Abstract
Knirps and other short-range transcriptional repressors play critical roles in patterning the early Drosophila embryo. These repressors are known to bind the CtBP corepressor, but their mechanism of action is poorly understood. We purified functional recombinant Knirps protein from transgenic embryos to identify possible cofactors that contribute to this protein’s activity. The protein migrates in a complex of approximately 450 kDa, and was found to copurify with the Rpd3 histone deacetylase protein during a double affinity purification procedure. Association of Rpd3 with Knirps was dependent on the presence of the CtBP-dependent repression domain of Knirps. Previous studies of an rpd3 mutant had not shown defects in the pattern of expression of even-skipped, a target of the Knirps repressor. However, in embryos doubly heterozygous for knirps and rpd3, a marked increase in the frequency of defects in the Knirps-regulated posterior domain of even-skipped expression was found, indicating that Rpd3 contributes to Knirps repression activity in vivo. This finding implicates deacetylation in the mechanism of short-range repression in Drosophila.
Transcriptional repression is critical for the patterned expression of developmentally regulated genes in Drosophila and other metazoans. Transcriptional repressors can exhibit a range of effects; repression can be transient or persist over long periods of time, and repressors can work locally (short-range) or influence the activity of cis regulatory elements over 1 kbp away (long-range). Repressors act through multiple biochemical pathways, including interference with activation domains, remodeling of chromatin, and direct interactions with the basal transcriptional machinery (1–3). One of the best characterized systems of developmental gene regulation involving transcriptional repressors is that present in the Drosophila blastoderm embryo, where repressors encoded by “gap genes” control the presegmental expression of pair rule genes, including even-skipped (eve). This pair-rule gene is controlled by five modular enhancers present 5’ and 3’ of the transcription unit, each of which are regulated by short-range repressors, including Giant, Krüppel, and Knirps (4). These short-range repressors all interact with the C-terminal binding protein (CtBP)1 corepressor and exhibit similar functional properties, including range of action, generally less than 100 bp from activators or the basal promoter (5–7).
Knirps is a member of the nuclear receptor superfamily, possessing this family’s characteristic Zn finger DNA binding domain, which is coupled to a unique C-terminus that interacts with CtBP (8). Knirps represses genes via CtBP-dependent and –independent activities. The combined repression activities play an especially important role on enhancers that are less sensitive to Knirps (7, 9–11). A gradient of the Knirps protein in the posterior of the embryo is responsible for simultaneously setting the boundaries of eve stripes 3, 4, 6, and 7. These multiple thresholds are achieved by differential sensitivity of the enhancers to Knirps, such that the domains of expression of eve stripe 4/6 are wholly contained within a region of the embryo that has sufficient Knirps protein to repress the eve stripe 3/7 (12).
CtBP is a widely conserved dimeric corepressor that interacts with a variety of transcription factors, recognizing a short peptide motif (PMDLS in Knirps) in the binding partner, reviewed in (13). CtBP proteins bear striking similarities to α-hydroxy acid dehydrogenases, and like these enzymes bind to NAD/NADH (14, 15). Binding of the cofactor has been observed to stimulate dimer or higher order complex formation in vitro, and has been suggested to function as a metabolic switch in vivo, allowing cells to tune patterns of gene expression to nuclear levels of NADH (16). Loss of NADH binding through mutagenesis of conserved glycines of the dinucleotide binding cleft compromises repression activity by this protein, preventing dimerization, and interaction with target transcription factors (10, 14). In addition, residues important for enzymatic function in the related dehydrogenases are conserved in metazoan CtBP proteins, although the possible enzymatic function of CtBP is poorly understood. Two reports find a weak dehydrogenase activity associated with the protein, but the conserved histidine residue of the catalytic triad is not required for transcriptional repression in vivo (10, 14, 17).
Mammalian CtBP physically associates with a variety of chromatin modifying proteins, including histone deacetylases, histone methyl transferases, and a polyamine oxidase that was recently found to mediate lysine demethylation (18, 19). These associated factors may be the main mediators of CtBP’s repression activity, although how a particular suite of CtBP associated activities may combine to regulate gene expression on endogenous circuits is not currently understood.
The physiological role of one CtBP-associated histone deacetylase enzyme, HDAC1 (also termed Rpd3 in yeast and Drosophila), has been extensively characterized with respect to gene regulation. In addition to association with CtBP, Rpd3 proteins have been found in complexes that include the Sin3, Sds3, SAP18 and SAP30 proteins (20–23). As a complex or individually, Rpd3/HDAC1 proteins are recruited to promoters to mediate repression by a variety of DNA-binding transcription factors, including Ume6 in yeast, and YY1 and Mad/Max in mammals (24–26). Rpd3 also associates with corepressors, including Groucho, SMRTER, Retinoblastoma, and E(z)-containing Polycomb complexes (27–31). Loss of Rpd3 leads to increased acetylation at target promoters, and increased transcription of many genes (32–34). In yeast and in metazoans, rpd3 mutants show defects in transcriptional regulation of cell cycle genes and firing of origins of replication (35–37). The Rpd3 protein can deacetylate histone H4 and H3 in vitro, and in mutant backgrounds, increases in histone H3 and H4 acetylation is observed (38–40). The extent of histone deacetylation in yeast appears to be very local, extending over a range of 1–2 nucleosomes (41). Rpd3 has also been suggested to function positively, suppressing the repressive effects of heterochromatin and in certain cases directly activating the transcription of specific genes, although in most cases this regulation is thought to be indirect (42–44).
Unlike the nonlethal phenotype observed in yeast, homozygous null rpd3 mutations in Drosophila are lethal in the larval stage (45). Many aspects of pair rule gene regulation in the embryo that are under the control of gap repressor proteins such as Knirps are not affected by loss of Rpd3. This finding prompted Mannervik and Levine to suggest that these short-range repressors do not require Rpd3 for their activity (46). However, Rpd3 has been linked to a number of repressors active in the Drosophila embryo. Loss of r p d 3 activity is observed to lead to misregulation of ftz and odd, genes regulated by the Even-skipped repressor, suggesting that Rpd3 might serve as a cofactor for Eve (46). In addition, the Runt repressor requires Rpd3 for repression of the engrailed gene during the later “maintenance” phase of repression (47). Biochemical studies showed that the long-range repressor Hairy associates with Rpd3 through the Groucho corepressor (48). Although rpd3 germline mutants do not have the dramatic embryonic phenotypes of gro and hairy mutants, gro shows strong dosage effect interactions with rpd3, suggesting that Rpd3 protein may play a partially redundant role in this cofactor’s function (48). Rpd3 is also found to copurify with a large complex containing the Polycomb group (PcG) gene products E(z) and Pcl, and misregulation in homeotic genes controlled by PcG proteins have been reported in an rpd3 mutant background (31, 49). These lines of evidence indicate that Rpd3 plays central roles in a number of repression activities in the Drosophila embryo.
Work from our laboratory has underscored the role of multiple repression activities that Knirps and other short-range repressors deploy in specific developmental settings (7, 9, 10, 50). These repression activities respond to a complex cis regulatory grammar relating to binding site number, affinity, and arrangement that dictates how a repressor functions in specific contexts (12, 51). We currently lack a deeper understanding of the molecular mechanisms underlying these phenomena, however. Therefore, to better understand the activity of Knirps repression, we have undertaken a biochemical and genetic study of Knirps associated proteins, and find contrary to previous indications that the Rpd3 protein may play an important role in mediating Knirps repression activity.
EXPERIMENTAL PROCEDURES
Transgenic flies carrying inducible, double-tagged Knirps genes
Details on the generation of transgenic flies expressing either full-length Knirps (1–429) or the N-terminal, CtBP-independent repression domain of Knirps (1–330) were reported previously (9). Each protein is double-tagged, carrying an N-terminal hexahistidine tag and a C-terminal double FLAG tag, and is expressed under the control of the hsp 70 promoter. Recombinant proteins are functional and can be expressed in embryos older than ~2 hours (9, and data not shown). To induce expression of recombinant Knirps proteins, transgenic embryos collected on apple juice plates at room temperature (22–23°C) were incubated for 30 minutes at 38°C in a 10-liter water bath to ensure rapid and even heating. After induction, embryos were immediately dechorionated and sonicated within 15 minutes from the end of heat-shock.
Western blotting analysis
Immunoblotting was performed using a tank transfer system (Mini Trans-Blot® Cell, BioRad 170–3930) and Immun-Blot™ PVDF membranes (BioRad 162–0177). Antibody incubation was in TBST (20 mM Tris-HCl, pH 7.5, 120 mM NaCl, 0.1% Tween-20) supplemented with 5% (w/v) nonfat dry milk as blocking agent. SuperSignal® West Pico chemiluminescent substrate (Pierce 34080) was used for detecting horseradish peroxidase (HRP) on immunoblots. FLAG M2 monoclonal antibody (Sigma F3165) was used at 1:10,000 dilution. Rabbit polyclonal antiserum against Drosophila Rpd3 (kindly provided by D. Wassarman, University of Wisconsin) (52) was used at 1:5,000 dilution. Rabbit polyclonal antiserum against Drosophila CtBP (dCtBP) was generated against full-length dCtBP and used at 1:20,000 dilution. To generate this antiserum, recombinant full-length, hexahistidine-tagged Drosophila CtBP (dCtBP 1-479) from pET15bCtBPL vector was expressed in E. coli BL21-CodonPlus™ RIL competent cells (Stratagene #230240) and purified on Ni-NTA agarose beads (Qiagen 30210) according to the manufacturer’s instructions. dCtBP protein (400μg) in 0.2 ml PBS buffer (1.9 mM NaH2PO4, 8.1 mM Na2HPO4, 154 mM NaCl, pH 7.2) was mixed with an equal volume of Titermax Gold adjuvant (Sigma T2684) and injected subcutaneously at multiple sites in a New Zealand female rabbit. Two secondary boosts were performed similarly after 4 and 12 weeks. ImmunoPure® Goat Anti-Mouse HRP-conjugated antibody (Pierce 31430) was used at 1:20,000 dilution. Goat Anti-Rabbit HRP-conjugated antibody (BioRad 170-6515) was used at 1:10,000 dilution.
Drosophila embryo nuclear extract preparation
0–12 hour old embryos from the hskni1-429.3 line were collected on grape juice plates from two population cages. For each extraction, two 0–12 hour collections (the first one stored 12 hours at 13°C) were pooled together. 20–40 gm of embryos were either dechorionated and processed immediately or transferred on a 155-mm Petri dish and floated on a 38°C water bath for 60 min. Heat-shocked embryos were recovered for 30 min at room temperature, prior to dechorionation and homogenization. Drosophila standard nuclear extracts (DSNE) were made according to Soeller et al. (53).
Co-immunoprecipitation (CoIP) experiments
200 μl of nuclear extracts (30 μg/μl of total protein) from embryos overexpressing full-length Knirps were incubated overnight at 4°C with 4 μl (4.9μg/μl) of α-FLAG M2 monoclonal antibody or an equivalent amount of mouse IgG antibody on a rotating wheel. 1 ml of washing buffer (150 mM NaCl, 50mM Hepes pH 7.9, 0.5 mM EDTA, 10% glycerol, 1mM DTT, 1 mM PMSF, 1mM Na-metabisulfite, 1mM benzamidine, 10μM pepstatin A) was added and each sample was supplemented with 10 μl of pre-equilibrated protein G-agarose beads (Cat. #16-266, Upstate) and incubated for 3 hours at 4°C on a rotator. Beads were washed for four times (10 minutes each time) with 1 ml of washing buffer, resuspended in Laemmli buffer, and boiled for 5 min at 95°C.
Lambda protein phosphatase treatment
Crude embryo lysates were prepared from embryos expressing full-length Knirps (hskni1-429.3) and subjected to treatment with increasing amounts of lambda;-phosphatase. For each reaction, 20 μl of crude embryo lysate (280 μg of total protein) from 2–4 hour hskni1-429.3 embryos subjected to 30 minutes heat-shock was incubated with 0, 20, 80, 400 or 1600 units of lambda;-phosphatase (New England Biolabs, P0753S) in 1X lambda;-phosphatase buffer (50 mM Tris-HCl pH 7.5, 0.1 mM Na2EDTA, 5 mM DTT, 0.01% Brij 35) supplemented with 2 mM MnCl2, in a final volume of 50 μl. To limit the activity of endogenous phosphatases, reactions were carried out at 4°C for 1 hour. Phosphatase activity was blocked by addition of 20-mM sodium orthovanadate (Sigma, S-6508). Reactions were stopped by the addition of Laemmli buffer followed immediately by incubation at 95°C for 5 min. Proteins were resolved by 8% SDS-PAGE and recombinant Knirps was detected by Western blot using anti-FLAG M2 antibody.
Double affinity purification of recombinant Knirps proteins
0–12 hour embryos were collected at room temperature (22–23°C) and heat-shocked for 30 min at 38°C as described. 2–4 grams of dechorionated embryos were resuspended in 40 ml lysis buffer (150 mM NaCl, 50 mM Hepes pH 7.9, 10% glycerol, 10 mM imidazole, 20 mM ß-mercaptoethanol, 1 mM PMSF, 1 mM Na-metabisulfite, 1 mM benzamidine, 10 μM pepstatin A) and sonicated using a Branson-250 Sonifier (4 cycles, 20–30 pulses/cycle, output 6, duty cycle 60%, 3 min on ice between cycles, using a medium-tip). Lysates were cleared by centrifugation (20 min at 27,000xg) and 2 ml of washed and pre-equilibrated Ni-NTA agarose beads (His Select™ HC Nickel, Sigma 6611) were added to the supernatant. After 6 hours at 4°C on a rotating wheel, beads were washed three times with 50 ml of lysis buffer supplemented with 20-mM imidazole and transferred to a 5-ml tube. Proteins were eluted twice using 3 ml of lysis buffer supplemented with 150 mM imidazole. The eluates were pooled and diluted to 50 ml with 150 mM NaCl, 50 mM Hepes pH 7.9, 10% glycerol, 0.2 mM EDTA, 2 mM DTT, 1 mM Na-metabisulfite, 1 mM benzamidine, 10 μM pepstatin A, 1 mM PMSF. 200–300μl of Protein-G agarose beads (Upstate, 16–266) covalently coupled with anti-FLAG M2 antibody (2 mg of antibody per ml of wet beads) were added to the solution and incubated for 12–18 hours at 4°C on a rotating wheel. Beads were washed three times with 50 ml of the same buffer, transferred to a microfuge tube and proteins were eluted with 1.2 ml of buffer containing 0.2% N-lauroyl-Sarkosine, (Sigma L-5777). Protein samples were TCA precipitated using 4 mg/ml Na-deoxycholate (Sigma D-6750) as carrier, resuspended in Laemmli buffer and heated 5 min at 95°C. For each purification experiment, one or two negative controls were used in parallel: either heat-shocked, non-transgenic yellow, white67 ( yw67) embryos, or transgenic embryos from the hs-kni line that was not heat-shocked.
Chromatographic identification of the Knirps complex
To determine the apparent molecular size of recombinant Knirps, whole-cell extracts from embryos expressing full-length Knirps were subjected to gel filtration chromatography. 0–12 hour embryos from hskni1-429.3 were heat-shocked 30 min and a crude lysate was prepared essentially as described above, using a lysis buffer containing 100 mM NaCl, 50 mM Hepes pH 7.9, 5% glycerol, 0.1 mM EDTA, 1 mM DTT, 1 mM Na-metabisulfite, 1 mM benzamidine, 10 μM pepstatin A, and 1 mM PMSF. Lysates were centrifuged at 27,000xg for 20 min and 300 μl of cleared lysate (6 mg of total protein) was loaded onto a pre-equilibrated Superdex-200 HR 10/30 column (Amersham, 17-1088-01) and eluted with 1.5 volume of lysis buffer at the flow rate of 0.4 ml/min using the ÄKTAexplorer 100 system (Amersham). Fractions (0.5ml) were collected and analyzed by Western blot for the presence of recombinant Knirps, CtBP and Rpd3. The same column was loaded with size markers (MW-GF-1000, Sigma) and run using identical conditions to determine in which fractions the different markers elute.
Genetic interaction between knirps and rpd3.
To test for a genetic interaction between kni and rpd3, transheterozygous flies for kni and rpd3 were generated and the expression pattern of the Knirps target gene even-skipped (eve) was monitored by in situ hybridization as previously described (9). kni9 (Bloomington stock #3332) carries a null mutation in Knirps and was previously used to test a genetic interaction between kni and CtBP (8). kni7G (Tübingen stock # Z334) is a loss of function mutation caused by a point mutation (C48S) in the DNA binding domain (54). rpd304556 (Bloomington stock 11633) is a strong hypomorphic mutation caused by a P-element insertion in the 5’ untranslated region of rpd3, resulting in a severe reduction in Rpd3 expression (46). rpd3def24 (kindly provided by Stewart Frankel) is a null allele caused by excision of a P-element, resulting in a deletion of ~870 bp in rpd304556 in the 5’ coding region of the gene (45). Heterozygous phenotypes for each kni and rpd3 allele were noted after crossing balanced lines to a yw67 strain. Half of embryos from outcrosses to wild-type stocks are heterozygous, thus the observed frequency of eve stripe abnormalities in kni heterozygous embryos (14% for kni9, 21% for kni7G) indicates that the penetrance is 28–42%.
RESULTS
Expression and phosphorylation of recombinant full-length Knirps in Drosophila embryos
Because many transcriptional repressors function via recruitment of additional proteins, we sought to identify proteins that might physically interact with the Knirps protein. Knirps is expressed in limited areas, times, and cell types during development, therefore to facilitate purification, we increased the levels of this protein by conditionally overexpressing it in embryos under the control of the hsp70 heat shock promoter. The protein was expressed with a N-terminal hexahistidine tag and a C-terminal FLAG epitope tag to allow affinity purification. We first determined that the transgenic Knirps protein was biologically active by confirming that it specifically represses the transcription of genes known to be direct targets of knirps (9). The protein was present at undetectable levels prior to heat shock, and was easily detected by Western blotting of crude embryo lysates (Figure 1). We noted that after induction, FLAG M2 antibody cross-reacting bands of approximately 60–80 kDa were present (Figure 1, lane 2). To determine whether the species of different mobility were different polypeptides or whether they might represent the effect of posttranslational modification, phosphatase was added to the extract. Incubation with lambda phosphatase caused these bands to collapse to a single band of approximately 60 kDa. (Fig. 1, lanes 3–7). Inclusion of the phosphatase inhibitor sodium orthovanadate in the reaction prevented this change, indicating that the slower mobility forms of Knirps are phosphorylation products, not distinct polypeptides (Fig. 1, lanes 8 and 9). In later experiments we found that Knirps is also dephosphorylated by endogenous phosphatase activity in the extract upon prolonged incubation (not shown).
Fig. 1.
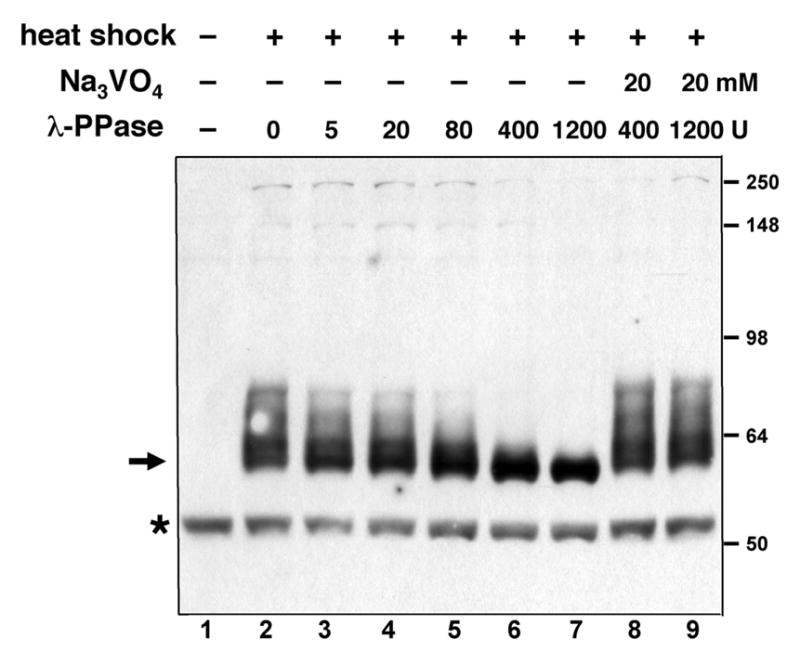
Phosphorylation of recombinant Knirps protein in Drosophila embryos. FLAG-tagged Knirps protein was expressed in 2–4 hour Drosophila embryos and lysates were analyzed by Western blot. Before heat-shock induction, no specific band is visible (lane 1). After heat shock, a set of bands from 60–80 kDa is visible (arrow, lane 2). These bands collapse to a single species upon incubation of lysates with increasing amount of lambda phosphatase (lanes 3–7), but not in the presence of the phosphatase inhibitor sodium orthovanadate (lanes 8–9). A nonspecific product is marked with the asterisk.
Knirps protein present in high molecular weight fractions with Rpd3 protein
Next, we characterized the chromatographic behavior of the induced Knirps protein by passing crude embryo lysates over a gel filtration column whose elution profile had been precalibrated with molecular weight standards. The recombinant protein eluted in fractions corresponding to approximately 450 kDa, indicating that this protein was present in higher order complexes, as the monomer would have the mass of approximately 50–60 kDa. (Fig. 2A). Previous work has demonstrated that Knirps binds to the CtBP cofactor, which exists as products of 40 and 50 kDa, produced from alternatively spliced transcripts (10). A CtBP dimer binding to Knirps would be expected to produce a complex of only ~150 kDa, therefore we tested for the presence of additional proteins using antibodies to candidate proteins. Based on the known association of vertebrate CtBP with histone deacetylases and the frequent association of repressors with HDACs, we tested whether the class I HDAC Rpd3 protein is present in Knirps-containing fractions. We probed fractions from the gel filtration column by Western blot using polyclonal antibodies against Drosophila Rpd3, and found that this protein is detectable in fractions containing Knirps, as well as in lower molecular weight fractions (Fig. 2B). By analyzing a larger amount of the fractions containing Knirps protein on Western blots, we confirmed that the Knirps peak eluted at 450 kDa, and noted that some protein trailed off in smaller fractions (Fig. 2C). We found that Rpd3 protein, as well as the CtBP corepressor, was also present in these fractions (Fig. 2D). These results show that both CtBP and Rpd3 cofractionate with Knirps, and are possibly in a complex with this repressor. The presence of Rpd3 in other fractions is consistent with the protein being associated with a variety of different complexes in vivo (24–31).
Fig. 2.
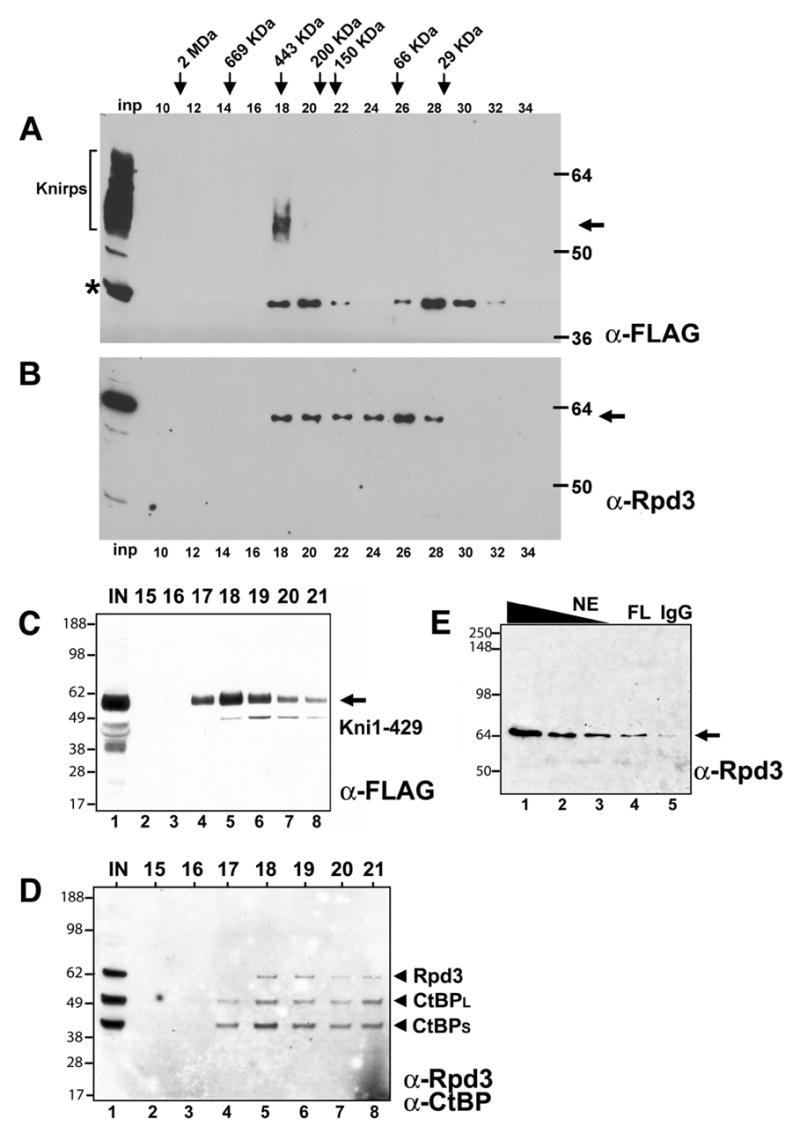
Recombinant Knirps protein is present in a large molecular weight complex, cofractionating with CtBP and Rpd3. Crude extracts containing FLAG-tagged Knirps protein was passed over a Superdex 200 gel filtration column, and even numbered fractions were assayed by Western blot. A, Peak Knirps levels were detected in fraction 18 with anti-FLAG antibodies. Inp, input. Asterisk, nonspecific band. Phosphorylated Knirps species in input lane marked by bracket. B, Rpd3 protein was detected in fraction 18, and in smaller molecular weight complexes. C, Larger amounts of fractions around peak fraction 18 were assayed by Western blot, showing presence of Knirps protein in flanking fractions. D, Rpd3 and CtBP proteins were detected in these Knirps-containing fractions. E, Immunoprecipitation of Knirps protein from embryo nuclear extract using anti-FLAG antibody (FL, lane 4) enriches Rpd3 protein over levels obtained by immunoprecipitation using nonspecific IgG (lane 5). Rpd3 protein present in nuclear extract starting material (NE) is shown in lanes 1–3. Blot was probed with anti-Rpd3 antibodies.
Rpd3 immunoprecipitates with Knirps
To test whether Rpd3 might be physically associated with Knirps, we performed immunoprecipitations on embryo nuclear extracts containing the FLAG-tagged Knirps (Fig. 2E). M2 anti-FLAG antibody-or nonspecific IgG coupled– protein G agarose beads were added to the extract, washed, and eluted material was analyzed by Western blotting using anti-Rpd3 antibodies. Rpd3 was enriched in the immunoprecipitate using the anti-FLAG antibody, suggesting that Knirps and Rpd3 are in the same physical complex. A much smaller amount of Rpd3 was detected in the immunoprecipitation performed with the nonspecific antibodies (Fig. 2E, lane 4 vs. 5).
Co-purification of Rpd3 with Knirps during double affinity purification procedure
To more extensively characterize a putative Knirps-Rpd3 interaction, we next tested whether Rpd3 copurified with Knirps in a double affinity purification scheme (Fig. 3). Knirps protein was expressed in 0–12 hour embryos using a 30 min. heat pulse, and crude lysates were prepared from these embryos. The lysates were incubated with Ni-NTA beads to isolate the protein based on its N-terminal hexahistidine affinity tag. After washing, proteins were eluted from the beads with imidazole, and eluates were diluted in binding buffer and incubated with anti-FLAG epitope M2 antibody coupled to Protein G beads (Fig. 3A). After washing the beads, proteins bound to the antibody beads were stripped off in a buffer containing Sarkosyl detergent, and fractions were analyzed by Coomassie or silver staining, and Western blotting. As expected, most proteins do not bind to the Ni-NTA matrix (Fig. 3A, lanes 1, 2). The low protein concentrations after this first step made it difficult to determine the exact fold enrichment, but a substantial decrease in complexity of the fraction was apparent, including removal of the abundant ~43 kDa yolk proteins (Fig 3A, lanes 1 vs. 6). A substantial fraction of the Knirps protein was not recovered on the Ni-NTA matrix (Fig. 3B, lane 2); increasing the binding time or amount of the matrix did not improve recovery, suggesting that some of this protein may be unable to associate, perhaps because of loss of the N-terminal hexahistidine portion of the protein (S. Payankaulam, unpublished observations). In contrast, most of the Knirps protein applied to the M2 antibody matrix in the second step appears to have bound, as indicated by the substantially depleted levels in the remaining fraction (Fig. 3B, lane 11). After the immunopurification step, the Knirps-containing fractions appear to have a less complex pattern of proteins, especially among lower molecular weight species (Fig. 3A, lanes 10 vs. 15–17).
Fig. 3.
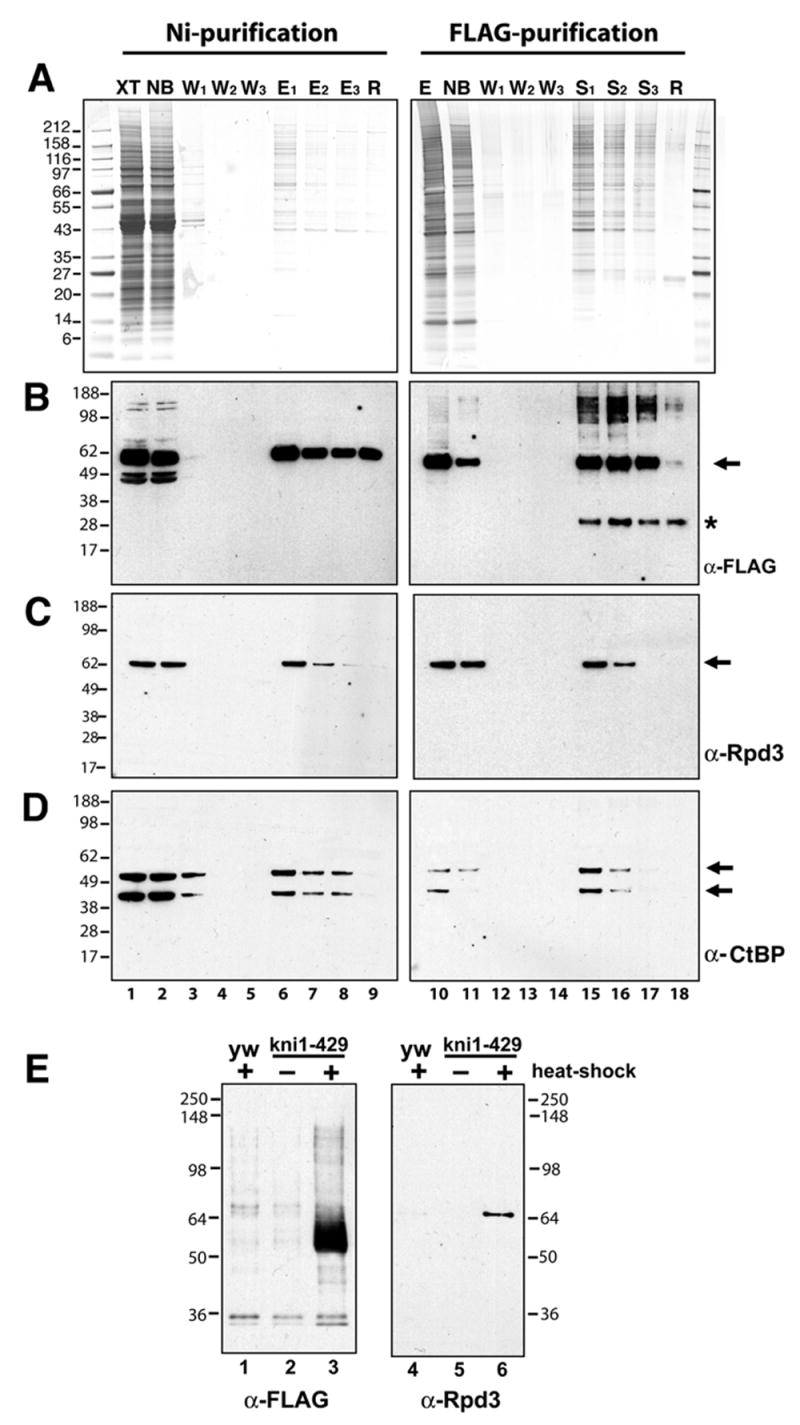
Double affinity purification of Knirps protein reveals copurification of CtBP and Rpd3 proteins. Knirps protein was purified by sequential binding to Ni-NTA affinity resin and anti-FLAG antibody coupled to protein G sepharose beads. A, Coomassie (left) and silver (right) stained gels showing protein composition of crude lysate (XT, lane 1), nonbound material (NB, lane 2), wash fractions 1–3 (W, lanes 3–5; lanes 1–5, 0.007% of total), imidazole eluates from Ni-NTA beads (E1–3, lanes 6–8, 0.13% of total) and material retained on beads after elution (R, lane 9). Panel on the right shows the pooled Ni-NTA eluates (E, lane 10), material that did not bind to anti-FLAG antibody sepharose (NB, lane 11), three washes (W, lanes 12–14; lanes 10–14, 0.16% of total), material eluted from antibodies with Sarkosyl (S, lanes 15–17), and material remaining on beads (R, lane 18; lanes 15–18, 0.08% of total). B, Western blot with anti-FLAG antibody reveals presence of Knirps protein (arrow) during fractionation. Only a fraction of Knirps in the crude lysate is retained on Ni-NTA, however, most of the Ni-NTA purified Knirps subsequently binds to the anti-FLAG beads. Asterisk indicates nonspecific band. C, Western blot with anti-Rpd3 antibodies. A portion of this protein cofractionates with Knirps through the two affinity purification steps. D, Western blot with anti-CtBP antibody reveals fractionation of this corepressor; both short and long forms of the protein copurify with recombinant Knirps protein. E, E, After double affinity purification, Rpd3 is present exclusively in fractions containing recombinant Knirps. Shown are Western blots of proteins from heat-shocked non-transgenic embryos, or from uninduced or heatshock induced transgenic embryos. Double affinity purifications were performed in parallel using equivalent amounts of embryos. Left panel: no anti-FLAG staining material (Knirps protein) is found in fractions from control embryos (lanes 1–2), while a strong band is found in fractions from extracts containing induced Knirps protein (lane 3). Right panel: Rpd3 protein is recovered only in fractions from induced, Knirps expressing embryos (lane 3) and not from controls (lanes 1–2).
Western blotting revealed that Rpd3 copurified with Knirps protein through both of these steps (Fig. 3C). A substantial portion of Rpd3 protein was also present in the nonbound fractions, possibly because of its association with the unbound Knirps or with other complexes (Fig. 3C, lane 2). We noted that both 40 and 50 kDa species of CtBP protein also copurified with Knirps (Fig. 3D). To further test whether the association of Knirps and Rpd3 was specific, we carried out double immunopurifications using extracts from embryos that did not carry the Knirps transgene, or from embryos that carried the transgene but were not induced to express it (Fig. 3E). FLAG-tagged Knirps was recovered only from embryos carrying the transgene that had been induced to express it (Fig. 3E, lane 3), and Rpd3 protein was detected only in these fractions (Fig. 3E, lane 6). This result indicates that purification of Rpd3 from crude extracts was specifically dependent on the presence of Knirps, and confirms that these proteins associate in embryo extracts.
Knirps C-terminal CtBP-dependent repression domain is required for interaction with Rpd3
The N-terminal portion of Knirps, residues 1–330, contains a CtBP-independent repression activity capable of repressing knirps target genes, albeit with lower efficiency (9). A separable CtBP-dependent repression activity that requires a canonical CtBP-binding motif is located in the C terminus (7). We sought to determine whether the C-terminal portion of Knirps is required for association with Rpd3 by testing the association of this protein with the N-terminal region of Knirps during the double affinity purification. This protein was expressed and purified with the same scheme as was used previously (Fig. 4). In the first step, a substantial portion of the Knirps 1–330 protein bound to the Ni-NTA matrix, while no detectable CtBP proteins were present (Fig. 4A, lane 3 vs. 4B, lane 3). Rpd3 protein was found in the material eluted from the Ni-NTA matrix (Fig. 4C, lane 3); however, in the second round of affinity purification, this protein was found almost exclusively in the unbound fraction (4C, lanes 6), while most of the Knirps 1–330 protein was retained in the bound fraction (Fig. 4A, lane 7–8). This result suggests that the C-terminus of Knirps, residues 331–429, is required for efficient interaction with Rpd3. This portion of Knirps also contains the CtBP binding domain, therefore the interaction of Knirps with Rpd3 might be mediated partially or wholly through CtBP. We cannot rule out that the initial copurification of Rpd3 might be a reflection of a weak interaction with the N-terminal portion of Knirps, but clearly the strong association seen with full length Knirps requires the CtBP binding portion of the molecule.
Fig. 4.
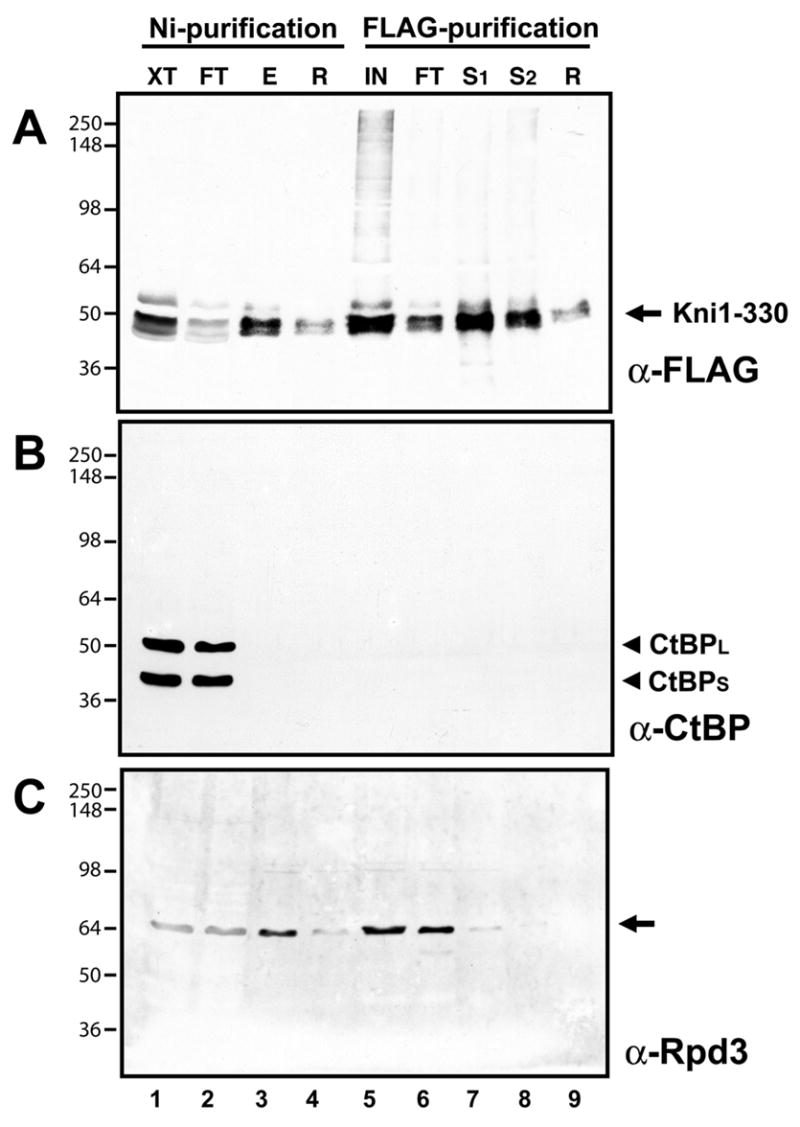
Rpd3 does not copurify through two step affinity purification with N-terminal, CtBP-independent repression domain of Knirps. Double affinity purification was conducted with extracts from embryos expressing the his6- and FLAG-tagged Knirps 1–330 protein, which is active for repression (9). A, Western blot showing Ni-NTA and anti-FLAG purification of recombinant Knirps 1–330 protein. Crude lysate (XT, lane 1, 0.16% of the total), material not bound (FT, lane 2, 0.16% of the total), imidazole eluate from Ni-NTA resin (E, lane 3, 0.3% of the total), material remaining on Ni-NTA, not eluted by imidazole (R, lane 4, 0.2% of the total), pooled eluates from Ni-NTA (IN, lane 5, 20% of the total), material not bound by anti-FLAG protein G sepharose beads (FT, lane 6, 20% of the total), material eluted from antibody beads with Sarkosyl (S1–2, lanes 7–8, 20% of the total), and material remaining on beads (R, lane 9, 20% of the total). B, CtBP protein that is present in the crude extract (lane 1) does not copurify with Knirps, and is instead detected only in the unbound fraction (lane 2). Western blot performed using anti-CtBP antibodies. C, Western blot using anti-Rpd3 antibodies shows presence of Rpd3 protein in fractions. Some of the Rpd3 protein present in the initial fraction does bind to the Ni-NTA column and co-purifies with Knirps in this first step (lane 3), but this protein does not copurify with Knirps 1–330 in the second step, and is found mainly in the unbound fraction (lane 6).
Functional interaction between rpd3 and knirps for segmental gene expression in the Drosophila blastoderm embryo
Our biochemical experiments suggest that Rpd3 protein may form a complex with Knirps, therefore we tested whether the rpd3 and knirps genes show functional interactions in vivo. Previous experiments had indicated that reduction in Rpd3 protein levels alone did not have observable effects on repression by Knirps and other short-range repressors (46). However, the multiple repression activities of the Knirps protein may have masked the reduction in Rpd3 activity. Therefore, we measured the effects of depleting both Rpd3 and Knirps simultaneously in doubly heterozygous mutant embryos. As a measure of Knirps activity, we monitored the expression of even-skipped (eve). This segmentation gene is expressed in seven transverse stripes in the blastoderm embryo under the control of separate, modular enhancers (Fig. 5A, B). Two of the eve transcriptional enhancers are direct targets of Knirps; the eve stripe 3/7 enhancer is highly sensitive to Knirps, while the eve stripe 4/6 enhancer is repressed only in regions containing higher levels of Knirps, and is less sensitive to ectopic Knirps (9, 12). In heterozygous backgrounds for two different alleles of rpd3, almost all of the embryos showed a wild-type eve pattern (Table I). As noted previously, eve expression is perturbed in some embryos that are heterozygous for knirps (8). We found that in kni9 or kni7G heterozygotes, some embryos have aberrant expression of eve, exhibiting ectopic expression between stripes 4–6 (Fig. 5B, C), or in other embryos, a loss of stripe 5 expression (Fig. 5E, F). In the double heterozygous cross, the proportion of embryos showing these aberrant patterns is greatly increased, with up to one third of the embryos exhibiting a stripe 4–6 fusion or loss of stripe 5 expression (Table I). For the kni9 allele, the number of embryos exhibiting aberrant eve patterns doubled in both rpd3 backgrounds. The increase is much greater than would be expected if the effects of kni and rpd3 mutations were additive. Heterozygosity in rpd3 also substantially increased the frequency of eve stripe irregularities in the kni7G background. In general, the alterations in eve expression were qualitatively similar in the kni heterozygous and kni/rpd3 double heterozygous backgrounds, indicating that reduction of Rpd3 levels pushes the expression pattern closer toward a threshold already approached by the reduction in Knirps, rather than imposing an entirely novel phenotype. We conclude that Knirps repression activity is compromised when the levels of Rpd3 protein are decreased. This finding supports the idea that Rpd3 is a functional component of a Knirps repression complex, and is consistent with the idea that short-range repressors mediate at least some of their activity via local chromatin deacetylation.
Fig. 5.
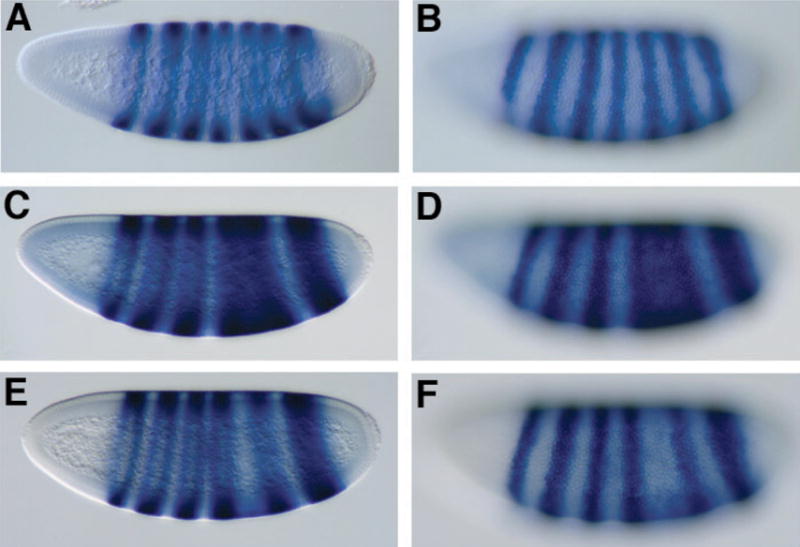
eve expression patterns reveal genetic interaction between rpd3 and knirps in doubly heterozygous embryos. eve expression was analyzed by in situ hybridization of embryos from wild-type strains (A, B) or from crosses of rpd3 and kni heterozygous mutants (C–F). A, B Wild-type eve expression in a mid cycle 14 embryo; C, D eve expression showing fusion of eve stripes 4–6, characteristic of a large percentage of embryos from double heterozygous cross and a smaller fraction of kni heterozygous embryos; this embryo from kni9/+, rpd3def24/+ cross. E, F attenuation of eve stripe 5 embryo characteristic of a large percentage of embryos from double heterozygous cross and a smaller fraction of embryos from kni heterozygotes; this embryo from kni9/+, rpd3def24/+ cross. Identical phenotypes were observed in crosses with kni7G and rpd04556 (Table I). Embryos were fixed and hybridized with antisense eve RNA to visualize expression of the eve gene, and are oriented with dorsal up, anterior pole to the left. A, C, E parasagittal sections; B, D, F surface images of the embryos at left.
Table I.
Quantitation of expression patterns of even-skipped (eve) in blastoderm embryos mutant for knirps and rpd3. eve expression patterns were scored in blastoderm embryos derived from crosses of heterozygous individuals carrying mutations in rpd3 and/or knirps. Heterozygosity at the rpd3 locus for two different alleles had little effect on eve patterning (the mutants were outcrossed to a wild-type strain with yw markers). Heterozygosity at the knirps locus led to 14–21% of blastoderm embryos exhibiting a fused 4/6 or missing stripe 5 phenotype; the frequency of this phenotype increased approximately 1.5 – 2 fold in the rpd3 heterozygous backgrounds. rpd3def24 is a null allele and rpd3 04556 is a P-element insertion that strongly reduces rpd3 expression. Values do not add to 100% in some cases because other, heterogeneous and difficult to classify phenotypes were observed in a small number of embryos.
| wild type eve pattern (%) | fused or missing stripe 5 (%) | n | |
|---|---|---|---|
| rpd3 04556 x yw67 | 94 | 2.5 | 231 |
| rpd3 04556 x kni9 | 64 | 32 | 331 |
| rpd3 04556 x kni7G | 64 | 29 | 316 |
| rpd3def24 x yw67 | 98.5 | 0.5 | 219 |
| rpd3def24 x kni9 | 72 | 28 | 294 |
| rpd3def24 x kni7G | 66 | 33 | 314 |
| kni9 x yw67 | 85 | 14 | 283 |
| kni7G x yw67 | 79 | 21 | 258 |
DISCUSSION
Rpd3 as a Knirps co-repressor
Our study indicates that the Knirps repressor is present in a complex with the Rpd3 histone deacetylase, and that the portion of Knirps required for strong association with Rpd3 includes the CtBP binding C-terminus. CtBP proteins, both long and short forms, are found in the affinity purified Knirps fractions along with Rpd3 (Fig. 3). Therefore, the association of Rpd3 might be entirely via CtBP, which is known to directly contact Knirps via a small PMDLSMK motif in the C terminus of Knirps. Indeed, the copurification of mammalian CtBP with class I histone deacetylases indicates that such a direct association is possible (although that study did not rule out the possibility that HDAC proteins were present in the CtBP complex due to a direct association with DNA binding transcription factors such as Zeb, which was also found in the purified complex) (18). The apparent molecular mass of the Knirps complex of 450 kDa suggests that additional cofactors are yet to be identified; these may include Sin3, Sds3, SAP18, and SAP30, which have been previously identified in Rpd3-containing complexes, or chromatin modifying activities such as histone methyl transferases or demethylases, as were found in the vertebrate CtBP complex (18, 20, 21). Genetic studies of sap18 indicate that this protein is not required for correct pair rule gene patterning, which indicates that short-range repressors are still active, but as we have shown in this study, dosage sensitivity studies may be required to reveal more subtle functional relationships (55).
Previous genetic studies have not uncovered a connection between the Rpd3 histone deacetylase and Drosophila short-range repressors, however, it was recognized that due to the presence of multiple histone deacetylases, loss of Rpd3 might be compensated for by other HDACs (46). Biochemical tests have also failed to draw a link between short-range repressors and deacetylation; repression mediated by Knirps domains and CtBP was found to be resistant to trichostatin A under conditions where the Groucho corepressor, which is known to bind to Rpd3, is partially inhibited (11). However, these experiments were carried out in transiently transfected cells, and the exact mechanism of repression might differ from that found on native chromatin. In addition, the multiple repression activities of Knirps might mask a partial loss of activity in this system, so that an Rpd3 dependence might only be revealed in certain native genetic contexts.
The genetic interaction between knirps and rpd3 by itself does not distinguish between a direct or indirect interaction model; perturbations to the general chromatin environment might alter the ability of Knirps to function in a nonspecific manner. However, studies point to Rpd3 as playing a very specific role in modifying chromatin in a local fashion, unlike the general effects seen with some histone acetyltransferases (41, 44). In yeast, recent studies point to Rpd3 recruitment to a discrete subset of genes that are bound by Ume6 and Swi4/6 transcription factors (44, 56). In Drosophila, Rpd3 is bound to euchromatic regions estimated to encompass 2–13% of genes in polytene chromosomes, and knock down experiments in Drosophila S2 cells of the Sin3 cofactor of Rpd3 show that only 364 of 13,000 genes examined are induced (52, 57). Thus, it seems likely that effects on Knirps activity are not due to gross alterations of bulk chromatin. The direct physical association with the Knirps complex that we have demonstrated indicates that Rpd3 is likely to co-occupy promoters during repression, a model that we are currently testing by chromatin immunoprecipitation assays.
Genetic effects of kni and rpd3 on eve expression patterns.
Knirps binds directly to both the eve stripe 4/6 and 3/7 enhancers, however the higher number and affinity of binding sites in stripe 3/7 appear to allow this element to respond to very low levels of the Knirps protein, and to remain repressed even when the CtBP corepressor is absent (9, 12). Thus, stripes 3 and 7 appear to form normally in all embryos, even those with reduced doses of Knirps and Rpd3 (Fig. 5). The more weakly repressed stripe 4/6 enhancer element is thus a more sensitive measure of reduced Knirps activity; some embryos exhibit ectopic expression between stripes 4–6, similar to the phenotype observed in CtBP mutant embryos. In other embryos, the borders of stripes 4 and 6 do appear to form, possibly responding to an increase in Knirps protein levels or the eve autoactivating element. Here, the aberrant phenotype manifests itself as a reduced or absent eve stripe 5. This reduction in stripe 5 expression is unlikely to be due to a direct interaction between the Knirps protein and the stripe 5 enhancer, because this enhancer lacks any Knirps binding sites, and is never affected in a background in which Knirps is ectopically expressed, unlike stripes 4/6 and 3/7 (9, 12). Knirps functional interaction with the hunchback, krüppel and giant gap genes, whose repressive activies also shape eve expression, are a likely explanation for the failure of eve stripe 5 to form properly in the double heterozygous background.
Repression Mechanism
Detailed molecular studies of Rpd3-mediated repression in yeast reveal interesting properties that are fully concordant with the types of repression mediated by Knirps. Deckert and Struhl found that the range of activity of Rpd3 complexes on reporters was strongly distance dependent, with loss of repression when the linear distance between activators and repressors exceeded 200 bp (58). Studies indicate that this distance is consistent with the range of histone deacetylation mediated by Rpd3 in yeast of 150–250 bp, or one to two nucleosomes (41). These ranges are strikingly similar to those observed for Knirps, CtBP, and Giant in Drosophila (6, 7, 10). One aspect of repression may be different between these two systems; in yeast, repression mediated by Rpd3 coincided with loss of TBP binding, but not activator binding (59). Short-range repression mediated by Rpd3 via Knirps and other short-range repressors is not likely to influence binding of basal machinery, because repression of individual enhancers is observed under conditions where the basal promoter is active, and responding to signals from other enhancers. Thus, unlike the situation studied in yeast, in Drosophila, short-range repressors are very likely to influence the ability of activators to access the template, for their repression efficiency is dictated by the number, position, and affinity of the activator binding sites (51). It is possible that Rpd3 mediates similar molecular changes to the chromatin template in both yeast and flies, and the individual context in which the repressors are found dictate the actual effects on basal machinery or neighboring activator proteins. The local activity of Rpd3, in any event, would be entirely consistent with a “quenching” mode of repression, involving interference with the activity of local activators, as opposed to long-range, but transient “hitchhiking” effects on the basal machinery, whereby repressors would not disturb activators bound close by (60).
Long-range vs. short-range repression
In contrast to the short-range modifications mediated by Rpd3 in yeast, and Knirps in Drosophila, the long-range Drosophila repressor Hairy is able to influence activity of cis regulatory elements from long distances (61). Surprisingly, the Hairy protein also recruits the Rpd3 deacetylase, via the Groucho corepressor. Two possible explanations provide a basis for such different functional outputs from repressor complexes that recruit Rpd3; first, other mechanisms, not dependent on Rpd3, may account for Hairy’s long range of activity. For example, Groucho also interacts with histone H1, which may explain its Rpd3-independent repression activity (27). In addition, Hairy independently interacts with the Sir2 HDAC, which may be involved in long-range repressive effects (62). Second, Groucho has been found to multimerize, which may facilitate its spreading along the template, and potentially tethering Rpd3 to multiple locations (63). This would allow the same molecular mechanism to be active on a larger portion of the gene, effectively extending its range from the original Hairy binding site.
Although histone deacetylases have been implicated in numerous repression complexes, our study is the first to directly implicate Rpd3 in the activity of short-range Drosophila repressors. Further studies to identify the protein complexes present at repressed promoters in the embryo will provide a richer understanding of the mechanisms by which these repressors execute their functions during development.
Acknowledgments
We thank Z. Ullah and R.W. Henry for assistance in gel exclusion chromatography, D. Wassarman, S. Frankel for reagents, the Bloomington Stock Center for fly stocks, M. Buckley, P. Mani, and C. Martinez for useful comments on the manuscript, and E. Fernandez-Villatoro for technical assistance.
Footnotes
This work was supported by NIH grant GM56976 to D.N.A.
The abbreviations used are: CtBP, C-terminal binding protein; NAD, nicotinamide adenine dinucleotide; HDAC, histone deacetylase; eve, even-skipped; Ni-NTA, Ni(2+)-nitrilotriacetic acid; HRP, horseradish peroxidase; DTT, dithiothreitol; PMSF, phenylmethylsulfonyl fluoride; EDTA ethylene diamine tetraacetic acid; TCA, trichloroacetic acid; SDS-PAGE, sodium dodeecyl sulfate polyacrylamide gel electrophoresis; TBP, TATA binding protein.
References
- 1.Arnosti DN. In: Handbook of Experimental Pharmacology. Gossen M, Kaufmann J, Triezenberg SJ, editors. Springer; Heidelberg: 2004. pp. 33–67. [Google Scholar]
- 2.Gaston K, Jayaraman PS. Cell Mol Life Sci. 2003;60:721–741. doi: 10.1007/s00018-003-2260-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thiel G, Lietz M, Hohl M. Eur J Biochem. 2004;271:2855–2862. doi: 10.1111/j.1432-1033.2004.04174.x. [DOI] [PubMed] [Google Scholar]
- 4.Fujioka M, Emi-Sarker Y, Yusibova GL, Goto T, Jaynes JB. Development. 1999;11:2527–2538. doi: 10.1242/dev.126.11.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nibu Y, Zhang H, Bajor E, Barolo S, Small S, Levine M. EMBO J. 1998;23:7009–7020. doi: 10.1093/emboj/17.23.7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hewitt GF, Strunk BS, Margulies C, Priputin T, Wang XD, Amey R, Pabst BA, Kosman D, Reinitz J, Arnosti DN. Development. 1999;6:1201–1210. doi: 10.1242/dev.126.6.1201. [DOI] [PubMed] [Google Scholar]
- 7.Keller SA, Mao Y, Struffi P, Margulies C, Yurk CE, Anderson AR, Amey RL, Moore S, Ebels JM, Foley K, Corado M, Arnosti DN. Mol Cell Biol. 2000;19:7247–7258. doi: 10.1128/mcb.20.19.7247-7258.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nibu Y, Zhang H, Levine M. Science. 1998;5360:101–104. doi: 10.1126/science.280.5360.101. [DOI] [PubMed] [Google Scholar]
- 9.Struffi P, Corado M, Kulkarni M, Arnosti DN. Development. 2004;131:2419–2429. doi: 10.1242/dev.01075. [DOI] [PubMed] [Google Scholar]
- 10.Sutrias-Grau M, Arnosti DN. Mol Cell Biol. 2004;24:5953–5966. doi: 10.1128/MCB.24.13.5953-5966.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryu JR, Arnosti DN. Nucleic Acids Res. 2003;31:4654–4662. doi: 10.1093/nar/gkg491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clyde DE, Corado MS, Wu X, Pare A, Papatsenko D, Small S. Nature. 2003;426:849–853. doi: 10.1038/nature02189. [DOI] [PubMed] [Google Scholar]
- 13.Chinnadurai G. BioEssays. 2003;1:9–12. doi: 10.1002/bies.10212. [DOI] [PubMed] [Google Scholar]
- 14.Kumar V, Carlson JE, Ohgi KA, Edwards TA, Rose DW, Escalante CR, Rosenfeld MG, Aggarwal AK. Mol Cell. 2002;4:857–869. doi: 10.1016/s1097-2765(02)00650-0. [DOI] [PubMed] [Google Scholar]
- 15.Nardini M, Spanò S, Cericola C, Pesce A, Massaro A, Millo E, Luini A, Corda D, Bolognesi M. EMBO J. 2003;22:3122 – 3130. doi: 10.1093/emboj/cdg283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Q, Piston DW, Goodman RH. Science. 2002;5561:1895–1897. doi: 10.1126/science.1069300. [DOI] [PubMed] [Google Scholar]
- 17.Balasubramanian P, Zhao LJ, Chinnadurai G. FEBS Lett. 2003;537:157–160. doi: 10.1016/s0014-5793(03)00119-4. [DOI] [PubMed] [Google Scholar]
- 18.Shi Y, Sawada J, Sui G, Affar el B, Whetstine JR, Lan F, Ogawa H, Luke MP, Nakatani Y, Shi Y. Nature. 2003;6933:735–738. doi: 10.1038/nature01550. [DOI] [PubMed] [Google Scholar]
- 19.Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA, Shi Y. Cell. 2004;119:941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 20.Kasten MM, Dorland S, Stillman DJ. Mol Cell Biol. 1997;17:4852–4858. doi: 10.1128/mcb.17.8.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lechner T, Carrozza MJ, Yu Y, Grant PA, Eberharter A, Vannier D, Brosch G, Stillman DJ, Shore D, Workman JL. J Biol Chem. 2000;275:40961–40966. doi: 10.1074/jbc.M005730200. [DOI] [PubMed] [Google Scholar]
- 22.Dorland S, Deegenaars ML, Stillman DJ. Genetics. 2000;154:573–586. doi: 10.1093/genetics/154.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Sun ZW, Iratni R, Erdjument-Bromage H, Tempst P, Hampsey M, Reinberg D. Mol Cell. 1998;1:1021–1031. doi: 10.1016/s1097-2765(00)80102-1. [DOI] [PubMed] [Google Scholar]
- 24.Kadosh D, Struhl K. Cell. 1997;3:365–371. doi: 10.1016/s0092-8674(00)80217-2. [DOI] [PubMed] [Google Scholar]
- 25.Yang WM, Inouye C, Zeng Y, Bearss D, Seto E. Proc Natl Acad Sci U S A. 1996;93:12845–12850. doi: 10.1073/pnas.93.23.12845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sommer A, Hilfenhaus S, Menkel A, Kremmer E, Seiser C, Loidl P, Luscher B. Curr Biol. 1997;7:357–365. doi: 10.1016/s0960-9822(06)00183-7. [DOI] [PubMed] [Google Scholar]
- 27.Chen G, Fernandez J, Mische S, Courey AJ. Genes Dev. 1999;17:2218–2230. doi: 10.1101/gad.13.17.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pile LA, Schlag EM, Wassarman DA. Mol Cell Biol. 2002;14:4965–4976. doi: 10.1128/MCB.22.14.4965-4976.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis PW, Beall EL, Fleischer TC, Georlette D, Link AJ, Botchan MR. Genes Dev. 2004;18:2929–2940. doi: 10.1101/gad.1255204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magnaghi-Jaulin L, Groisman R, Naguibneva I, Robin P, Lorain S, Le VJP, Troalen F, Trouche D, Harel-Bellan A. Nature. 1998;391:601–605. doi: 10.1038/35410. [DOI] [PubMed] [Google Scholar]
- 31.Tie F, Prasad-Sinha J, Birve A, Rasmuson-Lestander A, Harte PJ. Mol Cell Biol. 2003;9:3352–3362. doi: 10.1128/MCB.23.9.3352-3362.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robyr D, Suka Y, Xenarios I, Kurdistani SK, Wang A, Suka N, Grunstein M. Cell. 2002;109:437–446. doi: 10.1016/s0092-8674(02)00746-8. [DOI] [PubMed] [Google Scholar]
- 33.Kurdistani SK, Robyr D, Tavazoie S, Grunstein M. Nat Genet. 2002;31:248–254. doi: 10.1038/ng907. [DOI] [PubMed] [Google Scholar]
- 34.Sabet N, Volo S, Yu C, Madigan JP, Morse RH. Mol Cell Biol. 2004;24:8823–8833. doi: 10.1128/MCB.24.20.8823-8833.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aggarwal BD, Calvi BR. Nature. 2004;430:372–376. doi: 10.1038/nature02694. [DOI] [PubMed] [Google Scholar]
- 36.Vogelauer M, Rubbi L, Lucas I, Brewer BJ, Grunstein M. Mol Cell. 2002;10:1223–1233. doi: 10.1016/s1097-2765(02)00702-5. [DOI] [PubMed] [Google Scholar]
- 37.Aparicio JG, Viggiani CJ, Gibson DG, Aparicio OM. Mol Cell Biol. 2004;24:4769–4780. doi: 10.1128/MCB.24.11.4769-4780.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rundlett SE, Carmen AA, Suka N, Turner BM, Grunstein M. Nature. 1998;392:831–835. doi: 10.1038/33952. [DOI] [PubMed] [Google Scholar]
- 39.Rundlett SE, Carmen AA, Kobayashi R, Bavykin S, Turner BM, Grunstein M. Proc Natl Acad Sci U S A. 1996;93:14503–14508. doi: 10.1073/pnas.93.25.14503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kadosh D, Struhl K. Genes Dev. 1998;6:797–805. doi: 10.1101/gad.12.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kadosh D, Struhl K. Mol Cell Biol. 1998;9:5121–5127. doi: 10.1128/mcb.18.9.5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Nadal E, Zapater M, Alepuz PM, Sumoy L, Mas G, Posas F. Nature. 2004;427:370–374. doi: 10.1038/nature02258. [DOI] [PubMed] [Google Scholar]
- 43.De Rubertis F, Kadosh D, Henchoz S, Pauli D. Nature. 1996;6609:589–591. doi: 10.1038/384589a0. [DOI] [PubMed] [Google Scholar]
- 44.Robert F, Pokholok DK, Hannett NM, Rinaldi NJ, Chandy M, Rolfe A, Workman JL, Gifford DK, Young RA. Mol Cell. 2004;16:199–209. doi: 10.1016/j.molcel.2004.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mottus R, Sobel RE, Grigliatti TA. Genetics. 2000;154:657–668. doi: 10.1093/genetics/154.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mannervik M, Levine M. Proc Natl Acad Sci U S A. 1999;12:6797–6801. doi: 10.1073/pnas.96.12.6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wheeler JC, VanderZwan C, Xu X, Swantek D, Tracey WD, Gergen JP. Nat Genet. 2002;1:206–210. doi: 10.1038/ng942. [DOI] [PubMed] [Google Scholar]
- 48.Chen G, Courey AJ. Gene. 2000;1–2:1–16. doi: 10.1016/s0378-1119(00)00161-x. [DOI] [PubMed] [Google Scholar]
- 49.Chang YL, Peng YH, Pan IC, Sun DS, King B, Huang DH. Proc Natl Acad Sci U S A. 2001;98:9730–9735. doi: 10.1073/pnas.171325498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strunk B, Struffi P, Wright K, Pabst B, Thomas J, Qin L, Arnosti DN. Dev Biol. 2001;2:229–240. doi: 10.1006/dbio.2001.0454. [DOI] [PubMed] [Google Scholar]
- 51.Kulkarni MM, Arnosti DN. Mol Cell Biol. 2005;25:3411–3420. doi: 10.1128/MCB.25.9.3411-3420.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pile LA, Wassarman DA. EMBO J. 2000;22:6131–6140. doi: 10.1093/emboj/19.22.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soeller WC, Poole SJ, Kornberg T. Genes Dev. 1988;1:68–81. doi: 10.1101/gad.2.1.68. [DOI] [PubMed] [Google Scholar]
- 54.Gerwin N, La RA, Sauer F, Halbritter HP, Neumann M, Jackle H, Nauber U. Mol Cell Biol. 1994;12:7899–7908. doi: 10.1128/mcb.14.12.7899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singh N, Zhu W, Hanes SD. Dev Biol. 2005;278:242–254. doi: 10.1016/j.ydbio.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 56.Humphrey EL, Shamji AF, Bernstein BE, Schreiber SL. Chem Biol. 2004;11:295–299. doi: 10.1016/j.chembiol.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 57.Pile LA, Spellman PT, Katzenberger RJ, Wassarman DA. J Biol Chem. 2003;278:37840–37848. doi: 10.1074/jbc.M305996200. [DOI] [PubMed] [Google Scholar]
- 58.Deckert J, Struhl K. Mol Cell Biol. 2001;8:2726–2735. doi: 10.1128/MCB.21.8.2726-2735.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Deckert J, Struhl K. Mol Cell Biol. 2002;18:6458–6470. doi: 10.1128/MCB.22.18.6458-6470.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arnosti DN, Gray S, Barolo S, Zhou J, Levine M. EMBO J. 1996;14:3659–3666. [PMC free article] [PubMed] [Google Scholar]
- 61.Barolo S, Levine M. EMBO J. 1997;10:2883–2891. doi: 10.1093/emboj/16.10.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosenberg MI, Parkhurst SM. Cell. 2002;109:447–458. doi: 10.1016/s0092-8674(02)00732-8. [DOI] [PubMed] [Google Scholar]
- 63.Song H, Hasson P, Paroush Z, Courey AJ. Mol Cell Biol. 2004;24:4341–4350. doi: 10.1128/MCB.24.10.4341-4350.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


