SYNOPSIS
Objectives
The Centers for Disease Control and Prevention recommend screening individuals at risk for hepatitis C virus (HCV) infection. However, few published data describe outcomes of individuals with antibody to HCV (anti-HCV) identified through screening programs. The purpose of this study was to assess rates of medical evaluation and HCV treatment, change in alcohol consumption, and barriers to medical care after testing anti-HCV positive through a public screening program.
Methods
Anti-HCV positive individuals identified through San Diego sexually transmitted disease (STD) clinics and an HIV test site screening program were informed of positive test results, provided education and referral, and contacted by telephone three, six, and ≥12 months later.
Results
From September 1, 1999, to December 31, 2001, 411 anti-HCV positive individuals were newly identified, of whom 286 (70%) could be contacted ≥ three months after receipt of test results (median length [range] of follow-up 14 [3–35] months). Of these 286, 156 (55%) reported having received a medical evaluation, of whom 19 (12%) began HCV treatment. Of 132 who reported drinking alcohol before diagnosis, 100 (76%) reported drinking less after diagnosis. Individuals with medical insurance at diagnosis were more likely than those without insurance to obtain a medical evaluation during follow-up (75 [68%] of 111 vs. 70 [45%] of 155; p<0.001). Among those who did not obtain an evaluation, the most commonly reported reason was lack of insurance.
Conclusions
Only about half of newly identified anti-HCV positive individuals received a medical evaluation, although 76% reported drinking less alcohol. Identifying ways to improve medical access for those who are anti-HCV positive could improve the effectiveness of screening programs.
Hepatitis C virus (HCV) is the most common chronic bloodborne infection in the United States, with approximately 2.7 million Americans (1.3%) chronically infected,1 most of whom are unaware of their infection.2 Alcohol consumption is known to increase the likelihood of cirrhosis among HCV-infected individuals.3 Without treatment and moderation or avoidance of alcohol, 10% to 15% of those chronically infected with HCV will develop liver cirrhosis within 20 years of infection.4 Indeed, HCV infection is currently the most common indication for liver transplantation in the United States.4 The Centers for Disease Control and Prevention (CDC) recommend screening individuals at risk for hepatitis C, including current or former injection drug users (IDUs) and recipients of blood transfusions or organ transplants prior to July 1992.5
For a screening program to be effective, it must either improve patient prognosis by allowing access to medical care at an earlier, more treatable stage of disease, as in the case of Pap smear screening for cervical cancer, or prevent the spread of infection within a population, as in sexually transmitted disease (STD) screening and treatment of individuals at high risk. Because the majority of those infected with HCV are asymptomatic until advanced liver disease develops and therapeutic interventions may be more successful in early disease, HCV screening could improve patients' prognoses by allowing earlier access to HCV treatment and encouraging decreased alcohol consumption. Awareness of HCV infection through screening could also increase acceptance of recommended vaccines that protect the liver from other hepatitis viruses. HCV screening could also theoretically decrease the spread of HCV in the population, particularly if knowledge of infection were to prompt HCV-infected IDUs to stop using drugs or inject more safely, because 60% of new HCV infections are estimated to occur because of injection drug use.6 However, data are limited regarding whether individuals with antibody to HCV (anti-HCV) who are identified through screening programs access medical care, receive hepatitis A and B vaccination, decrease alcohol consumption, or change injection drug use behavior.2 To answer this question, this study prospectively followed anti-HCV positive individuals identified through HCV screening programs at STD clinics and an HIV test site in San Diego County, California.
METHODS
Hepatitis C Screening Program
All patients seeking care at the main San Diego County STD clinic were offered anti-HCV testing from September 1, 1999, to April 30, 2000. Selective screening criteria were established on the basis of an analysis of these data.7 For the remainder of the enrollment period (May 1, 2000, to December 31, 2001), anti-HCV testing was offered only to STD clinic patients with one or more of the following risk factors for HCV: injection drug use (past or present), blood transfusion before 1992, commercial sex work (women only), or sex with an injection drug user or person known to be chronically infected with hepatitis B or C. Selective screening using the same risk factor criteria was also offered at three part-time satellite STD clinics during this period. From October 1, 2000, to December 31, 2001, confidential anti-HCV testing was also offered to all clients at an anonymous HIV counseling and testing site in San Diego.
Individuals were considered to be anti-HCV positive if they had HCV antibody detected by enzyme immunoassay (EIA; HCV 3.0, released 1996, Ortho Diagnostic Systems, Inc., Raritan, NJ, www.orthoclinical.com) and confirmed by recombinant immunoblot assay (RIBA® 3.0, released 1999; Chiron Corp., Emeryville, CA, www.chiron.com), or if they had a history of injection drug use (IDU), a positive EIA test, and no RIBA® performed. Confirmatory RIBA® testing of IDUs was discontinued in 2000 when it was determined that the positive predictive value of an EIA test in an IDU was ≥97%.7 Hepatitis B vaccination was offered to all STD clinic patients during the study period. Hepatitis A vaccination was offered to anti-HCV positive STD clinic patients starting in April 2000.
STD program disease investigators informed patients of positive anti-HCV test results, conducted a brief baseline interview (77% in person, 23% by telephone), and provided education emphasizing the need for further medical evaluation, alcohol cessation, hepatitis A and B vaccination, and the prevention of HCV spread. Anti-HCV positive individuals were also given a packet of information that recommended abstaining from alcohol and obtaining a follow-up medical evaluation from either their regular physician or a community clinic (sliding fee scale). The packet included names, addresses, and phone numbers of community clinics, public assistance programs, groups conducting clinical trials, support groups, and other community organizations. No facilities in San Diego County provide non-emergent hepatitis C-related medical care to uninsured individuals without payment in advance of service provision.
Follow-up
Staff attempted to contact patients by telephone three, six, and ≥12 months later to assess medical care access and alcohol and drug use, and to provide referral information as needed. Standard public health strategies were used to locate patients, both initially for notification of the positive test result and later for follow-up. These strategies included calling and sending a letter to the address on file at the clinic, and, if the phone number and/or address were incorrect, checking state Department of Motor Vehicles, local phone company, and county (e.g., public assistance and court) records for more current information. Field visits to the patient's home were performed initially if necessary to notify the patient of the positive result, but were not performed to obtain follow-up.
Analysis
Data were entered and analyzed in Epi Info 6.04.8 Medical insurance was defined as private insurance, public insurance (including Medicaid or County Medical Services, a program in San Diego County that provides medical coverage for otherwise medically indigent patients meeting certain criteria), military medical coverage, or other insurance as defined by the patient. Follow-up hepatitis A and B vaccination status was determined by self-report; most vaccinations were likely received in the STD clinic, but some could have been from outside medical providers. Predictors of obtaining a medical evaluation among anti-HCV positive individuals were assessed using relative risks, with adjustment for confounding by Mantel-Haenszel stratification. P values <0.05 were considered statistically significant. Human Subjects Review at CDC determined that this study was program evaluation, not research, and that Institutional Review Board approval was not necessary.
RESULTS
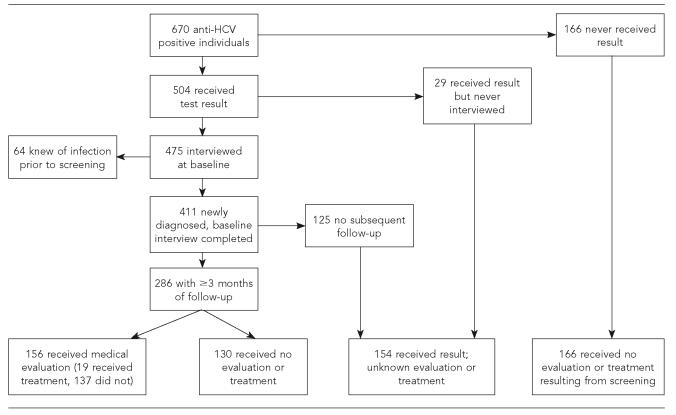
During the study period, 670 anti-HCV positive patients were identified, including 168 (5%) of 3,461 patients tested during the period of universal STD clinic screening, 352 (24%) of 1,463 tested during the period of selective STD clinic screening, and 150 (7%) of 2,148 tested during universal HIV test site screening. Of all 670 anti-HCV positive patients, 504 (75%) were documented to have received their test result and 475 (71%) were interviewed at baseline by disease investigators (Figure). After 64 patients who reported having known of their infection before this screening were excluded, 411 patients with newly diagnosed anti-HCV positive test results were identified. Of these 411 patients, 286 (70%) could be contacted ≥3 months after the receipt of test results and the remainder could not be located (n=95), were incarcerated (n=14), refused a follow-up interview (n=12), or were deceased (n=4).
Figure.
Anti-HCV-positive patient flow chart, San Diego, California, 1999–2003
Among the 670 anti-HCV positive individuals, those who tested at satellite STD clinics were less likely to have ≥3 months of follow-up than those from other sites (19% [29/150] vs. 49% [257/520]; p<0.001) because logistic problems prevented disease investigators from interviewing some patients from these outlying sites. Patients older than 40 years of age were more likely to have ≥3 months of follow-up data than younger patients (50% [160/321] vs. 36% [126/349]; p<0.001). Follow-up proportions were similar by sex, race/ethnicity, and history of injection drug use (data not shown).
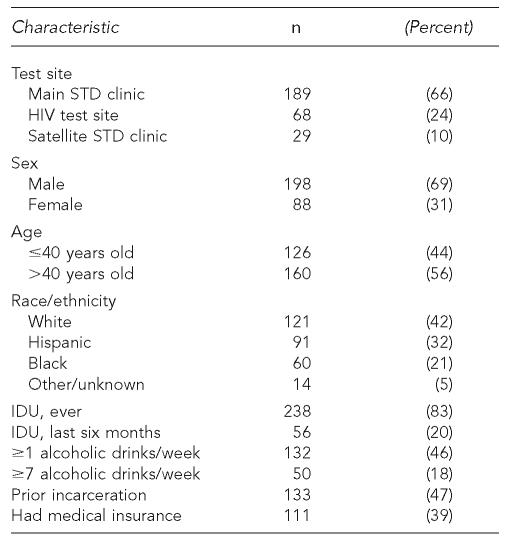
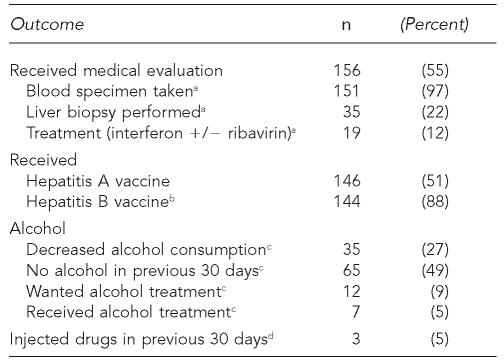
Among the 286 patients newly identified with anti-HCV and ≥3 months of follow-up, the median length of follow-up was 14 months (range: 3–35 months). Baseline characteristics of these 286 patients are shown in Table 1. Patients had a median age of 41 years (range: 20–63 years) with 81% aged 30–49 years. By the time of last follow-up, 156 (55%) had received a medical evaluation, of whom 19 (12%) had received hepatitis C treatment (interferon +/– ribavirin), and 146 (51%) had received ≥1 dose of hepatitis A vaccine (Table 2). Of 163 individuals who lacked serologic evidence (antibody to hepatitis B core antigen) of prior hepatitis B infection, 144 (88%) had received ≥1 dose of hepatitis B vaccine. Among the 140 individuals who reported the date of their first medical evaluation, 101 (72%) had their first evaluation within three months of receiving their test result, and 113 (81%) within six months. Of 132 individuals who reported drinking ≥1 alcoholic drink per week before diagnosis, 35 (27%) reported decreased alcohol consumption (as measured in drinks per week) and 65 (49%) reported no alcohol consumption in the 30 days before their most recent follow-up. Approximately three months after the receipt of test results with HCV education, 100% of 163 individuals asked knew that alcohol could damage the liver. Only three (5%) of 56 individuals who reported IDU in the six months prior to diagnosis reported injecting drugs within 30 days of their most recent follow-up.
Table 1.
Baseline characteristics of 286 individuals with newly identified anti-HCV and $3 months of follow-up, San Diego, California, 1999–2003
STD = sexually transmitted disease
IDU = injection drug use
Table 2.
Outcomes among 286 individuals with newly identified anti-HCV and ≥3 months of follow-up, San Diego, California, 1999–2003
Denominator is 156 individuals who received medical evaluation.
Denominator is 163 individuals who lacked serologic evidence of prior infection (anti-HBc).
Denominator is 132 individuals who reported drinking $1 alcoholic drink per week prior to HCV diagnosis.
Denominator is 56 individuals who reported injecting drugs in the six months prior to HCV diagnosis.
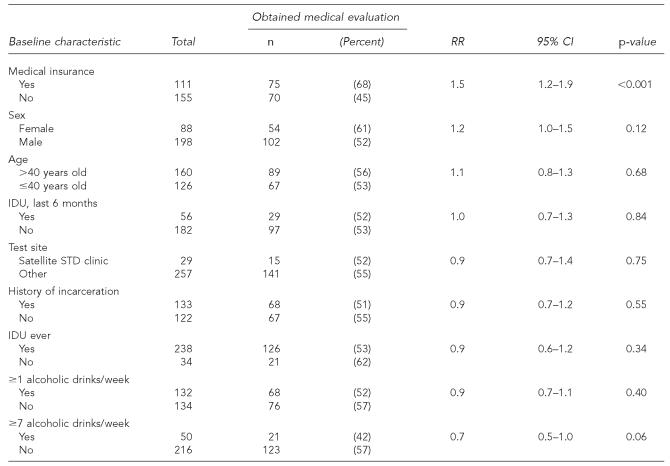
The major predictor of obtaining a medical evaluation was having medical insurance at baseline (Table 3). Race/ethnicity, sex, age, test location, IDU, and history of incarceration did not substantially affect the likelihood of medical evaluation, while individuals who reported drinking ≥7 alcoholic drinks per week at baseline tended to be less likely to have a medical evaluation during follow-up. Controlling for baseline alcohol consumption did not alter the observed association between having medical insurance at baseline and receiving a medical evaluation during follow-up (adjusted relative risk [ARR] = 1.5; 95% confidence interval [CI] 1.2, 1.8).
Table 3.
Predictors of obtaining medical evaluation among anti-HCV-positive patients, San Diego, California, 1999–2003
NOTE: Total denominators vary slightly because some individuals did not answer all questions.
RR = relative risk
CI = confidence interval
IDU = injection drug use
Barriers to medical evaluation were also assessed by directly asking the 130 patients who had not had a medical evaluation why they had not. Sixty-two (48%) gave lack of insurance as the reason that they had not had a medical evaluation, 24 (18%) cited lack of time, 15 (12%) felt evaluation was not important, 17 (13%) gave other reasons, and for 12 (9%) the reason was unknown. Eighty-nine individuals who had not had a medical evaluation ≥12 months after receiving a diagnosis were asked, “If you had a case manager to help you arrange medical care, do you think you would have had a medical evaluation for hepatitis C?” Sixty-five (73%) responded “yes,” and of these, 50 (77%) wanted help obtaining insurance, 48 (74%) wanted help finding a physician, and 45 (69%) wanted help arranging payment for medical care.
DISCUSSION
This report documents the largest prospective follow-up of individuals newly identified with anti-HCV through publicly funded screening programs and shows that multiple barriers exist between screening and HCV treatment. First, getting results to patients was difficult; only 75% of HCV-infected individuals received their result. The less than ideal result receipt rate is typical of individuals seen in public STD clinics and HIV test sites; in 1998, only 63% of HIV-infected individuals tested at publicly funded sites received their results.8 Similarly, a 1997 study of chronic hepatitis B in San Diego found that only 66% of individuals who had a positive hepatitis B surface antigen and were tested by private providers knew their test result.9 Obtaining accurate locating information from patients when the test is done and using standard public health techniques to notify patients who do not return for results are important steps in making sure that patients receive results. In 2003, after emphasizing the importance of recording accurate patient locating information at the time of hepatitis screening and shortening the time between testing and result availability, 83% of HCV-positive patients tested in the main STD clinic received their results.
Second, among newly identified anti-HCV positive patients with at least three months of follow-up, only slightly more than half (55%) received a medical evaluation and only a very small proportion (12%) of these started treatment during a median of 14 months of follow-up. The proportion of anti-HCV positive patients who meet current indications for treatment is unknown. Data from a liver clinic showed that 25% of referred anti-HCV positive patients were started on treatment,10 while data from a Veterans Administration study showed that approximately 40% of individuals with detectible HCV RNA (thus approximately 34% of antibody-positive patients) were considered treatment candidates.11
Even though relatively few anti-HCV positive patients were started on treatment, a majority (76%) of baseline alcohol drinkers reported decreased alcohol consumption in the 30 days prior to last follow-up, most (88%) susceptible individuals received hepatitis B vaccine, and about half (51%) received hepatitis A vaccine. Because hepatitis A and B can have a more fulminant course in HCV-infected individuals and alcohol consumption can increase the likelihood of cirrhosis,3 these outcomes are likely to positively influence the health of anti-HCV positive individuals identified through the screening program.
Eighty-three percent of anti-HCV positive individuals were current or former IDUs, higher than the 60% estimated by other investigators,6 likely because of the screening criteria used. IDUs typically have difficulty accessing medical care and qualifying for government assistance programs such as Medicaid.12,13 Treating former IDUs with HCV can be challenging but has been successfully reported by different groups.14–18 Screening programs that appropriately target this population should determine whether infected individuals are able to access medical care and obtain treatment. The majority of IDUs in this population had not injected drugs in the six months before baseline, suggesting that STD clinics and HIV test sites may be a good place to identify former IDUs who may be better treatment candidates than current users.
This study has several limitations. These results may not be generalizable to HCV screening conducted at other sites, such as drug treatment facilities and physician's offices, where both the proportion of patients testing positive and the proportion following through with medical care may differ. Because some individuals did not answer all questions, some data were missing on baseline characteristics, which could have biased our assessment of predictors of obtaining a medical evaluation. However, since only 7% of individuals were missing baseline information on medical insurance, this is unlikely to have changed our conclusion that lack of medical insurance is associated with not obtaining a medical evaluation. Although most (76%) baseline alcohol drinkers with follow-up available reported drinking less or no alcohol in the 30 days before their most recent follow-up, whether this self-reported information is reliable, sustainable over time, or a result of the screening program is unknown. The apparent decrease in injection drug use should not be interpreted as causally related to the screening program because individuals actively using drugs may have been less likely to have been reached for follow-up. In addition, some individuals tested at the STD clinic had recently entered drug treatment and may have injected drugs within the six months prior to presentation, but stopped injecting prior to HCV screening.
Assessing the outcomes of publicly funded screening programs is essential. This study found that having medical insurance increased the likelihood of getting a medical evaluation by 50% and that the majority of those who didn't get an exam wanted help obtaining insurance and/or paying for a medical evaluation. This study demonstrates a clear unmet need for medical care in this population. Obtaining an initial medical evaluation, following through with the necessary medical work-up before treatment can be considered, maintaining adherence to treatment, and maintaining sobriety can all be difficult tasks. Anti-HCV positive individuals could benefit from case management similar to the publicly funded case management available for those infected with HIV.19 Such a program should be developed and rigorously evaluated to determine its impact on medical, alcohol, and drug use outcomes.
Acknowledgments
The authors thank Lissa Bentulan, Gilbert “Andy” Valasquez, and Craig Sturak for their help with data collection, and Julie Magri, MD, MPH for her comments on the manuscript.
Footnotes
This study was partially supported by funding from the Viral Hepatitis Integration Project (VHIP) cooperative agreement U50/CCU919053-01, Centers for Disease Control and Prevention.
The findings and conclusions in this report of those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556–62. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- 2.Chou R, Clark EC, Helfand M Preventive Services Task Force (US) Screening for hepatitis C virus infection: a review of the evidence for the U.S. Preventive Services Task Force. Ann Int Med. 2004;140:465–79. doi: 10.7326/0003-4819-140-6-200403160-00014. [DOI] [PubMed] [Google Scholar]
- 3.Ostapowicz G, Watson KG, Locarnini SA, Desmond PV. Role of alcohol in the progression of liver disease caused by hepatitis C virus. Hepatology. 1998;27:1730–35. doi: 10.1002/hep.510270637. [DOI] [PubMed] [Google Scholar]
- 4.National Institutes of Health Consensus Development Conference Statement: management of hepatitis C 2002—June 10–12, 2002. Hepatology. 2002;36:S3–20. doi: 10.1053/jhep.2002.37117. [DOI] [PubMed] [Google Scholar]
- 5.Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR-19):1–39. [PubMed] [Google Scholar]
- 6.Alter MJ. Hepatitis C virus infection in the United States. J Hepatol. 1999;31(Suppl 1):88–91. doi: 10.1016/s0168-8278(99)80381-x. [DOI] [PubMed] [Google Scholar]
- 7.Gunn RA, Murray PJ, Brennan CH, Callahan DB, Alter MJ, Margolis HS. Evaluation of screening criteria to identify individuals with hepatitis C virus infection among sexually transmitted disease clinic clients: results from the San Diego Viral Hepatitis Integration Project. Sex Transm Dis. 2003;30:340–4. doi: 10.1097/00007435-200304000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (US) Atlanta: CDC; 2001. Epi Info: version 6.04. [Google Scholar]
- 9.Centers for Disease Control and Prevention. Atlanta: CDC; 2001. [cited 2005 May 3]. HIV counseling and testing in publicly funded sites annual report 1997 and 1998. Available from: URL: http://www.cdc.gov/hiv/pubs/cts98.pdf. [Google Scholar]
- 10.Weinberg MS, Gunn RA, Mast EE, Gresham L, Ginsberg M. Preventing transmission of hepatitis B virus from people with chronic infection. Am J Prev Med. 2001;20:272–6. doi: 10.1016/s0749-3797(01)00298-7. [DOI] [PubMed] [Google Scholar]
- 11.Falck-Ytter Y, Kale H, Mullen KD, Sarbah SA, Sorescu L, McCullough AJ. Surprisingly small effect of antiviral treatment in patients with hepatitis C. Ann Intern Med. 2002;136:288–92. doi: 10.7326/0003-4819-136-4-200202190-00008. [DOI] [PubMed] [Google Scholar]
- 12.Cheung RC, Currie S, Shen H, Ho SB, Bini EJ, Anand BS, et al. Chronic hepatitis C in Latinos: natural history, treatment eligibility, acceptance, and outcomes. Am J Gastroenterol. 2005;100:2186–93. doi: 10.1111/j.1572-0241.2005.00240.x. [DOI] [PubMed] [Google Scholar]
- 13.Riley ED, Wu AW, Junge B, Marx M, Strathdee SA, Vlahov D. Health services utilization by injection drug users participating in a needle exchange program. Am J Drug Alcohol Abuse. 2002;28:497–511. doi: 10.1081/ada-120006738. [DOI] [PubMed] [Google Scholar]
- 14.Chitwood DD, Comerford M, McCoy HV. Satisfaction with access to health care among injection drug users, other drug users, and nonusers. J Behav Health Serv Res. 2002;29:189–97. doi: 10.1007/BF02287705. [DOI] [PubMed] [Google Scholar]
- 15.Sylvestre DL. Treating hepatitis C in methadone maintenance patients: an interim analysis. Drug Alcohol Depend. 2002;67:117–23. doi: 10.1016/s0376-8716(02)00010-8. [DOI] [PubMed] [Google Scholar]
- 16.Dalgard O, Bjoro K, Hellum K, Myrvang B, Skaug K, Gutigard B, et al. Treatment of chronic hepatitis C in injecting drug users: 5 years' follow-up. Eur Addict Res. 2002;8:45–9. doi: 10.1159/000049487. [DOI] [PubMed] [Google Scholar]
- 17.Sylvestre DL. Treating hepatitis C virus infection in active substance users. Clin Infec Dis. 2005;40(Suppl 5):S321–4. doi: 10.1086/427447. [DOI] [PubMed] [Google Scholar]
- 18.Matthews G, Kronborg IJ, Dore GJ. Treatment for hepatitis C virus infection among current infection drug users in Australia. Clin Infect Dis. 2005;40(Suppl 5):S325–9. doi: 10.1086/427448. [DOI] [PubMed] [Google Scholar]
- 19.Litwin AH, Soloway I, Gourevitch MN. Integrating services for injection drug users infected with hepatitis C virus with methadone maintenance treatment: challenges and opportunities. Clin Infect Dis. 2005;40(Suppl 5):S339–45. doi: 10.1086/427450. [DOI] [PubMed] [Google Scholar]
- 20.Katz MH, Cunningham WE, Fleishman JA, Andersen RM, Kellogg T, Bozzette SA, Shapiro MF. Effect of case management on unmet needs and utilization of medical care and medications among HIV-infected individuals. Ann Intern Med. 2001;135(8 Pt 1):557–65. doi: 10.7326/0003-4819-135-8_part_1-200110160-00006. [DOI] [PubMed] [Google Scholar]