Abstract
Background
Naive coronary vessels may appear to have intimal thickening histologically characteristic of cardiac allograft vasculopathy (CAV). This study appraises the experimental and clinical impact of this observation.
Methods
Tissue sections from 12 naïve hearts of miniature swine, 13 native porcine hearts of recipients of heterotopic cardiac allografts, three native human hearts, and 3 human hearts with CAV were compared with light microscopy and morphometric analysis. Results were also compared with morphometric data previously gathered from 3 grafts in a standard experimental model of CAV (rejectors) and 3 grafts harvested from swine rendered tolerant to their donor hearts (chimeras).
Results
In the naïve and native porcine hearts, the prevalence of CAV "mimics” was 0–6.94% (mean ± s.d. (m) =1.99 ± 1.97%) and 0–7.57% (m=2.97 ± 2.20%), respectively (P=0.12). The prevalence of CAV in the grafts of porcine rejectors and chimeras was 9.9–14.8% (m = 12.4 ± 2.5%) and 0.6–4.5% (m = 2.6 ± 2.0%), respectively (P < 0.05). “CAV” in the chimeras was similar in prevalence to that of the naïve and native hearts. In native human hearts and human grafts, the prevalence was 1.86–2.00 (m=1.95 ± 0.08) and 9.09–17.50% (m=12.80 ± 4.29%), respectively (P=0.01).
Conclusions
Smooth muscle bundles inside the internal elastic laminae are similarly prevalent in human and porcine coronary vasculature. Their histological similarity to intimal thickening of CAV could lead to an inaccurate distinction between graft tolerance and CAV in clinical, as well as experimental, studies of heart transplantation.
INTRODUCTION
With the advent of effective immunosuppressive agents, which have reduced the loss of allografts due to acute rejection, cardiac allograft vasculopathy (CAV) is the chief cause of allograft failure after the first year of transplantation1–3. Originally observed more than thirty years ago4, CAV in cardiac allografts is manifest by accelerated, diffuse coronary artery disease, which has important morphologic differences from native vessel atherosclerosis5. CAV is characterized as progressive, concentric intimal thickening by donor-derived6,7 smooth muscle cells and extracellular matrix, leading to severe allograft ischemia and causing over 50% of late deaths in some transplant centers8.
An experimental large animal model using miniature swine with defined MHC loci (termed SLA, for swine leukocyte antigens) has been developed by a selective breeding program over the last 30 years9. This unique model has offered a means of studying the development of CAV in a MHC-defined manner. As we have previously reported10, the majority of vascular lesions in this model reveal intimal expansion by α-smooth muscle actin-positive spindle cells and extracellular matrix. The lesions, at the time of graft failure, typically lack inflammatory cells and maintain an intact endothelium. The severity and prevalence of CAV have been considered useful parameters in comparing the effectiveness of different protocols of heart transplantation (HT) in this large animal model.
In our standardized morphometric analysis of vessels in a harvested graft, 24 tissue sections are routinely examined. On occasion, we have observed vessels in naïve porcine hearts that appear to have intimal thickening histologically characteristic of CAV. Furthermore, in some cases, apparent lesions of CAV were observed in a relatively few number of sections. These findings led to occasional uncertainty in the diagnosis and quantification of CAV in experimental allografts, a potentially important caveat in comparing the effectiveness of various protocols of HT. This study appraises the impact of these observations on our assessment of experimental CAV and tests for a clinical correlation.
MATERIAL AND METHODS
Animals
Three to four month-old swine with intra-MHC recombinant haplotypes were derived from spontaneous recombination events during the breeding of heterozygotes as part of the breeding program11. Genotyping has been controlled by strict pedigree breeding and confirmed by microcytotoxicity testing with allospecific antisera.
All animal care and procedures complied with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH publication 86–23, revised 1985).
Non-transplanted animal hearts
An effort was made to quantify the prevalence of vessels with histologic morphology resembling that of CAV in naive hearts taken from pigs that had undergone no experimental treatment. Likewise, native (non-transplanted) hearts from recipients of heterotopic porcine heart allografts were examined similarly to determine any effect of experimental treatment on the prevalence of CAV mimics.
Twelve hearts of randomly selected, untreated miniature swine (naïve hearts) and 13 orthotopic hearts (native hearts) of recipients of heterotopic cardiac allografts were harvested at the time of sacrifice of the animals, which ranged 3–4 months in age. The allograft recipients received hearts that were transplanted across an MHC matched, minor antigen mismatched barrier (n = 5), an MHC class I barrier (n = 7), or an MHC class II barrier (n = 1). Treatment of these recipients varied according to seven different experimental protocols.
Cardiac transplantation in animals
Hearts were implanted heterotopically into partially inbred miniature swine, as previously described12. Briefly, donor and recipient animals underwent general anesthesia, heparinization, and preparation for long-term vascular access. The donor pulmonary artery and aorta were sewn end-to-side to the recipient inferior vena cava and aorta, respectively. Epicardial pacemaker wires were placed and brought out through the skin. Graft function was followed daily by direct transabdominal palpation and electrocardiography (EK/5A, Burdick Corp, Milton, WI). Allografts were harvested when loss of graft function was documented.
Allograft data are from hearts transplanted into two groups of three miniature swine, which have been described in recent publications. Rejectors were class I disparate allograft recipients treated with a 12-day course of cyclosporin A (CsA), starting on the day of HT12. A second group of hearts were transplanted into MHC-matched, minor antigen-disparate recipients made chimeric to minor antigens by treatment with peripheral hematopoietic progenitor cells (PHPC) from their respective donors, as described previously13. CsA was given through a gastric tube one day prior to and continued for 30 days after PHPC infusion. Rejection of skin grafts documented maintenance of a selectively immunocompetent state in each of the chimeras.
Human hearts
Three native hearts harvested at autopsy were studied to obtain histomorphometric data comparable to that of the naïve animal hearts. The deceased individuals included 2 males and 1 female, who were 9–18 (mean 13.3) years old. Two of them had myocarditis, and one had died suddenly with Wolf-Parkinson-White syndrome. Although the hearts examined in this study were not normal, vascular disease was not an issue in any of the three hearts.
Three cardiac allografts were harvested at autopsy from patients who had succumbed to CAV. The deceased were three males, who were 49–59 (mean 53.7) years old at the time of death. One patient was 4 years post-HT, and two were 6 years out.
Histomorphometric analysis
Using an established protocol, three parallel, 0.5 cm thick, cross-sections of heart were sampled midway between the left atrioventricular groove and apex and parallel to the right atrioventricular groove. A total of twenty-four sections of myocardium were cut for analysis, the same representative sections from each of the three slices of heart. In each slice, sections of left anterior descending, circumflex, and right coronary arteries were taken. Additional sections included posteromedial and anterolateral papillary muscles, lateral wall of left ventricle, interventricular septum, and anterior right ventricular wall.
Cardiac tissue obtained at autopsy was fixed in 10% formalin and subsequently embedded in paraffin. Five-micron, representative histologic sections were cut from paraffin blocks, stained with hematoxylin and eosin (H&E) and Verhoeff elastic stain and studied by light microscopy.
Morphometry was done to quantify the prevalence and severity of CAV-like morphology. Cardiac tissue sections were cut in a standardized fashion except in two of the human allografts, which were cut prior to our established protocol. Each tissue section was scanned with a 10X objective lens until the entire section was examined. All vessels with intact muscular walls, no branching distortions, and a clear presence or absence of CAV-like morphology were included in the study. The morphometric analysis included all vessels showing intimal thickening. These vessels demonstrated intimal expansion by spindle cells (smooth muscle-like cells), with or without extracellular matrix, inside the internal elastic laminae. They included epicardial and myocardial arteries and veins ranging from 20 μm to 2 mm in diameter.
Images of vessels with morphologic features of CAV were captured to a Power Macintosh 7300/200 computer by a Hitachi 3-CCD Color Camera (model HV-C20) attached to a Nikon Eclipse E600 microscope. With digital imaging (IPLab Spectrum, Signal Analytics Corporation, Vienna, VA), images were then analyzed by manual color segmentation by tracing the endothelial surface (intima), internal elastic lamina, and external elastic lamina of each vessel that demonstrated intimal thickening. From segmented images, intimal and luminal areas were computed, allowing calculation of percent stenosis of each vessel lumen. Prevalence of CAV-like morphology was defined as the percent of vessels with apparent lesions of CAV, i.e., [number of vessels with CAV-like morphology/number of vessels examined] x 100. Severity of CAV-like morphology quantified the apparent percent stenoses of involved vessels, i.e., [intimal area/intimal area + luminal area] x 100. The prevalence and severity reported for each group represent the mean of prevalences and severities of all animals in each group. The data were stored digitally.
Statistical analysis
All results are reported as mean ± standard deviation (s.d.). Statistical differences between the mean values within the experimental and clinical groups were determined by using a Student's T-test. Scatter plots were derived according to standard methods.
RESULTS
Animal data
Naïve porcine hearts were studied to quantify the prevalence of vessels with morphologic features of CAV despite the de facto absence of allograft vasculopathy. Native hearts from recipients of heterotopic allografts were similarly studied to seek evidence that vasculopathy might somehow be induced by experimental treatment protocols.
In 3444 and 3118 vessels assessed in naïve and native porcine hearts, respectively, the prevalence of intimal thickening (Figure 1) was 0–6.94% (mean ± s.d. (m) =2.0±2.0%) in the naïve hearts and 0–7.57% (m=3.0±2.2%) in the native hearts (P=0.12); mean severity was 81.5±7.5% and 86.4±4.5% (P=0.06), respectively. Hence, the prevalence of vessels with CAV-like morphology was found to be small but notable, and there is no statistically significant difference between prevalence and severity of CAV-like morphology in naïve and native porcine hearts.
Figure 1.
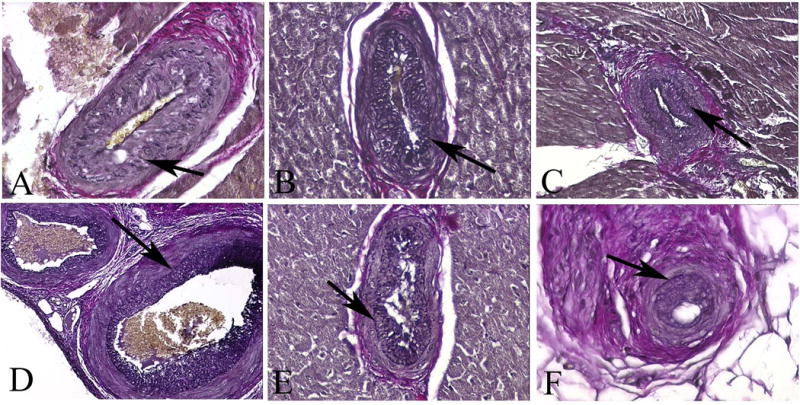
Porcine coronary arteries of three naïve (A–C) and three native (D–F) hearts. The vessels reveal morphologic features of CAV in experimental grafts (elastic stains) (arrows mark internal elastic laminae). (A–C) Myocardium, x200; (D–E) Myocardium, x600; (F) Epicardium x600.
As we have previously reported13, the prevalence of CAV in 1547 vessels of rejectors and 6195 vessels of the chimeras (Figure 2) was 9.9–14.8% (m=12.4 ± 2.5%) and 0.6–4.5% (m=2.6 ± 2.0%), respectively (P<0.05), while the mean severity of CAV in the two groups was similar (64.8±15.2% and 60.3±9.9%) (P=0.69), respectively). These data are compared with the prevalence of intimal thickening in a scatter plot in Figure 3. As in the naive and native control hearts, vascular intimal thickening consisted of smooth muscle cells in a similarly dense pattern with a random or more regular orientation inside the internal elastic lamina. In addition, there was a small amount of extracellular matrix, but intimal mononuclear cells were rarely observed.
Figure 2.
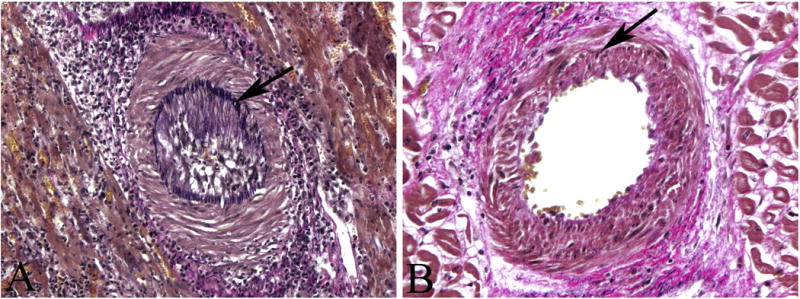
Typical features of CAV seen in two porcine heterotopic allografts (elastic stains) (arrows mark internal elastic laminae). (A) Myocardium in a rejector’s graft, x200; (B) Myocardium in a graft from a chimeric recipient, x320.
Figure 3.
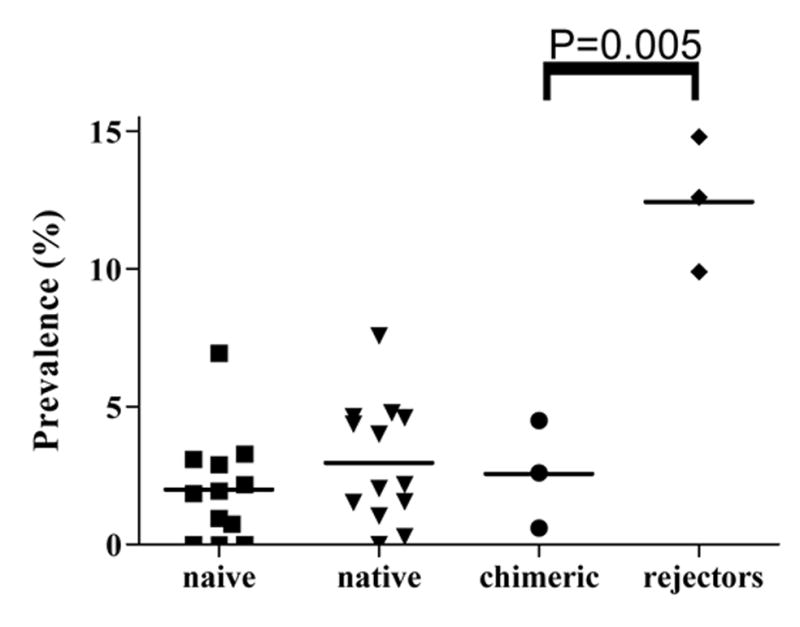
A scatter plot comparing the prevalence of vessels with intimal thickening in the non-transplanted and transplanted porcine hearts. The prevalence in the grafts of the rejectors is higher than that of the chimeric animals as well as that of the naïve and native hearts.
Interestingly, the prevalence of CAV in the rejectors, in which the experimental protocol is known not to result in graft tolerance, is more than the prevalence of intimal thickening in the naïve and native porcine hearts; however, the prevalences of intimal thickening in the naïve and native porcine hearts are similar to the prevalence of what was considered to be CAV in the chimeras, in which the treatment protocol does result in graft tolerance. Prior to the current study, the prevalence of CAV in the chimeras was considered to be relatively low compared to that of the rejectors. However, the current study suggests that statistically there is no CAV in chimeras at all.
Human data
To test for a clinical correlation of these experimental data, three native human pediatric hearts were examined to compare with the data obtained from the naïve and native porcine hearts. Of 1344 vessels in these hearts, the prevalence of intimal thickening (Figure 4) was 1.86–2.00 (m=2.0 ± 0.1), similar to that in the non-transplanted porcine hearts; mean severity was 48.0±1.9%.
Figure 4.
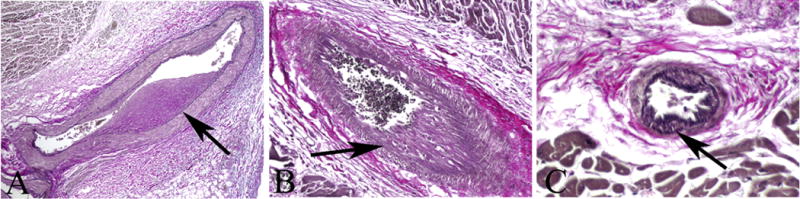
Human coronary arteries of three native hearts. The vessels reveal morphologic features that could be confused with those CAV (elastic stains) (arrows mark internal elastic laminae). (A) Epicardium, 50; (B) Myocardium, x200; (C) Myocardium, 400.
Likewise, a study of human allografts with documented severe CAV was performed to check for a distinction between real vasculopathy and a normally variant appearance of intimal thickening. In 845 vessels assessed in the human grafts, the prevalence of CAV (Figure 5) was 9.1–17.5% (m=12.80 ± 4.3%); mean severity was 72.0±5.0%. As in experimental rejectors, the prevalence of CAV is clearly higher (P<0.05) than the prevalence of apparent intimal thickening in the native human hearts (Figure 6). Human and animal data are compared in Table 1.
Figure 5.
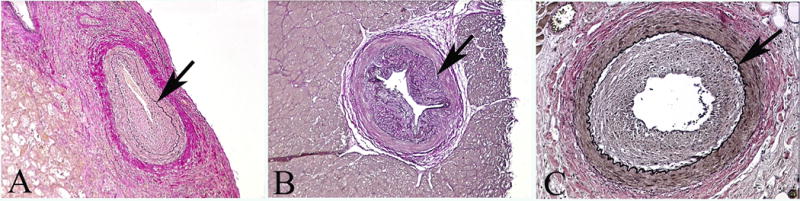
Typical features of CAV seen in three human orthotopic allografts (elastic stains) (arrows mark internal elastic laminae). (A) Epicardium, 100; (B) Myocardium, x100; (C) Myocardium, 200.
Figure 6.
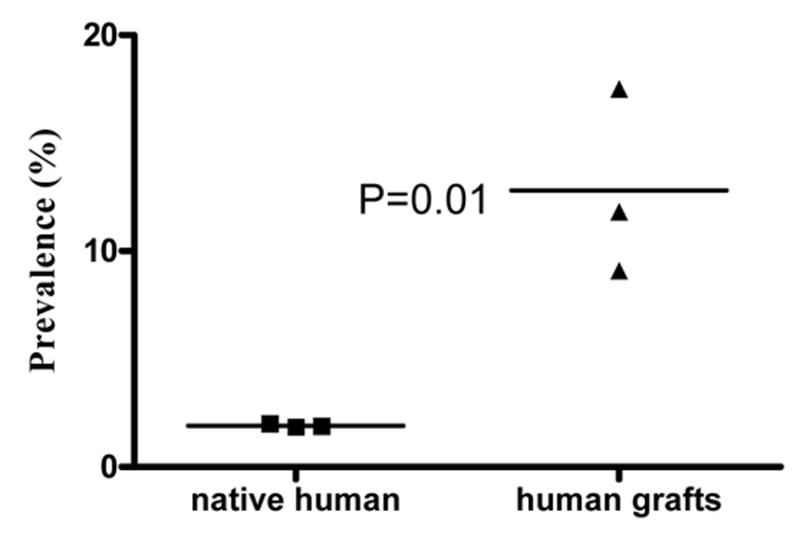
A scatter plot comparing the prevalence of vessels with intimal thickening in the native human hearts. The prevalence in the human grafts is higher than that of the native human hearts.
Table 1.
Comparison of prevalence and severity of CAV-like morphology.
| Mean Prevalence (%) | Mean Severity (%) | |
|---|---|---|
| Naive pig hearts | 2.0 ± 2.0 | 81.5 ± 7.5 |
| Native pig hearts | 3.0 ± 2.2 | 86.4 ± 4.5 |
| Rejector pig hearts | 12.4 ± 2.5 | 64.8 ± 15.2 |
| Chimera pig hearts | 2.6 ± 2.0 | 60.3 ± 9.9 |
| Human control hearts | 2.0 ± 0.1 | 48.0 ± 1.9 |
| Human allograft hearts | 12.8 ± 4.3 | 72.0 ± 5.0 |
DISCUSSION
Principal Findings
To our knowledge, this report is the first to quantify the small but notable prevalence of vessels in naïve porcine epicardium and myocardium, which have morphologic features of CAV in experimental heterotopic porcine allograft hearts. Longitudinally oriented cushions of smooth muscle have been characterized in human coronary arteries as normal14 and as pathologic findings15–20. They have also been observed in rhesus monkeys and rabbits21–24. In the only other known report of this finding in porcine coronary arteries, Whelan et al25 described these “coronary endocardial cushions” in humans as well as seven additional mammalian species and suggested that these cushions may play a functional role in intramural coronary arterial blood flow and predispose to ischemic heart disease.
Vessels with these muscular cushions, particularly if they produce a more or less concentric morphology in cross-section, could clearly mimic histological features of CAV. This study provides convincing evidence that intimal thickening observed in the naïve miniature swine hearts and native hearts of recipients of porcine allografts reflect the presence of morphologic variants of normal coronary arteries, viz., smooth muscle cell cushions inside internal elastic laminae, that have previously been described in human hearts. The validity of this conclusion is supported by the prevalence of intimal thickening found in the native human hearts, which was similar to that of the non-transplanted porcine hearts. As anticipated, we observed no evidence of intimal smooth muscle cell cushions in veins of naive or native hearts.
The development of severe cardiac allograft vasculopathy in pediatric recipients has recently been shown to be more common than previously thought26, 27. Because of the relatively young ages of the experimental animals and human cadavers from which the native (control) hearts were studied, native vessel atherosclerosis was not observed in these coronary arteries. Likewise, the typical phenotype of native vessel atherosclerosis was not observed in the human allografts. Such a finding could have confounded histologic interpretation in our study. Fortunately, we were able to focus on CAV versus normal smooth muscle cushions as the cause of intimal thickening.
The second important finding in this study is related to the impact of this morphologic variant in coronary arteries on the assessment of various models of HT in a large animal model such as the miniature swine. In retrospect, a very regular arrangement of smooth muscle cells inside internal elastic lamina is characteristic of longitudinal intimal cushions. We have no reason to believe that the procedure of transplantation itself alters the morphology of longitudinally oriented smooth muscle cushions since the neatly regular pattern of smooth muscle cells seen in some naive and native vessels has also been observed in some vessels of allograft hearts.
However, as illustrated in Figure 1 and Figure 4, in a notable number of vessels in naive and native hearts, the smooth muscle cells expanding intimae lacked this neatly regular pattern. Ignoring this finding could result in a diagnosis of CAV when, in fact, no CAV is present. As shown in our comparison of morphometric data of the rejectors and chimeras, a cursory assessment suggests that CAV was present in both groups albeit significantly lower in the chimeras. On the basis of this study, one could argue that CAV was indeed present in the rejectors but, in fact, did not reach statistical significance in the chimeras, an assessment which might alter one’s evaluation of the latter experimental protocol.
Finally, the study indicates that muscular cushions in human coronary arteries can potentially affect one’s assessment of prevalence of CAV in human grafts. The diagnosis of CAV on a limited amount of cardiac tissue could be invalidated by morphologic variants of normal coronary vessels. Furthermore, although intimal thickening of CAV is frequently concentric, lesions of CAV are not necessarily concentric. Longitudinally oriented smooth muscle cushions vary in morphology, and, depending on the manner in which a vessel with these cushions is cut in cross-section, an apparent intimal thickening might be concentric or eccentric. The importance of taking 10–15 sections of myocardium from allografts at autopsy, i.e., enough to allow one to assess 100–200 vessels, is clearly established by the findings of this study. When the prevalence of CAV has then been calculated, one must then adjust for the prevalence of morphologic variants of normal coronary vessels, based on the assessment of naïve hearts, before being decisive about the actual prevalence of CAV.
Conclusion
We confirm that longitudinally oriented smooth muscle bundles inside internal elastic laminae, previously described as a normal variant in human coronary vascular morphology, are also present in porcine epicardial and myocardial vessels. In histological cross-section, they can closely mimic intimal thickening of CAV. This occurrence can lead to an inaccurate distinction between CAV and graft tolerance in experimental protocols of heart transplantation. Furthermore, because of CAV mimics, a failure to quantify vascular lesions in allograft hearts at the time of autopsy may result in an erroneous diagnosis and assessment of severity of CAV in clinical studies as well.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shirwan H. Chronic allograft rejection. Do the TH2 cells preferentially induced by indirect alloantigen recognition play a dominant role? Transplantation. 1999;68:715–26. doi: 10.1097/00007890-199909270-00001. [DOI] [PubMed] [Google Scholar]
- 2.Yamani MH, Yousufuddin M, Starling RC, et al. Does acute cellular rejection correlate with cardiac allograft vasculopathy? J Heart Lung Transplant. 2004;23:272–76. doi: 10.1016/S1053-2498(03)00189-X. [DOI] [PubMed] [Google Scholar]
- 3.Andersen HØ. Heart allograft vascular disease, an obliterative vascular disease in transplanted hearts. Atherosclerosis. 1999;142:243–63. doi: 10.1016/s0021-9150(98)00291-3. [DOI] [PubMed] [Google Scholar]
- 4.Thomson JG. Production of severe atheroma in a transplanted human heart. Lancet. 1969;2:1088–92. doi: 10.1016/s0140-6736(69)90700-4. [DOI] [PubMed] [Google Scholar]
- 5.Valantine H. Cardiac allograft vasculopathy after heart transplantation risk factors and management. J heart Lung Transplant. 2004;23:S187–93. doi: 10.1016/j.healun.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Atkinson C, Horsley J, Rhind-Tutt S, et al. Neointimal smooth muscle cells in human cardiac allograft coronary artery vasculopathy are of donor origin. J Heart Lung Transplant. 2004;23:427–435. doi: 10.1016/S1053-2498(03)00222-5. [DOI] [PubMed] [Google Scholar]
- 7.Beranek JT. Are neointimal smooth muscle cells in human cardiac allograft vasculoopthy of donor origin? J Heart Lung Transplant. 2005;24:1121–2. doi: 10.1016/j.healun.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 8.Haverich A. Cyclosporine in heart transplantation. Transplant Proc. 1992;24:82–4. [PubMed] [Google Scholar]
- 9.Sachs DH, Leight G, Cone J, Schwarz S, Stuart L, Rosenberg S. Transplantation in miniature swine. I. Fixation of the major histocompatibility complex. Transplantation. 1976;22:559–67. doi: 10.1097/00007890-197612000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Stuart L, Houser Isabel M McMorrow, Christian LeGuern, et al. Histomorphometric Comparison of Cardiac Allograft Vasculopathy in Miniature Swine. J Heart Lung Transplant. 2004;23:50–60. doi: 10.1016/s1053-2498(03)00065-2. [DOI] [PubMed] [Google Scholar]
- 11.Pennington LR, Lunney JK, Sachs DH. Transplantation in miniature swine: VIII recombination within the major histocompatibility complex of miniature swine. Transplantation. 1981;31:66–75. [PubMed] [Google Scholar]
- 12.Madsen JC, Sachs DH, Fallon JT, Weissman NJ. Cardiac allograft vasculopathy in partially inbred miniature swine. 1. Time course, pathology and dependence on immune mechanisms. J Thorac Cardiovasc Surg. 1996;111:1230–39. doi: 10.1016/s0022-5223(96)70226-x. [DOI] [PubMed] [Google Scholar]
- 13.Schwarze ML, Menard M, Fuchimoto Y, Huang CA, Houser S, Mawulawde K, Allison BA, Sachs DH, Madsen JC. Mixed hematopoietic chimerism induces long term tolerance to cardiac allografts in miniature swine. Ann Thorac Surg. 2000;70:131–38. doi: 10.1016/s0003-4975(00)01564-2. [DOI] [PubMed] [Google Scholar]
- 14.Amenta PS. Elias-Pauly’s histology & human microanatomy. 5. New York: John Wiley and Sons; 1987. pp. 198–200. [Google Scholar]
- 15.Gross L, Epstein EZ, Kugel MA. Histology of the coronary arteries and their branches in the human heart. Am M Pathol. 1934;10:253–74. [PMC free article] [PubMed] [Google Scholar]
- 16.Dock W. The predilection of atherosclerosis for the coronary arteries. JAMA. 1946;131:875–8. doi: 10.1001/jama.1946.02870280001001. [DOI] [PubMed] [Google Scholar]
- 17.Velican C, Velican D. Intimal thickening in developing coronary arteries and its relevance to atherosclerotic involvement. Atherosclerosis. 1976;23:345–55. [Google Scholar]
- 18.Velican D, Velican C. Study of fibrous plaques occurring in the coronary arteries of children. Atherosclerosis. 1979;33:201–15. doi: 10.1016/0021-9150(79)90117-5. [DOI] [PubMed] [Google Scholar]
- 19.Velican C, Velican D. The precursors of coronary atherosclerotic plaques in subjects up to 40 years old. Atherosclerosis. 1980;37:33–46. doi: 10.1016/0021-9150(80)90091-x. [DOI] [PubMed] [Google Scholar]
- 20.Rahlf G. Intramyocardial microarteriopathy. Virchows Arch Path Anat Histol. 1980;388:289–311. doi: 10.1007/BF00430860. [DOI] [PubMed] [Google Scholar]
- 21.Stary HC, McMillan GC. Kinetics of cellular proliferation in experimental atherosclerosis. Arch Pathol. 1970;89:173–83. [PubMed] [Google Scholar]
- 22.Stary HC, Strong JP. The fine structure of non-atheroslerotic intimal thickening, of developing and of regressing atherosclerotic lesions at the bifurcation of the left coronary artery. Adv Exp Med Biol. 1976;67:89–108. doi: 10.1007/978-1-4614-4618-7_5. [DOI] [PubMed] [Google Scholar]
- 23.Stary HC. The origin in atherosclerotic lesions of extracellular lipid and debris and their elimination during regression. International Symposium: State of Prevention and Therapy in Junan Athersclerosis and Animal Models. 1977:39–53. [Google Scholar]
- 24.Stary HC, Malinov MR. Ultrastructure of experimental coronary artery atherosclerosis in cynomolgus macaques. Atherosclerosis. 1982;43:151–75. doi: 10.1016/0021-9150(82)90019-3. [DOI] [PubMed] [Google Scholar]
- 25.Whelan NL, Subramanian R, Jin J, Keith IM. Intramyocardial arterial cushions of coronary vessels in animals and humans: morphology, occurrence and relation to heart disease. J Vasc Res. 1996;33:209–24. doi: 10.1159/000159149. [DOI] [PubMed] [Google Scholar]
- 26.Fyfe DA, Ketchum D, Lewis R, Sabatier J, Kanter K, Mahle W, Vincent R. Tissue Doppler imaging detects severely abnormal myocardial velocities that identify children with pre-terminal cardiac graft failure after heart transplantation. J Heart Lung Transplant. 2006;25:510–7. doi: 10.1016/j.healun.2005.11.453. [DOI] [PubMed] [Google Scholar]
- 27.Seipelt IM, Pahl E, Seipelt RG, Mavroudis C, Backer CL, Stellmach V, Cornwell M, Crawford SE. Neointimal inflammation and adventitial angiogenesis correlate with severity of cardiac allograft vasculopathy in pediatric recipients. J Heart Lung Transplant. 2005;24:1039–45. doi: 10.1016/j.healun.2004.07.005. [DOI] [PubMed] [Google Scholar]


