Abstract
Adrenomedullin (AM) is a potent vasodilator peptide in plasma at picomolar levels. Polymorphisms in the human AM gene have been associated with genetic predisposition to diabetic nephropathy and proteinuria with essential hypertension, and numerous studies have demonstrated that endogenous AM plays a role in protecting the heart and kidneys from fibrosis resulting from cardiovascular disease. Elevated plasma levels of AM are associated with pregnancy and sepsis and with cardiovascular stress and hypertension. However, there are no reports of the effects of genetic differences in the expression of the endogenous AM gene and of gender on blood pressure in these circumstances or on the pathological changes accompanying hypertension. To address these questions, we have generated mice having genetically controlled levels of AM mRNA ranging from ≈50% to ≈140% of wild-type levels. These modest changes in AM gene expression have no effect on basal blood pressure. Although pregnancy and sepsis increase plasma AM levels, genetically reducing AM production does not affect the transient hypotension that occurs during normal pregnancy or that is induced by treatment with lipopolysaccharide. Nor does the reduction of AM affect chronic hypertension caused by a renin transgene. However, 50% normal expression of AM enhances cardiac hypertrophy and renal damage in male, but not female, mice with a renin transgene. These observations suggest that the effect of gender on the role of AM in counteracting cardiovascular damage in humans merits careful evaluation.
Keywords: cardiovascular, pregnancy, cardiac hypertrophy, renal fibrosis, gene targeting
Adrenomedullin (AM) is a 52-aa peptide vasodilator which circulates in the plasma as a protein-bound hormone and can influence many biological processes (1). The gene coding for AM is widely expressed throughout the body, most highly in endothelial cells and vascular smooth muscle cells (2, 3). Consequently, highly vascularized tissues, such as the placenta, lung, heart, and kidney cause elevated plasma AM levels when their secretion of AM peptide is stimulated by conditions such as pregnancy, sepsis, and cardiovascular disease or by inflammatory cytokines or hypoxia.
AM is most widely known for its vasodilatory properties (4). Bolus injection or infusion of AM in humans (5–10) and in several animal species (11–17) causes prolonged, dose-dependent vasorelaxation and hypotension. Yet the dose, route, and duration of exogenous administration required to elicit a systemic effect on blood pressure (BP) varies widely among published studies (8–10). Moreover, it is possible that bolus infusions of the unbound peptide may not accurately replicate the functions of the endogenous protein-bound peptide. Consequently, it is important to determine whether modest changes in expression of the endogenous AM gene affect the maintenance of BP during health and disease.
Endogenous AM plays a role in protecting the heart and kidneys from damage during cardiovascular stresses such as hypertension and its associated cardiac hypertrophy, myocardial infarction, heart failure, and atherosclerosis (18, 19). Thus, comparisons between male mice with genetically reduced levels of AM (AM+/−) and wild-type mice (AM+/+) have demonstrated that the reduced level of endogenous AM increases the damage to heart and renal tissue caused by aortic banding (20), angiotensin II infusion (20, 21), bilateral renal artery clamping (22), and atherosclerosis (23). However, although the expression of the AM gene is transcriptionally regulated via estrogen-responsive elements in its promoter region, the effects of different levels of AM in female mice have not been reported. Moreover, in our previous studies, we have observed that the regulation of AM gene expression during cardiovascular stress is highly affected by gender (24, 25). Thus, it is clearly important to determine the effects of differences in AM gene expression in females, a point that is further emphasized by our demonstration that female mice with only one copy of the gene have serious reproductive defects involving abnormal implantation and fetal growth restriction (26).
To study the effects of genetic differences in AM levels and of gender, we have therefore generated mice with AM peptide levels ranging from ≈50% to ≈140% of wild type, caused by their having one (AM+/−), two (AM+/+), or three copies (AM+/Duplication) of the AM gene. The basal BPs were determined in male and female mice of these three genotypes under normal conditions and in AM+/− females during normal pregnancy. To simulate the effects of sepsis, we administered lipopolysaccharide (LPS) to AM+/− males and females. To evaluate the effects of chronic hypertension, we introduced a renin transgene into the AM+/− mice by breeding. We also evaluated the effects of both reduced AM levels and of gender on the degree of cardiovascular damage caused by the renin-induced hypertension. These modest alterations of AM gene expression do not affect basal BP or the changes in BP accompanying normal pregnancy or after treatment with LPS or consequent to chronic hypertension. However, male, but not female, mice with 50% expression of AM develop greater degrees of cardiac hypertrophy and renal damage when they are hypertensive than do wild-type mice.
Results
Generation of AM Gene-Titration mice.
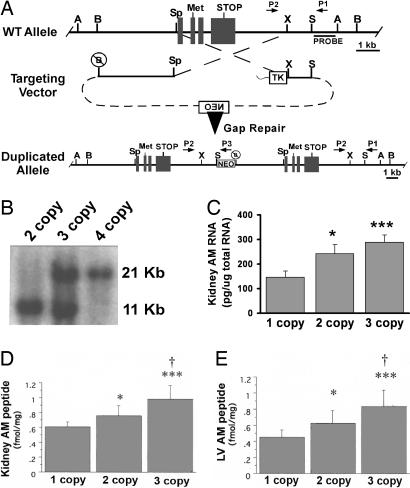
To generate mice with increased AM peptide levels that are within physiological ranges and genetically controlled, we used gene targeting to duplicate the AM gene within its endogenous locus on mouse chromosome 7. The targeting vector (Fig. 1A) used the same regions of homology as were previously used for generating mice with a targeted deletion of the AM gene (27), but by using a different arrangement of the DNA fragments, the cross-over event leads to duplication of the AM gene. The second copy of the AM gene on chromosome 7 contains ≈3.7 kb of 5′ flanking promoter sequence. The duplicated allele was detected by Southern blot analysis using a genomic probe fragment located outside the areas of homology (Fig. 1B). Wild-type mice were designated two-copy (AM+/+, one copy of the AM gene on each chromosome), heterozygote mice for the gene duplication were designated three-copy (AM+/Duplication, one copy of the AM gene on the wild-type chromosome and two copies on the targeted chromosome), and homozygous mice were designated four-copy (AMDuplication/Duplication, two copies of the AM gene on each targeted chromosome).
Fig. 1.
Generation of AM gene-titration mice. (A) Targeting strategy used to generate mice with a duplication of the AM gene on chromosome 7. The targeting vector consisted of regions of homology that flank the entire AM gene so that all sequences between the endogenous 5′ BamH1 site and 3′ Sac site were duplicated in the second copy. Primers to identify ES cells are labeled P1, P2, and P3. (B) Southern blot of correctly targeted ES cells by using genomic DNA digested with BamH1 and the probe sequence depicted in A. The wild-type allele is 11 kb, and the targeted allele is 21 kb. (C) Levels of AM RNA in kidney of one-, two-, and three-copy mice measured by quantitative RT-PCR. (D) Levels of AM peptide in kidney of one-, two-, and three-copy mice measured by RIA. (E) Levels of AM peptide in left ventricle (LV) of one-, two-, and three-copy mice measured by RIA. A, AvrII; B, BamH1; Sp, Spe; X, Xba; S, SacI. ∗, P < 0.05 vs. one-copy; ∗∗∗, P < 0.001 vs. one-copy; †, P < 0.05 vs. two-copy. n > 5 for each genotype in C–E. Error bars represent SEMs.
Mice with only one copy of the AM gene have been described (27) and were heterozygous for a targeted deletion of the AM gene (AM+/−). These one-copy mice produce ≈50% wild-type levels of AM mRNA and peptide.
To confirm that the series of AM gene-titration mice with different copy numbers of the AM gene resulted in modest yet physiologically relevant changes in AM gene expression, quantitative RT-PCR for AM was performed on total RNA isolated from adult kidneys. As expected, and shown in Fig. 1C, AM one-copy mice had approximately half the levels of AM RNA compared with AM two-copy mice, whereas AM three-copy mice had ≈120% more AM RNA than AM two-copy mice. AM four-copy mice had ≈140% more AM RNA than AM two-copy mice (data not shown). AM peptide radioimmunoassays were performed on protein extracts from kidney and left ventricle and showed a significant and dose-dependent increase in AM peptide levels between the one-, two-, and three-copy mice (Fig. 1D).
Basal BP Is Unaffected by AM Gene Copy Number.
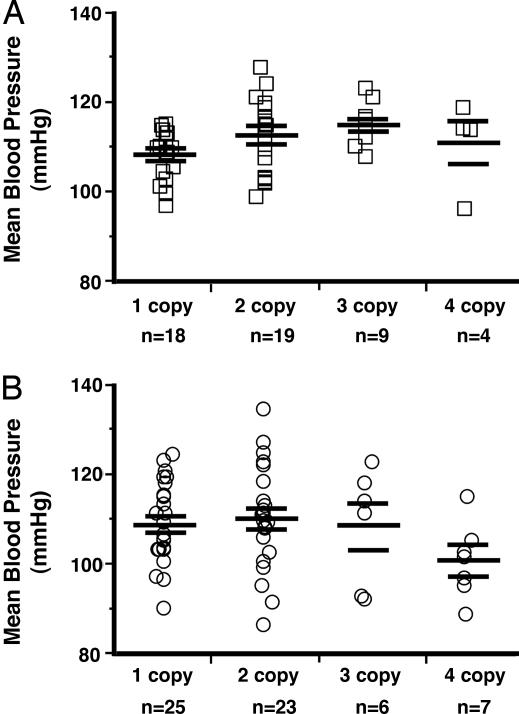
To determine whether modest, genetically induced changes in AM gene expression affect the basal BP of conscious, restrained animals, BPs of the AM gene-titration mice were measured for 6 consecutive days by using a computerized tail cuff system. As shown in Fig. 2A, we found no significant differences in the basal BPs of male mice with one to four copies of the AM gene (one copy, 108 ± 1; two-copy, 113 ± 2; three-copy, 115 ± 2; four-copy, 111 ± 5 mmHg; P = 0.07). Fig. 2B shows comparable results for the female AM gene-titration mice (one-copy, 109 ± 2; two-copy, 110 ± 2; three-copy, 109 ± 5; four-copy, 101 ± 3 mmHg; P = 0.2). Taken together, the results in Fig. 2 demonstrate that genetically controlled levels of AM peptide over the range of ≈50–140% wild-type levels has no effect on the basal BPs of male or female mice.
Fig. 2.
Mean BPs are unaffected by AM gene copy number. (A) Mean BPs of conscious AM gene-titration male mice measured by computerized tail cuff. (B) Mean BPs of conscious AM gene-titration female mice measured by computerized tail cuff.
Reduced AM Expression Does Not Affect the Transient Hypotension During Pregnancy or After LPS Treatment.
Because pregnancy and sepsis cause the greatest increases in AM levels, and because both conditions are accompanied with hypotension, we next asked whether a genetic reduction in AM moderates this hypotension.
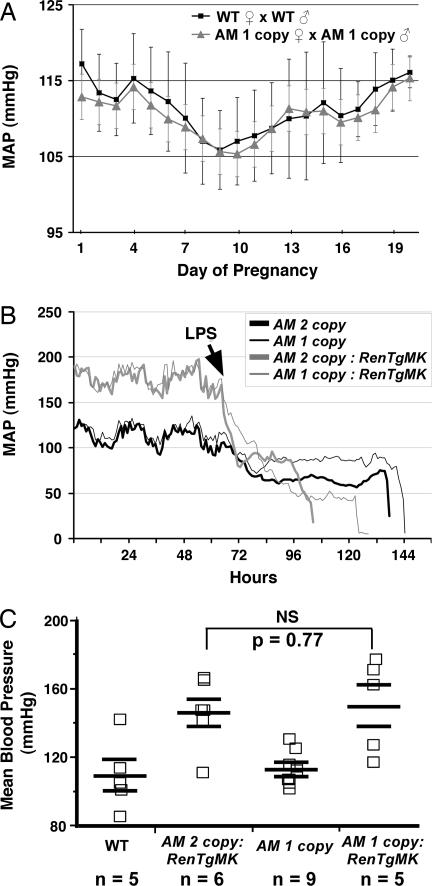
To measure BPs during pregnancy, we used radio telemetry in AM one-copy and wild-type female mice. This method allowed for the continuous, uninterrupted monitoring of basal BP in unrestrained and unanesthetized animals over a period of several weeks. In agreement with the results from our tail-cuff studies, we found no significant differences in basal BPs between wild-type and AM one-copy mice before pregnancy (data not shown) or at any time during pregnancy (Fig. 3A). Similar to humans, both wild-type and AM one-copy female mice displayed a characteristic drop in BP during midgestation (pregnancy day 7–10), which recovered to, but never exceeded the prepregnancy BP levels by the end of gestation. Moreover, because AM one-copy male mice were bred to AM one-copy female mice, we can also conclude that the presence of AM-null embryos within a litter does not influence maternal blood pressure during pregnancy. Therefore, genetic reduction of AM by as much as ≈50% in mice does not affect the systemic changes in BP that occur transiently during the course of a normal pregnancy.
Fig. 3.
Physiologically induced changes in BP are unaffected by AM gene copy number. (A) Mean arterial pressures (MAP) of wild-type (squares and line) and AM one-copy (triangles and line) female mice during pregnancy, measured by radiotelemetry. (B) Mean arterial pressures (MAP) of male AM two-copy (thick lines) and one-copy mice (thin lines) that were either wild-type (black) or targeted for the RenTgMK transgene (gray lines) before and after injection of LPS, measured by radiotelemetry. (C) Mean BPs of male mice that were wild type, AM two-copy (i.e., wild type):RenTgMK, AM one-copy, or AM one-copy:RenTgMK. In all cases, there were no significant effects of AM gene copy number on BP changes.
To determine whether genetically reduced levels of AM affect the degree of acute hypotension associated with septic shock, telemetry was used to monitor the BPs of wild-type and AM one-copy male mice that were injected i.p. with LPS to induce septic shock. Some of the mice in this experiment were also heterozygous for a renin transgene (RenTgMK, described below), so that they had elevated BPs before induction of septic shock. As shown in Fig. 3B, before injection of LPS, all mice displayed normal diurnal circadian rhythms in BP. Injection of LPS caused an immediate and dramatic fall in BP to ≈50–80 mmHg over 2–3 days. However, we found no significant difference in the overall drop in BP between AM two-copy and AM one-copy mice. Therefore, genetic reduction of AM in mice has no effect on the transient hypotension that occurs during induced septic shock.
Reduced AM Expression Does Not Affect the Chronic Hypertension in Mice Overexpressing Renin.
To determine whether a reduction in AM gene copy number can affect the development of hypertension caused by a genetic increase in renin production, we crossed the AM one-copy mice to mice that carry a targeted insertion of a transgene that expresses renin at high and constant levels from the liver. A single copy of the transgene, RenTgMK, elevates plasma renin levels and causes hypertension, cardiac hypertrophy, cardiovascular end-organ damage, and early death (25). Fig. 3C shows that, compared with wild-type mice, a single copy of the RenTgMK transgene causes an increase in BP of ≈45 mmHg (wild type, 108 ± 9; AM two-copy:RenTgMK, 145 ± 8 mmHg). Reducing the copy number of the AM gene to one copy had no significant effect on the hypertension induced by the RenTgMK (AM one-copy:RenTgMK, 149 ± 12 mmHg; P = 0.77 compared with AM two-copy:RenTgMK). Moreover, we again find no significant differences in BPs between AM one-copy and wild-type mice (AM one-copy, 112 ± 3 mmHg; P = 0.65 compared with wild type). Similar results were obtained for female mice of the same genotypes: wild type, 98 ± 7 versus AM one-copy, 96 ± 5 mmHg (P = 0.9); AM two-copy:RenTgMK, 139 ± 6 versus AM one-copy:RenTgMK, 133 ± 5 mmHg (P = 0.4). Therefore, genetic reduction of endogenous AM in male or female mice has no effect on the chronic hypertension that is induced by genetic overexpression of a renin transgene.
Taken together, the data in Fig. 3 demonstrate that when physiological conditions associated with elevated plasma AM levels in humans (pregnancy, sepsis, and hypertension) are reproduced in mice, the genetic reduction of AM has no effect on BP.
Genetic Reduction of AM Exacerbates Cardiovascular Damage in Male, but Not Female, Mice with Chronic Hypertension.
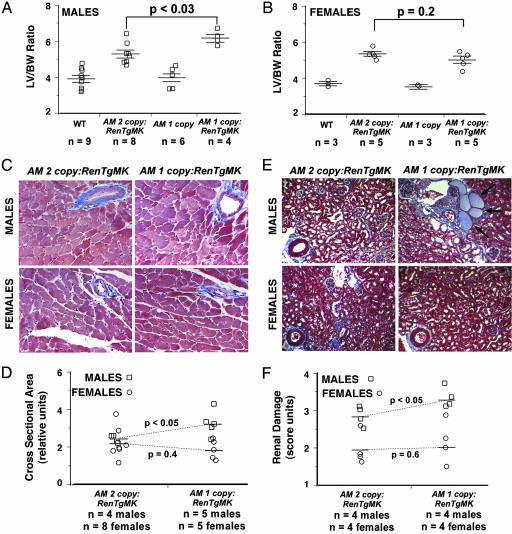
Studies have demonstrated that male mice heterozygous for a targeted deletion of the AM gene are more susceptible than wild-type mice to cardiac hypertrophy, fibrosis, and renal damage induced by aortic constriction or infusion of angiotensin II. However, female mice were not examined in these studies. To determine whether gender affects the protective functions of AM during cardiovascular disease, we characterized male and female mice that were either wild type or heterozygous for the AM gene deletion and either wild type or heterozygous for the presence of the RenTgMK transgene. Fig. 4A shows that male mice with only one copy of AM and the RenTgMK transgene have a significantly greater degree of cardiac hypertrophy (as measured by left ventricular-to-body weight ratio) compared with male mice with two copies of the AM gene and the RenTgMK transgene. In contrast, in female mice, there was no significant increase in the degree of cardiac hypertrophy between AM one- and two-copy animals that were heterozygous for the RenTgMK transgene (Fig. 4B) compared with their nontransgenic sisters. Histological characterization of the left ventricles from the mice revealed similar degrees of coronary artery fibrosis independent of gender and across all genotypes (Fig. 4C). Nevertheless, when we used computerized morphometry to measure the cross-sectional area of the hypertrophied myocytes, we found that whereas male AM one-copy:RenTgMK mice had significantly larger myocytes than AM two-copy:RenTgMK mice, female mice of comparable genotypes did not (Fig. 4D). Thus the male, but not the female, mice develop greater cardiac hypertrophy as a result of chronic hypertension when they have one copy of the AM gene compared with gender-matched animals with two copies of the AM gene.
Fig. 4.
AM one-copy male, but not female, mice suffer greater degree of cardiac hypertrophy in response to RenTgMK than wild-type mice. (A) Left ventricular (LV) to body weight (BW) ratio of 6- to 8-month-old male mice wild type, AM two-copy:RenTgMK, AM one-copy, or AM one-copy:RenTgMK showing a significant effect of reduced AM on left ventricular hypertrophy. (B) Left ventricular (LV) to body weight (BW) ratio of 6- to 8-month-old female mice wild type, AM two-copy:RenTgMK, AM one-copy, or AM one-copy:RenTgMK showing no significant effect of reduced AM on left ventricular hypertrophy. (C) Masson's trichrome staining of left ventricle shows coronary vessel fibrosis and enlarged myocytes. (D) Morphometric analysis of myocyte cross-sectional area shows that, compared with gender matched AM two-copy:RenTgMK control mice, male AM one-copy:RenTgMK mice have significantly larger myocytes than female AM one-copy:RenTgMK mice. (E) Masson's trichrome staining of kidney shows renal vascular fibrosis, glomerular sclerosis, and infiltration of immune cells that is more severe in male mice. Proteinacious casts (arrows) were abundant in AM one-copy:RenTgMK male mice, occasionally observed in AM two-copy:RenTgMK male mice but absent in female mice of similar age and genotype. (F) Renal damage induced by RenTgMK was scored by using arbitrary units for the severity of glomerular sclerosis, vascular fibrosis, interstitial fibrosis, and proteinacous casts. AM one-copy:RenTgMK male, but not AM one-copy:RenTgMK female, mice had significantly greater renal damage than age and gender-matched AM two-copy:RenTgMK mice.
A similar pattern of gender differences also emerged with respect to renal damage: renal damage in male, but not female, RenTgMK mice was increased by genetic reduction of AM. Thus, as shown in Fig. 4E, the degree of glomerular sclerosis and presence of proteinacious casts was markedly worse in male AM one-copy:RenTgMK mice compared with either male AM two-copy:RenTgMK mice or female mice. Histological scoring of renal damage revealed that the reduction of AM copy number did not significantly exacerbate the renal damage in female mice but caused a significant worsening of renal disease in male mice (Fig. 4F). Consistent with increased renal fibrosis in male AM one-copy:RenTgMK mice, we found a significant increase in the gene expression of the fibrotic marker transforming growth factor β in the kidneys of these mice compared with male AM two-copy:RenTgMK controls. In contrast, the gene expression levels of transforming growth factor β in the kidney were unaffected by the copy number of the AM gene in female mice (data not shown).
When we used the Fisher method (28) to test for the combined significance of these related but independent measures of cardiovascular damage, we found a highly significant overall difference in the severity of cardiovascular disease in male AM one-copy:RenTgMK mice (P < 0.02) but not in female AM one-copy:RenTgMK mice (P > 0.5), compared with gender-matched AM two-copy:RenTgMK control animals. Taken together, the results of Fig. 4 demonstrate that a reduction in endogenous levels of AM gene expression increases hypertension-related cardiovascular damage in male, but not in female, mice.
Discussion
We describe the generation of mice with a targeted duplication of the AM gene. When combined with mice that are heterozygous for a targeted deletion of the AM gene, these mice provide a genetically controlled series of animals with variations in AM gene expression that range from ≈50–140% wild-type levels. This “gene titration” is therefore useful for modeling any modest variations in AM gene expression that may occur in the human population because of genetic polymorphisms of the AM gene (29, 30). As opposed to conventional transgenic approaches where a tissue-specific promoter drives the expression of a randomly inserted transgene, the gene-titration model offers the advantage of retaining the normal location of the duplicated gene, driven by the endogenous promoter.
Plasma levels of AM rise dramatically in humans with a wide variety of cardiovascular conditions, and it has been hypothesized that elevated plasma AM may help regulate the changes in BP that accompany pregnancy, sepsis, and cardiovascular stress. Our experiments bear on this hypothesis because they test the effects of reduced AM gene expression on both transient hypotenstion and chronic hypertension.
Our results prove to be remarkably consistent and show that modest alterations in AM gene expression, either decreased or increased, have no effect on the basal BPs of male or female mice. This finding may be somewhat surprising given the wealth of information showing that AM infusions can elicit long-lasting depressor effects in several species of experimental animals (12, 31) and in humans (10). Shindo et al. (32) have also generated a line of mice with targeted deletion of the AM gene and reported that conscious and unrestrained heterozygous mice had elevated BPs but, in a later study, found no significant differences in BPs between unanesthetized, wild-type, and AM heterozygous mice by using a tail cuff method (21). It is therefore likely that the observed differences in BP in their first study (32) may have been due to different methods of BP measurement (33). A third group generated another line of AM gene-targeted mice and, consistent with our findings, has found no differences in basal BPs of AM heterozygous mice measured by either indirect tail cuff or direct catheterization of the carotid artery (34). Although AM four-copy mice with ≈140% wild-type expression of AM do not have decreased BP, transgenic mice with ≈8-fold overexpression of AM in the vasculature and 2.3-fold increase in plasma AM have lower BP that is normalized to wild-type levels when the mice are treated with a NOS inhibitor (23, 35). Thus, our analysis of AM gene-titration mice is consistent with previous studies and leads to the conclusion that modest alteration in endogenous AM gene expression does not affect basal BP in mice.
The action of AM peptide as a protective factor in the heart and kidney has been well established in male mice, but there have been no comparable studies in female mice. In our previous studies we have observed that changes in AM gene expression induced by several types of cardiovascular stress differ significantly in the two sexes (24, 25). For this reason, and because the AM gene is highly regulated by estrogen (36, 37), and because the AM one-copy female mice display profound reproductive defects (26), we suspected the established cardioprotective effects of endogenous AM might also be gender-dependent. Our results are consistent with previous studies in showing that the reduced levels of AM in AM one-copy male mice increase the cardiovascular damage induced by chronic hypertension. In marked contrast, in female mice, the degree of cardiovascular damage induced by the RenTgMK transgene is unaffected by the reduction in AM levels. Recently, Pearson et al. (38) found that estrogen and testosterone have different effects on the amount of AM secreted from angiotensin II-stimulated human endothelial cells, suggesting that an interplay between sex steroids and angiotensin II may account for the gender differences observed in our study. These findings clearly demonstrate that the tissue damage caused by hypertension is enhanced in male, but not in female, mice when AM levels are decreased. Our study suggests that the effect of gender on the beneficial role of AM in counteracting cardiovascular damage in the human population merits careful evaluation.
Materials and Methods
Additional information on materials and methods is available in supporting information (SI) Text. Standard gene-targeting methods were used to generate ES cells and mice with a targeted duplication of the AM gene (39). Male chimeric mice that transmitted the targeted allele were bred to 129S6/SvEv females to establish isogenic lines.
BPs were measured on unanesthetized, restrained mice by a computerized tail-cuff system, as described (40). Continuous recording of the BPs of unanesthetized, unrestrained animals was performed by radio telemetry.
To genetically induce hypertension and cardiac hypertrophy in the AM one-copy mice, heterozygous RenTgMK (25, 41, 42) mice were crossed to heterozygous AM one-copy mice. All mice were maintained on an isogenic SvEv129/S6 genetic background and were 6–8 months of age at the time of killing.
Experimental animals were 6–8 months of age and maintained on an isogenic 129S6/SvEv background. Control animals were wild-type, age- and gender-matched littermates. All experiments were approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill.
Statistical analyses for multiple comparisons were performed with one-way ANOVA using JMP Software (SAS Institute, Cary, NC). In all figures, error bars represent standard errors of the mean.
Supplementary Material
Acknowledgments
We thank Gleb Rozanov for technical assistance and Drs. Willis K. Samson, Howard Rockman, and Leighton R. James for helpful advice and discussions. This work was supported by the Burroughs Wellcome Fund and National Institutes of Health Grants HD046970 (to K.C.) and HL49277 (to O.S.).
Abbreviations
- AM
adrenomedullin
- BP
blood pressure
- LPS
lipopolysaccharide.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0611365104/DC1.
References
- 1.Gibbons C, Dackor R, Dunworth W, Fritz-Six K, Caron KM. Mol Endocrinol. 2006 doi: 10.1210/me.2006-0156. [DOI] [PubMed] [Google Scholar]
- 2.Garayoa M, Bodegas E, Cuttitta F, Montuenga LM. Microsc Res Tech. 2002;57:40–54. doi: 10.1002/jemt.10050. [DOI] [PubMed] [Google Scholar]
- 3.Hinson JP, Kapas S, Smith DM. Endocr Rev. 2000;21:138–167. doi: 10.1210/edrv.21.2.0396. [DOI] [PubMed] [Google Scholar]
- 4.Brain SD, Grant AD. Physiol Rev. 2004;84:903–934. doi: 10.1152/physrev.00037.2003. [DOI] [PubMed] [Google Scholar]
- 5.Del Bene R, Lazzeri C, Barletta G, Vecchiarino S, Guerra CT, Franchi F, La Villa G. Clin Physiol. 2000;20:457–465. doi: 10.1046/j.1365-2281.2000.00284.x. [DOI] [PubMed] [Google Scholar]
- 6.Nagaya N, Satoh T, Nishikimi T, Uematsu M, Furuichi S, Sakamaki F, Oya H, Kyotani S, Nakanishi N, Goto Y, et al. Circulation. 2000;101:498–503. doi: 10.1161/01.cir.101.5.498. [DOI] [PubMed] [Google Scholar]
- 7.Oya H, Nagaya N, Furuichi S, Nishikimi T, Ueno K, Nakanishi N, Yamagishi M, Kangawa K, Miyatake K. Am J Cardiol. 2000;86:94–98. doi: 10.1016/s0002-9149(00)00836-5. [DOI] [PubMed] [Google Scholar]
- 8.Lainchbury JG, Cooper GJ, Coy DH, Jiang NY, Lewis LK, Yandle TG, Richards AM, Nicholls MG. Clin Sci (London) 1997;92:467–472. doi: 10.1042/cs0920467. [DOI] [PubMed] [Google Scholar]
- 9.Lainchbury JG, Troughton RW, Lewis LK, Yandle TG, Richards AM, Nicholls MG. J Clin Endocrinol Metab. 2000;85:1016–1020. doi: 10.1210/jcem.85.3.6422. [DOI] [PubMed] [Google Scholar]
- 10.Meeran K, O'Shea D, Upton PD, Small CJ, Ghatei MA, Byfield PH, Bloom SR. J Clin Endocrinol Metab. 1997;82:95–100. doi: 10.1210/jcem.82.1.3656. [DOI] [PubMed] [Google Scholar]
- 11.Khan AI, Kato J, Kitamura K, Kangawa K, Eto T. Clin Exp Pharmacol Physiol. 1997;24:139–142. doi: 10.1111/j.1440-1681.1997.tb01796.x. [DOI] [PubMed] [Google Scholar]
- 12.Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, Eto T. Biochem Biophys Res Commun. 1993;192:553–560. doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
- 13.Ishiyama Y, Kitamura K, Ichiki Y, Nakamura S, Kida O, Kangawa K, Eto T. Eur J Pharmacol. 1993;241:271–273. doi: 10.1016/0014-2999(93)90214-3. [DOI] [PubMed] [Google Scholar]
- 14.Cheng DY, DeWitt BJ, Wegmann MJ, Coy DH, Bitar K, Murphy WA, Kadowitz PJ. Life Sci. 1994;55:PL251–PL256. doi: 10.1016/0024-3205(94)00246-0. [DOI] [PubMed] [Google Scholar]
- 15.Kato T, Bishop AT, Wood MB. J Orthop Res. 1996;14:956–961. doi: 10.1002/jor.1100140616. [DOI] [PubMed] [Google Scholar]
- 16.Hjelmqvist H, Keil R, Mathai M, Hubschle T, Gerstberger R. Am J Physiol. 1997;273:R716–24. doi: 10.1152/ajpregu.1997.273.2.R716. [DOI] [PubMed] [Google Scholar]
- 17.Westphal M, Stubbe H, Bone HG, Daudel F, Vocke S, Van Aken H, Booke M. Biochem Biophys Res Commun. 2002;296:134–138. doi: 10.1016/s0006-291x(02)00821-5. [DOI] [PubMed] [Google Scholar]
- 18.Kuwasako K, Cao YN, Nagoshi Y, Kitamura K, Eto T. Peptides. 2004;25:2003–2012. doi: 10.1016/j.peptides.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Nishikimi T, Yoshihara F, Mori Y, Kangawa K, Matsuoka H. Hypertens Res. 2003;26(Suppl):S121–S127. doi: 10.1291/hypres.26.s121. [DOI] [PubMed] [Google Scholar]
- 20.Niu P, Shindo T, Iwata H, Iimuro S, Takeda N, Zhang Y, Ebihara A, Suematsu Y, Kangawa K, Hirata Y, Nagai R. Circulation. 2004;109:1789–1794. doi: 10.1161/01.CIR.0000118466.47982.CC. [DOI] [PubMed] [Google Scholar]
- 21.Niu P, Shindo T, Iwata H, Ebihara A, Suematsu Y, Zhang Y, Takeda N, Iimuro S, Hirata Y, Nagai R. Hypertens Res. 2003;26:731–736. doi: 10.1291/hypres.26.731. [DOI] [PubMed] [Google Scholar]
- 22.Nishimatsu H, Hirata Y, Shindo T, Kurihara H, Kakoki M, Nagata D, Hayakawa H, Satonaka H, Sata M, Tojo A, et al. Circ Res. 2002;90:657–663. doi: 10.1161/01.res.0000013697.55301.e7. [DOI] [PubMed] [Google Scholar]
- 23.Imai Y, Shindo T, Maemura K, Sata M, Saito Y, Kurihara Y, Akishita M, Osuga J, Ishibashi S, Tobe K, et al. Arterioscler Thromb Vasc Biol. 2002;22:1310–1315. doi: 10.1161/01.atv.0000024685.92243.e7. [DOI] [PubMed] [Google Scholar]
- 24.Kim HS, Lee G, John SW, Maeda N, Smithies O. Proc Natl Acad Sci USA. 2002;99:4602–4607. doi: 10.1073/pnas.072083799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caron KM, James LR, Kim HS, Knowles J, Uhlir R, Mao L, Hagaman JR, Cascio W, Rockman H, Smithies O. Proc Natl Acad Sci USA. 2004;101:3106–3111. doi: 10.1073/pnas.0307333101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li M, Yee D, Magnuson TR, Smithies O, Caron KM. J Clin Invest. 2006;116:2653–2662. doi: 10.1172/JCI28462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caron KM, Smithies O. Proc Natl Acad Sci USA. 2001;98:615–619. doi: 10.1073/pnas.021548898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fisher RA. Statistical Methods, Experimental Design, and Scientific Inference. New York: Hafner; 1972. [Google Scholar]
- 29.Kobayashi Y, Nakayama T, Sato N, Izumi Y, Kokubun S, Soma M. Hypertens Res. 2005;28:229–236. doi: 10.1291/hypres.28.229. [DOI] [PubMed] [Google Scholar]
- 30.Ishimitsu T, Tsukada K, Minami J, Ono H, Matsuoka H. Hypertens Res. 2003;26(Suppl):S129–S134. doi: 10.1291/hypres.26.s129. [DOI] [PubMed] [Google Scholar]
- 31.Charles CJ, Rademaker MT, Richards AM, Cooper GJ, Coy DH, Jing NY, Nicholls MG. Am J Physiol. 1997;272:R2040–R2047. doi: 10.1152/ajpregu.1997.272.6.R2040. [DOI] [PubMed] [Google Scholar]
- 32.Shindo T, Kurihara Y, Nishimatsu H, Moriyama N, Kakoki M, Wang Y, Imai Y, Ebihara A, Kuwaki T, Ju KH, et al. Circulation. 2001;104:1964–1971. doi: 10.1161/hc4101.097111. [DOI] [PubMed] [Google Scholar]
- 33.Kurtz TW, Griffin KA, Bidani AK, Davisson RL, Hall JE. Arterioscler Thromb Vasc Biol. 2005;25:478–479. doi: 10.1161/01.ATV.0000153088.15433.8f. [DOI] [PubMed] [Google Scholar]
- 34.Shimosawa T, Shibagaki Y, Ishibashi K, Kitamura K, Kangawa K, Kato S, Ando K, Fujita T. Circulation. 2002;105:106–111. doi: 10.1161/hc0102.101399. [DOI] [PubMed] [Google Scholar]
- 35.Shindo T, Kurihara H, Maemura K, Kurihara Y, Kuwaki T, Izumida T, Minamino N, Ju KH, Morita H, Oh-hashi Y, et al. Circulation. 2000;101:2309–2316. doi: 10.1161/01.cir.101.19.2309. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe H, Takahashi E, Kobayashi M, Goto M, Krust A, Chambon P, Iguchi T. J Mol Endocrinol. 2006;36:81–89. doi: 10.1677/jme.1.01825. [DOI] [PubMed] [Google Scholar]
- 37.Ikeda K, Arao Y, Otsuka H, Kikuchi A, Kayama F. Mol Cell Endocrinol. 2004;223:27–34. doi: 10.1016/j.mce.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Pearson L, Rait C, Nicholls M, Yandle T, Evans J. J Endocrinol. 2006;191:171–177. doi: 10.1677/joe.1.06815. [DOI] [PubMed] [Google Scholar]
- 39.Smithies O, Kim HS. Proc Natl Acad Sci USA. 1994;91:3612–3615. doi: 10.1073/pnas.91.9.3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krege JH, Hodgin JB, Hagaman JR, Smithies O. Hypertension. 1995;25:1111–1115. doi: 10.1161/01.hyp.25.5.1111. [DOI] [PubMed] [Google Scholar]
- 41.Caron KM, James LR, Lee G, Kim HS, Smithies O. Physiol Genomics. 2005;20:203–209. doi: 10.1152/physiolgenomics.00221.2004. [DOI] [PubMed] [Google Scholar]
- 42.Caron KM, James LR, Kim HS, Morham SG, Sequeira Lopez ML, Gomez RA, Reudelhuber TL, Smithies O. Proc Natl Acad Sci USA. 2002;99:8248–8252. doi: 10.1073/pnas.112222199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.