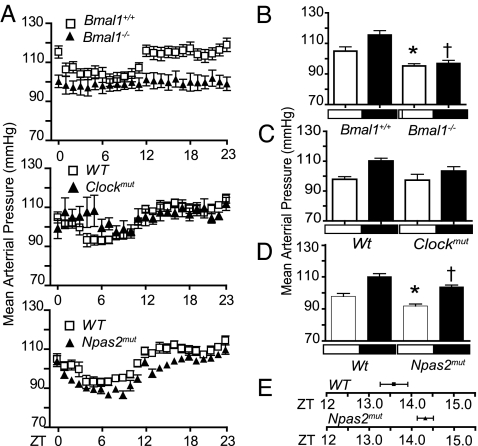
Using telemetry, mean arterial BP (MAP), heart rate (HR), and activity were recorded every 5 min under a light–dark (LD) regimen over 72 h in Bmal1−/−, Clockmut, and Npas2mut mice and WT littermate controls. A robust diurnal variation in MAP, HR, and activity was apparent in Bmal1+/+ and WT mice [Fig. 1A; supporting information (SI) Fig. 5 A and B]. BP, HR, and activity increase during the dark cycle (active period) relative to the light cycle (inactive period) (16). In contrast, the diurnal variation in MAP, HR, and activity was completely abolished in Bmal1−/− mice. Clockmut caused disruption of MAP during the light phase and HR during the dark phase. Npas2mut mice still maintained rhythmicity but had reduced MAP and HR, particularly during the hours surrounding the LD transition, with no effect on activity (Fig. 1A; SI Fig. 5 A and B).
Fig. 1.
BMAL1 and CLOCK and NPAS2 differ in severity of BP variation and maintenance according to the severity of the genotype. (A) MAP telemetry recordings averaged each hour from three 24-h periods in WT, Bmal1−/−, Clockmut, and Npas2mut mice. MAP recordings were significantly different (Kruskal–Wallis test) for Bmal1−/− (light phase, P < 0.05; dark phase, P < 0.0001) and Npas2mut mice (both light and dark phase. P < 0.0001). Clockmut mice were significantly different only during the light phase (P < 0.05). MAP recordings taken separately during light and dark phases in Bmal1+/+ vs. Bmal1−/− (B) (n = 8; ∗, P < 0.05; †, P < 0.001), WT vs. Clockmut (C) (n = 4–8) WT vs. Npas2mut mice (D) (n = 8; ∗, P < 0.05), and delayed acrophase (time of peak) of MAP rhythm in Npas2mut vs. WT (D) (n = 8, P < 0.05). White boxes denote lights on, and dark boxes denote lights off.
Telemetry recordings of Bmal1+/+ and WT mice taken separately during the LD phase revealed an expected elevation of MAP during the nocturnal active phase (P < 0.05) (Fig. 1 B and C) in WT and Bmal1+/+ but not in Bmal1−/− mice. Deletion of Bmal1 also resulted in a general hypotensive phenotype. MAP was lower in both the light (105 ± 2.8 vs. 95.27 ± 1.5 mmHg, P = 0.02) and dark phase (115.5 ± 2.9 vs. 96.9 ± 2, P = 0.0006) compared with littermate controls (Fig. 1B). Such a hypotensive phenotype was not observed in Clockmut mice, despite disruption of diurnal variability in MAP (Fig. 1C). Npas2mut mice are hypotensive, similar to Bmal1−/− with a significant reduction in MAP levels observed during both their resting (97.86 ± 1.8 vs. 91.84 ± 1.2, P = 0.03) and activity phases (110.2 ± 1.8 vs. 103.8 ± 1.2, P = 0.014) (Fig. 1D); this was also observed with tail-cuff plethysmography measurements at Zeitgeber time (ZT) 7 (SI Fig. 6). Despite maintenance of a circadian rhythm in the Npas2mut group, the acrophase (time of peak) of 24-h MAP was delayed in comparison to WT littermate controls (1:34 a.m. ± 19 min vs. 2:19 a.m. ± 11 min, P = 0.04) (Fig. 1E).
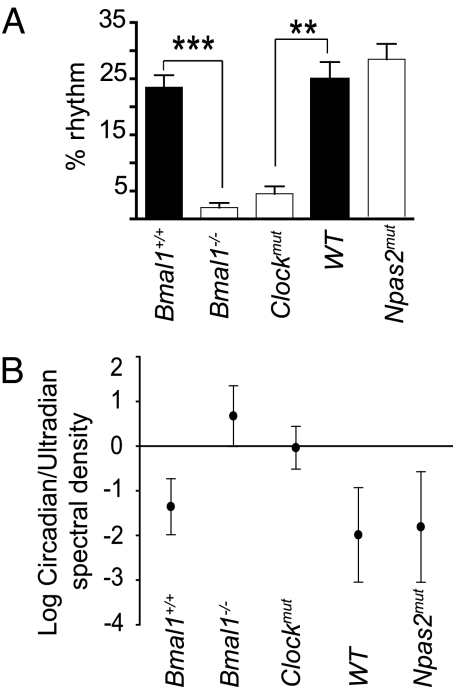
MAP data gathered over 3 days were fitted to a 24-h period harmonic; a percentage rhythm to this harmonic was scored as described (17). Deletion of Bmal1 (23.5 ± 2.1 Bmal1+/+ vs. 2.05 ± 0.8 Bmal1−/−; ∗∗∗, P = 0.0002) and mutation of Clock (24.9 ± 3.05 WT vs. 4.4 ± 1.3 Clockmut; ∗, P = 0.004) reduced significantly the 24-h period harmonic, whereas this was not altered significantly in Npas2mut mice (Fig. 2A). We estimated the strength of rhythmicity at each period by a spectrum density. A strong diurnal peak in both MAP and activity at the 24-h frequency was evident in all WT. Less distinct peaks of varying intensities corresponding to ultradian rhythms were visible in mice between 1 and 4 h. Spectral density values of corresponding circadian and ultradian frequencies were calculated in each mouse and a log ratio of circadian to ultradian spectral density plotted (Fig. 2B). Distinct ultradian rhythms were clearly stronger than any residual circadian rhythmicity in Bmal1−/− mice (Mann–Whitney test, P < 0.001). Dominant ultradian behavior has been observed in the common vole, Microtus arvalis (18). Unlike circadian rhythms driven from the SCN, ultradian rhythms are regulated by the arcuate nucleus and retrochiasmatic region of the hypothalamus (18). Similar to the hierarchical impact of gene manipulation on MAP and behavior, mutation of Clock exhibited roughly similar dominance of circadian and ultradian rhythms, whereas Npas2mut were indistinguishable from WT controls. Npas2mut mice were also subjected to a constant darkness (DD) regimen (data not shown). They remained rhythmic and hypotensive in DD, results indistinguishable from those gained in LD.
Fig. 2.
Circadian but not ultradian rhythmicity depends on BMAL1 and CLOCK. Twenty-four-hour period harmonic (A) and log ratio of circadian and ultradian spectal values (B) were calculated for Bmal1+/+, Bmal1−/[supi]−, Clockmut, WT, and Npas2mut mice.
Thus, these three genes, which function in the core clock, appear to play discrete but important roles in regulating vascular homeostasis and maintaining circadian variation of MAP. Although BMAL1 and CLOCK appear fundamental to circadian oscillation in BP, NPAS2 may function, in the presence of CLOCK, to define the precise timing of these rhythms. The recent observation that deletion, as opposed to mutation, of CLOCK does not render mice arrhythmic (19) is consistent with our previous observations (6) that NPAS2 can substitute as a heterodimeric partner of BMAL1 in a setting of CLOCK deficiency. Aside from their importance in diurnal variation in BP, the present studies also reveal an unexpected role for both BMAL1 and NPAS2 in the maintenance of BP, perhaps reflecting roles independent of the function of the molecular clock. The hierarchical contribution of the three genes to vascular rhythms in vivo is reflected by their impact on the oscillatory expression of the clock component per2 in response to serum shock of murine aortic vascular smooth muscle cells (mASMC) in culture. Although per2 fluctuates rhythmically in WT and Npas2mut mASMCs, cyclical gene expression was abolished in Bmal1−/− and Clockmut mASMC (SI Fig. 7).
Corresponding to the rhythms in MAP, those in HR were lost in Bmal1−/−, dampened in Clockmut, but preserved in Npas2mut mice (SI Fig. 5A). HR was significantly lower in Bmal1−/− and Clockmut mice in comparison to controls only during the active phase (SI Fig. 5C). Conversely, Npas2 mutation results in a significant reduction in HR during the resting phase (SI Fig. 5C). Thus, the baroreflex response to hypotension (20) appears to be diminished in both Bmal1−/− and Npas2mut mice. Disruption of the baroreflex by sympathectomy in rats results in a loss of circadian variation in MAP but not HR (21). Therefore, the molecular clock might exert its circadian control of BP oscillation in part via the baroreflex. We have reported previously that the baroreflex is subject to diurnal variation in humans (22). MAP and HR are increased in both the light and dark phases, and baroreflex sensitivity is enhanced in mice lacking CRY1 and CRY2, proteins that mediate an inhibitory feedback in the molecular clock (23). Consistent with these observations, BMAL1, CLOCK, and NPAS2, components of the positive loop of the clock, exert opposing actions on BP and HR.
The Molecular Clock Influences Sympathoadrenal Function.
BP and HR are under the control of various hormonal systems including the catecholamines, norepinephrine (NE) and epinephrine (Epi). Plasma (24) and urinary (25) levels of these catecholamines exhibit a diurnal variation, with higher levels during the active phase. NE and Epi were measured in plasma in terminal bleeds drawn from all three mouse models at ZT2 and ZT14 (Table 1). Corresponding to the hypotension that accompanied the asynchronous MAP phenotype in Bmal1−/− mice, both NE and Epi were significantly reduced at both ZT2 and ZT14 compared with Bmal1+/+ controls. Plasma NE and Epi were significantly lower at ZT2 in Npas2mut mice, which exhibit hypotension but preserve their circadian rhythm of MAP. These indices of sympathoadrenal function were not depressed in Clockmut mice, again corresponding to MAP.
Table 1.
The molecular clock influences sympathoadrenal and neuronal function
| ZT | Bmal1+/+ | Bmal1-/- | WT | Npas2mut | Clockmut | |
|---|---|---|---|---|---|---|
| NE | 2 | 23 ± 4 | 6 ± 1* | 39 ± 10 | 21 ± 3* | 47 ± 10 |
| NE | 14 | 17 ± 2 | 9 ± 2* | 33 ± 4 | 23 ± 5 | 33 ± 5 |
| Epi | 2 | 38 ± 8 | 4 ± 1* | 47 ± 6 | 27 ± 1* | 38 ± 5 |
| Epi | 14 | 29 ± 3 | 9 ± 3* | 52 ± 7 | 44 ± 14 | 33 ± 5 |
Values are represented as mean ± SEM; n = 4–6 per group.
*, P < 0.05 vs corresponding control group (nonparametric Mann–Whitney test).
Genes Relevant to Catecholamine Synthesis and Disposition Are Under the Control of the Molecular Clock.
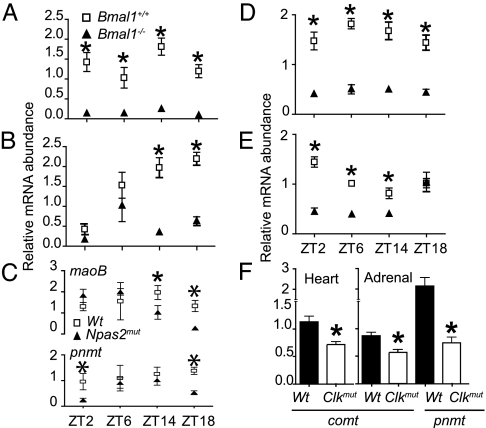
mRNA was harvested from adrenal glands and hearts of mice at ZT2, ZT6, ZT14, and ZT18 and analyzed for the catecholamine biosynthetic enzymes, tyrosine hydroxylase (th), dopamine β hydroxylase (dbh), and phenylethanolamine N-methyl transferase (pnmt) and the catecholamine-metabolizing enzymes catechol-O-methyl transferase (comt), and monoamine oxidase A and B. Expression of pnmt, the rate-limiting enzyme in the conversion of NE to Epi, was markedly depressed in both heart and adrenals in Bmal1−/− (Fig. 3 A and B) and to a lesser extent in Npas2mut at ZT2 and ZT18 (Fig. 3C). Drugs that inhibit pnmt reduce BP (26). The MAO enzymes account for rapid reuptake of catecholamines from the sympathetic nerve cleft, and inhibitors of maoB are under investigation as potential therapies for Parkinson's disease (27). Expression of maoB, just like pnmt, was dramatically reduced in the hearts and adrenals of Bmal1−/− mice (Fig. 3 D and E). Administration of specific inhibitors of maoB, including those not metabolized to amphetamines (which might act centrally to depress BP), also result in hypotension, probably reflecting accumulation of dopamine or the false transmitter, octopamine (27). Expression of maoB was depressed to a more limited extent in Npas2mut mice, evident only in adrenals at ZT14 and ZT18 (Fig. 3C). A limited investigation of expression in Clockmut mice at ZT14 showed reduced pnmt in adrenals and comt in both heart and adrenals (Fig. 3F). Although there was a significant change in pnmt and maoB expression in WT over time (one-way ANOVA), our analysis did not indicate a circadian profile. Furthermore, the pattern of pnmt expression in heart, assayed every 4 h over 48 h by microarray, was not circadian (unpublished data). Therefore, the influence of the molecular clock on pnmt, comt, and maoB is indirect; such an effect could account for the observed impact on resting and diurnal variation in BP. Despite extensive analysis, defects in expression of the other enzymes involved in catecholamine synthesis or disposition were not observed in the mutants.
Fig. 3.
Pnmt, maoB, and comt are under clock control in hearts and adrenals. mRNA was analyzed by real-time PCR at ZT2, ZT6, ZT14, and ZT18 for pnmt expression (A) and maoB expression (D) in hearts of Bmal1+/+ vs. Bmal1−/− mice (n = 4–8; ∗, P < 0.01) pnmt expression (B) and maoB expression (E) in adrenals of Bmal1+/+ vs. Bmal1−/− mice (n = 4–8; ∗, P < 0.01) maoB and pnmt expression in WT and Npas2mut (C) (n = 4–5; ∗, P < 0.05), and comt and pnmt expression in WT and Clockmut (F) (n = 4; ∗, P < 0.05).
RNA microarray analysis of aortas from Bmal1−/−, Npas2mut, and their respective WT was performed at circadian time 14. Decreased expression of a number of recognized targets of BMAL1, such as per1, per2, and D site albumin promoter-binding protein (dbp), was observed in Bmal1−/− mice (SI Fig. 8A). Interestingly, the most markedly up-regulated gene in Bmal1−/− aortae was npas2, corresponding to its increased expression in Bmal1−/− mASMCs after serum shock (SI Fig. 8C). Among the genes differentially regulated in aorta, but not in heart or adrenals, by Bmal1 deletion is comt, an enzyme that accounts for an extraneuronal low-affinity high-capacity “sink” for clearance of catecholamines. Unlike MaoB inhibitors, selective inhibitors of COMT do not alter BP in humans except in those with severe impairment of baroreflex function resulting from multisystem atrophy (28), in whom it may cause mild hypertension. On the other hand, Comt-deficient mice are resistant to salt induced hypertension (29). Comt itself exhibits a robust circadian profile of gene expression in both aorta and kidney but not the liver or SCN (30). Kininogen, another gene of potential relevance to BP control, was also dysregulated in aorta of Bmal1−/− mice (31) (SI Fig. 8A). Mutation of npas2 also disrupted aortic expression of a number of genes regulated by the molecular clock, such as per2, and casein kinase 1 delta (SI Fig. 8B). However, we failed to identify dysregulated expression of catecholamine-related genes, emphasizing the discrete mechanisms by which the individual clock proteins may influence cardiovascular function in a tissue-specific manner. No significant compensatory changes in bmal1 or clock expression were detected in the hypothalamus or brainstem of the mouse models (data not shown). Similar to the microarray data, npas2 expression was up-regulated in Bmal1−/− mice in the hypothalamus and brainstem (SI Fig. 8D). Therefore, it would appear that the effect on MAP and HR is not solely because of differential expression of Bmal1/Npas2/Clock in specific brain regions.
The Circadian Clock Modulates Selective Stress Responses.
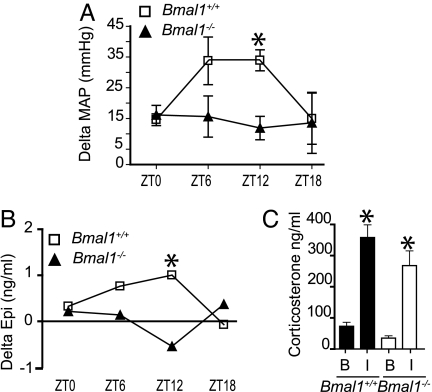
Restraint serves as a source of emotional stress for rodents that results in rapid sympathoadrenal activation (32). Bmal1−/− mice and corresponding controls were restrained for 15 min at separate clock times, and their pressor and sympathetic response was assessed. We observed a temporal variation in the pressor response to stress in Bmal1+/+ with an increased response at ZT6 and ZT12. However, similar to free running MAP (Fig. 4A), the temporal variation in the pressor response was abolished in Bmal1−/−, and the stress-induced alteration in BP was significantly lower at ZT12 (Fig. 4A). Plasma catecholamines were also measured. Similar to the pressor response, the increase in plasma Epi in response to stress occurred in a time-dependent fashion in Bmal1+/+ mice. This response was also lost with Bmal1 deletion (Fig. 4B). BMAL1 deletion did not affect the corticosterone response to stress at ZT12, in contrast to the pressor and plasma Epi response. Surprisingly, we did not detect a significant increase in NE in response to restraint in either Bmal1+/+ or Bmal1−/− mice (SI Fig. 9 A and B). This may reflect the size of the evoked response relative to the elevation in NE induced by the experimental conditions.
Fig. 4.
The circadian clock modulates pressor and hormonal but not adrenocortical responses to stress. (A) Telemetry recordings of MAP were taken at baseline and after restraint within each mouse. Delta MAP values were calculated for Bmal1+/+ and Bmal1−/− at ZT0, ZT6, ZT12, and ZT18, (n = 4–8; ANOVA, ∗, P < 0.05). (B) Serum was collected by retroorbital bleed from naïve (baseline) Bmal1+/+ and Bmal1−/− mice and naïve Bmal1+/+ and Bmal1−/− mice after 15-min restraint at ZT0, ZT6, ZT12, and ZT18, and delta Epi values were calculated for both Bmal1+/+ and Bmal1−/− responses (n, 4–8; ∗, P < 0.05). (C) Glucocorticoid analysis from serum collected as in B from animals at ZT12 at baseline (B) or immobilized (I).
Discussion
Surgical ablation of the SCN (33) disrupts circadian variation in BP, along with endocrine and behavioral rhythms. However, this approach results in lesions of varying size and specificity and affords no molecular insight into an interaction between the clock and BP. Besides elucidating distinct roles for three core clock genes in BP and its diurnal variation, we also implicate the molecular clock in the vascular response to stress, irrespective of its timing. Thus, the clock may influence the vascular response to stress indirectly, by controlling the underlying rhythm of BP on which asynchronous cues are imposed but also directly by modulating pressor response, irrespective of timing. Both effects reflect the influence of the clock on sympathoadrenal activation, which is activated in the integrated arousal response and many of its discrete elements, such as assumption of the upright posture, exercise, and emotional stress. The clock appears to exert effects on the vasculature by modulating both the synthesis and disposition of catecholamines. Consistent with the hemodynamic impact of inhibitors of both PNMT and MAOB (26, 27) and deletion of comt (28), depressed expression of these clock-controlled genes coincide here with hypotensive phenotypes. Indeed, given the nonredundant role of BMAL1 with either CLOCK or NPAS2 within the molecular clock, its impact on this humoral system may be more profound than suggested by single-gene disruptions.
There is some precedent to suggest a connection between the clock and sympathoadrenal function. An anatomical connection exists between the SCN and the adrenal gland by sympathetic nerves, as revealed by transneural virus tracing (34) and light-induced per1 induction of the adrenal gland (35). Tyrosine hydroxylase is up-regulated in the midbrain ventral tegmental area of Clockmut mice (36), and mice lacking dopamine β hydroxylase (Dbh) are hypotensive and lose some of their circadian variability in BP (37). However, in contrast to Bmal1−/− and Clockmut mice in the present study, they retain a functional clock and preserve their activity profiles and a diurnal variation in HR.
Circadian variation in BP is lost when the negative limb of the clock is disrupted. Thus, diurnal changes in BP, along with periodicity in activity, are lost in DD in Cry1−/−/Cry2−/− mice (23). Pertinent to our studies of emotional stress, the pressor response to the α1-adrenoreceptor agonist, phenylephrine is severely suppressed in these mice, irrespective of clock time (23), raising the possibility that clock genes might also influence the response to sympathoadrenal activation. Finally, prolonged changes in light exposure, such as constant light or DD, alter expression of the genes th, dbh, and pnmt.
We used restraint as a model of stress that causes sympathoadrenal activation in rodents (38). The underlying circadian rhythm, unconfounded by activity, conditions the magnitude of the sympathoadrenal and hypertensive response to a standardized stress. The most pronounced response to stress occurred in mice at the nadir of the rhythm, corresponding to the early-morning hours when clinical vascular events begin to peak. Indeed, one might expect this effect to be pronounced in patients with hypertension, in whom the amplitude of the diurnal variation in BP is exaggerated (39) or in those individuals who do not lower their BP at night. Such “nondippers” also lose their rhythm in sympathoadrenal function and, like hypertensives, are at increased risk of cardiovascular morbidity and mortality (39, 40). Deletion of Bmal1, irrespective of the timing of the immobilization, displayed a markedly reduced stress response. Takumi et al. (41) have recently reported that behavioral rhythms and the peripheral molecular oscillator are quite resistant to stress, but a stress-related increase in the expression of per1 mRNA induced by glucocorticoids is preserved. The present studies reveal that Bmal1 deletion does not affect the adrenocortical response to immobilization, analogous to our previous observations that the corticosterone response to the stress of insulin-induced hypoglycemia is also preserved in Bmal−/− mice (42).
In summary, we have shown that genes that subserve core functions in the molecular clock regulate differentially enzymes relevant to the synthesis and disposition of catecholamines. Disruption or deletion of clock genes results in alterations in BP, plasma NE and Epi, their diurnal variation, and, surprisingly, their response to immobilization stress. Clock-dependent effects on BP may also interact with diurnal variation of hemostatic variables, such as PAI-1 (43), to determine the diurnal incidence of cardiovascular events. The clock may influence the temporal incidence of clinical cardiovascular events, not only by regulating the magnitude of the early morning rise in BP, but also in the extent of the pressor response to environmental stress, which itself is conditioned by timing.
Materials and Methods
Animals.
Bmal1−/−, Clockmut, and Npas2mut mice and littermate controls were allowed to acclimatize to a 12/12-h LD cycle, lights on (7:00–19:00 h, ZT0–ZT12), lights off (19:00–7:00 h, ZT12–ZT24) for 2 weeks before surgical experimentation. Bmal1−/− and WT mice were housed singly for 1 wk in LD for restraint experiments, after which they were handled at approximately the same time of day for 5 continuous days, and experimental restraint protocol was carried out on sixth day in LD. Clockmut and Npas2mut mice used were fully backcrossed onto the C57BL/6J background and were both compared with the same group of C57BL/6J animals (WT). The Bmal1−/− colony was backcrossed to 5–6th generation and therefore compared with Bmal1+/+ littermate controls.
Carotid Artery Implantation of Telemetry Probes.
Animals were anesthetized by intramuscular injection (50 μl/25 g) of a mixture of ketamine (100 mg/kg, 300 μl) and xylazine (20 mg/kg, 150 μl) in 0.9% saline (450 μl). PA-C20 telemetry probes (Data Sciences, St. Paul, MN) were implanted by using the carotid artery placement as described (16). Telemetry recordings began 10 days after surgery.
Data Acquisition and Analysis.
Continuous 24-h systolic, diastolic, pulse pressure, HR, and activity were monitored in unrestricted animals with the use of the Dataquest IV system (Data Sciences), as described (44). Circadian rhythm analysis of the individual hourly MAP and HR data was performed with the nonlinear least-squares fitting program PHARMFIT (17), and the “best-fit” model was defined as the one with the lowest number of harmonics that had a confidence value of at least 0.05, as determined by the subprogram SYNOPS. All PHARMFIT analyses were based on data for 3 consecutive days, thus allowing measurements of the mean midline estimating statistic of rhythm for active and resting phases and acrophase (clock time of peak amplitude) of the 24-h adjusted rhythm. Fourier analyses were conducted on the first 2 days of MAP and activity measurements. Activity measurements were square root transformed to normalize the variance, assuming a Poisson distribution. Smoothed periodograms for typical mice were generated in which the strength of the rhythmicity at each cycle length is estimated by the spectral density.
Serum Shock.
Primary mASMCs from WT, Bmal1−/−, Clockmut, and Npas2mut mice were prepared and plated on 10-cm2 plates (3,500 cells/cm2) ≈6 days before the experiment and serum-shocked, as described (45).
Tissue Harvesting.
Mice were killed at prespecified times. The abdomen and thorax were opened and blood sample taken. The animal was perfused by the left ventricle with 10 ml of PBS, followed by 5 ml of diethyl pyrocarbonate-treated water (Fischer Scientific, Hampton, NH). The adrenals were harvested and immediately flash-frozen in liquid nitrogen. The aorta was exposed, as close to the heart as possible at the aortic arch, to just beyond the femoral artery bifurcation, by using a dissection microscope. It was removed and immediately flash-frozen along with the heart. Whole brains were extracted and allowed to soak in RNAlater (Ambion, Austin, TX) for ≈4 h, after which brainstem and hypothalamus regions were isolated.
RNA Analysis.
Total RNA was extracted from cells and tissues as described (46).
DNA Microarray Hybridization and Data Analysis.
Aortae were harvested from male mice at ZT14. Target cRNA was prepared from total RNA and hybridized by using 20 μg of cRNA onto Affymetrix (Santa Clara, CA) MOE 430V2 chips in accordance with the protocols described in the Affymetrix Gene-Chip Expression Analysis Technical Manual. Statistical analysis on the microarray data was carried out by using freely distributed software implemented in R 2.0.1 (www.cran.r-project.org and www.BioConductor.org). Specifically, the data were normalized by using GCRMA (47) with the default settings. Differential expression was analyzed by using linear models estimated by an Empirical Bayes procedure, as implemented in LIMMA (48) by using FDR (49) correction for multiple testing. Differentially expressed transcripts were reconfirmed by quantitative real-time PCR.
Quantitative real-time PCR was carried out as described (46). Primer sequences are described in SI Table 2.
Restraint Protocol.
Bmal1−/− and Bmal1+/+ mice were sampled undisturbed (baseline) or restrained in a prone position for 15 min immediately before sampling. Changes in MAP were measured within the same animal, whereas catecholamine analysis was performed by using separate animals. Animals were lightly anesthetized (isoflurane) and a 100-μl blood sample was removed by retroorbital plethysmography and plasma extracted for catecholamine analysis as detailed below.
Plasma Extraction for Terminal Catecholamine Analysis.
Blood samples were taken from the vena cava by using a 1-ml syringe containing 100 μl of heparin and plasma-extracted as described (42).
Catecholamine and Corticosterone Analysis.
Plasma Epi and NE were measured by using a commercially available enzyme immunoassay kit (Bi-CAT EIA, ALPCO Diagnostics, Salem, NH). Plasma corticosterone was measured by using a commercially available RIA kit (Corticosterone, 125I RIA, MP Biomedicals, Solon, OH).
Statistical Analysis.
Data are expressed as mean ± SEM. Differences between means were tested by using the ANOVA or a nonparametric Mann–Whitney test.
Supplementary Material
Acknowledgments
We thank Christopher A. Bradfield (University of Wisconsin, Madison) and Celeste Simon (University of Pennsylvania) for providing BMAL1−/− mice, Joseph S. Takahashi (Northwestern University, Evanston, IL) and Amita Sehgal (University of Pennsylvania) for Clockmut mice, and Steve L. McKnight (University of Texas Southwestern, Dallas) for providing Npas2mut mice. We are indebted to R. Daniel Rudic, Debu Chakravarti, and Teresa Reyes for helpful suggestions. We thank Weili Yan and John O'Donovan for technical help and Eileen Callaghan for assistance with figures. This work was supported by National Institutes of Health Grants HL70128 and HL62250. G.A.F. is the Elmer Bobst Professor of Pharmacology.
Abbreviations
- SCN
suprachiasmatic nucleus
- BP
blood pressure
- MAP
mean arterial BP
- HR
heart rate
- LD
light–dark
- ZT
Zeitgeber time
- DD
constant darkness
- mASMC
murine aortic vascular smooth muscle cells
- NE
norepinephrine
- Epi
epinephrine.
Footnotes
The authors declare no conflict of interest.
Data deposition: The microarray data reported in this paper have been deposited in the NCBI database (accession nos. GSE3849 and GSE3850).
This article contains supporting information online at www.pnas.org/cgi/content/full/0611680104/DC1.
References
- 1.Muller JE. Am J Hypertens. 1999;12:35S–42S. doi: 10.1016/s0895-7061(98)00278-7. [DOI] [PubMed] [Google Scholar]
- 2.Manfredini R, Boari B, Smolensky MH, Salmi R, Gallerani M, Guerzoni F, Guerra V, Maria Malagoni A, Manfredini F. Chronobiol Int. 2005;22:1121–1135. doi: 10.1080/07420520500398106. [DOI] [PubMed] [Google Scholar]
- 3.Hastings MH, Reddy AB, Maywood ES. Nat Rev Neurosci. 2003;4:649–661. doi: 10.1038/nrn1177. [DOI] [PubMed] [Google Scholar]
- 4.Weber MA, Fodera SM. Rev Cardiovasc Med. 2004;5:148–155. [PubMed] [Google Scholar]
- 5.Ralph MR, Foster RG, Davis FC, Menaker M. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- 6.Schibler U, Ripperger J, Brown SA. J Biol Rhythms. 2003;18:250–260. doi: 10.1177/0748730403018003007. [DOI] [PubMed] [Google Scholar]
- 7.Alvarez JD, Chen D, Storer E, Sehgal A. Biol Reprod. 2003;69:81–91. doi: 10.1095/biolreprod.102.011833. [DOI] [PubMed] [Google Scholar]
- 8.McNamara P, Seo SB, Rudic RD, Sehgal A, Chakravarti D, FitzGerald GA. Cell. 2001;105:877–889. doi: 10.1016/s0092-8674(01)00401-9. [DOI] [PubMed] [Google Scholar]
- 9.Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, Schibler U. Science. 2000;289:2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 10.Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davidson AJ, London B, Block GD, Menaker M. Clin Exp Hypertens. 2005;27:307–311. [PubMed] [Google Scholar]
- 12.Shearman LP, Sriram S, Weaver DR, Maywood ES, Chaves I, Zheng B, Kume K, Lee CC, van der Horst GT, Hastings MH, Reppert SM. Science. 2000;288:1013–1019. doi: 10.1126/science.288.5468.1013. [DOI] [PubMed] [Google Scholar]
- 13.Young MW, Kay SA. Nat Rev Genet. 2001;2:702–715. doi: 10.1038/35088576. [DOI] [PubMed] [Google Scholar]
- 14.Reppert SM, Weaver DR. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 15.Kume K, Zylka MJ, Sriram S, Shearman LP, Weaver DR, Jin X, Maywood ES, Hastings MH, Reppert SM. Cell. 1999;98:193–205. doi: 10.1016/s0092-8674(00)81014-4. [DOI] [PubMed] [Google Scholar]
- 16.Carlson SH, Wyss JM. Hypertension. 2000;35:E1–E5. doi: 10.1161/01.hyp.35.2.e1. [DOI] [PubMed] [Google Scholar]
- 17.Mattes A, Witte K, Hohmann W, Lemmer B. Chronobiol Int. 1991;8:460–476. doi: 10.3109/07420529109059182. [DOI] [PubMed] [Google Scholar]
- 18.van der Veen DR, Minh NL, Gos P, Arneric M, Gerkema MP, Schibler U. Proc Natl Acad Sci USA. 2006;103:3393–3398. doi: 10.1073/pnas.0507825103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Debruyne JP, Noton E, Lambert CM, Maywood ES, Weaver DR, Reppert SM. Neuron. 2006;50:465–477. doi: 10.1016/j.neuron.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 20.Cowley AW, Jr, Liard JF, Guyton AC. Circ Res. 1973;32:564–576. doi: 10.1161/01.res.32.5.564. [DOI] [PubMed] [Google Scholar]
- 21.Makino M, Hayashi H, Takezawa H, Hirai M, Saito H, Ebihara S. Circulation. 1997;96:1667–1674. doi: 10.1161/01.cir.96.5.1667. [DOI] [PubMed] [Google Scholar]
- 22.Hossmann V, FitzGerald GA, Dollery CT. Cardiovasc Res. 1980;14:125–129. doi: 10.1093/cvr/14.3.125. [DOI] [PubMed] [Google Scholar]
- 23.Masuki S, Todo T, Nakano Y, Okamura H, Nose H. J Physiol. 2005;566:213–224. doi: 10.1113/jphysiol.2005.086728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sauerbier I, von Mayersbach H. Horm Metab Res. 1977;9:529–530. doi: 10.1055/s-0028-1095589. [DOI] [PubMed] [Google Scholar]
- 25.Fu L, Patel MS, Bradley A, Wagner EF, Karsenty G. Cell. 2005;122:803–815. doi: 10.1016/j.cell.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 26.Kennedy B, Elayan H, Ziegler MG. Hypertension. 1993;21:415–419. doi: 10.1161/01.hyp.21.4.415. [DOI] [PubMed] [Google Scholar]
- 27.Abassi ZA, Binah O, Youdim MB. Br J Pharmacol. 2004;143:371–378. doi: 10.1038/sj.bjp.0705962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jordan J, Lipp A, Tank J, Schroder C, Stoffels M, Franke G, Diedrich A, Arnold G, Goldstein DS, Sharma AM, Luft FC. Circulation. 2002;106:460–465. doi: 10.1161/01.cir.0000022844.50161.3b. [DOI] [PubMed] [Google Scholar]
- 29.Helkamaa T, Mannisto PT, Rauhala P, Cheng ZJ, Finckenberg P, Huotari M, Gogos JA, Karayiorgou M, Mervaala EM. J Hypertens. 2003;21:2365–2374. doi: 10.1097/00004872-200312000-00026. [DOI] [PubMed] [Google Scholar]
- 30.Rudic RD, McNamara P, Reilly D, Grosser T, Curtis AM, Price TS, Panda S, Hogenesch JB, FitzGerald GA. Circulation. 2005;112:2716–2724. doi: 10.1161/CIRCULATIONAHA.105.568626. [DOI] [PubMed] [Google Scholar]
- 31.Ura N, Sasaki H. Nippon Rinsho. 2005;63(Suppl 3):392–397. [PubMed] [Google Scholar]
- 32.Kvetnansky R, Sabban EL. Ann NY Acad Sci. 1998;851:342–356. doi: 10.1111/j.1749-6632.1998.tb09008.x. [DOI] [PubMed] [Google Scholar]
- 33.Weaver DR. J Biol Rhythms. 1998;13:100–112. doi: 10.1177/074873098128999952. [DOI] [PubMed] [Google Scholar]
- 34.Buijs RM, Wortel J, Van Heerikhuize JJ, Feenstra MG, Ter Horst GJ, Romijn HJ, Kalsbeek A. Eur J Neurosci. 1999;11:1535–1544. doi: 10.1046/j.1460-9568.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- 35.Ishida A, Mutoh T, Ueyama T, Bando H, Masubuchi S, Nakahara D, Tsujimoto G, Okamura H. Cell Metab. 2005;2:297–307. doi: 10.1016/j.cmet.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 36.McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, Nestler EJ. Proc Natl Acad Sci USA. 2005;102:9377–9381. doi: 10.1073/pnas.0503584102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swoap SJ, Weinshenker D, Palmiter RD, Garber G. Am J Physiol. 2004;286:R108–R113. doi: 10.1152/ajpregu.00405.2003. [DOI] [PubMed] [Google Scholar]
- 38.Tjurmina OA, Armando I, Saavedra JM, Goldstein DS, Murphy DL. Endocrinology. 2002;143:4520–4526. doi: 10.1210/en.2002-220416. [DOI] [PubMed] [Google Scholar]
- 39.Millar-Craig MW, Bishop CN, Raftery EB. Lancet. 1978;1:795–797. doi: 10.1016/s0140-6736(78)92998-7. [DOI] [PubMed] [Google Scholar]
- 40.Verdecchia P, Schillaci G, Guerrieri M, Gatteschi C, Benemio G, Boldrini F, Porcellati C. Circulation. 1990;81:528–536. doi: 10.1161/01.cir.81.2.528. [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto T, Nakahata Y, Tanaka M, Yoshida M, Soma H, Shinohara K, Yasuda A, Mamine T, Takumi T. J Biol Chem. 2005;280:42036–42043. doi: 10.1074/jbc.M509600200. [DOI] [PubMed] [Google Scholar]
- 42.Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, FitzGerald GA. PLoS Biol. 2004;2:e377. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Minami Y, Horikawa K, Akiyama M, Shibata S. FEBS Lett. 2002;526:115–118. doi: 10.1016/s0014-5793(02)03159-9. [DOI] [PubMed] [Google Scholar]
- 44.Carlson SH, Osborn JW, Wyss JM. Hypertension. 1998;32:46–51. doi: 10.1161/01.hyp.32.1.46. [DOI] [PubMed] [Google Scholar]
- 45.Balsalobre A, Damiola F, Schibler U. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- 46.Curtis AM, Seo SB, Westgate EJ, Rudic RD, Smyth EM, Chakravarti D, FitzGerald GA, McNamara P. J Biol Chem. 2004;279:7091–7097. doi: 10.1074/jbc.M311973200. [DOI] [PubMed] [Google Scholar]
- 47.Wu Z, Irizarry RA. J Comput Biol. 2005;12:882–893. doi: 10.1089/cmb.2005.12.882. [DOI] [PubMed] [Google Scholar]
- 48.Wettenhall JM, Smyth GK. Bioinformatics. 2004;20:3705–3706. doi: 10.1093/bioinformatics/bth449. [DOI] [PubMed] [Google Scholar]
- 49.Klipper-Aurbach Y, Wasserman M, Braunspiegel-Weintrob N, Borstein D, Peleg S, Assa S, Karp M, Benjamini Y, Hochberg Y, Laron Z. Med Hypotheses. 1995;45:486–490. doi: 10.1016/0306-9877(95)90228-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.