Abstract
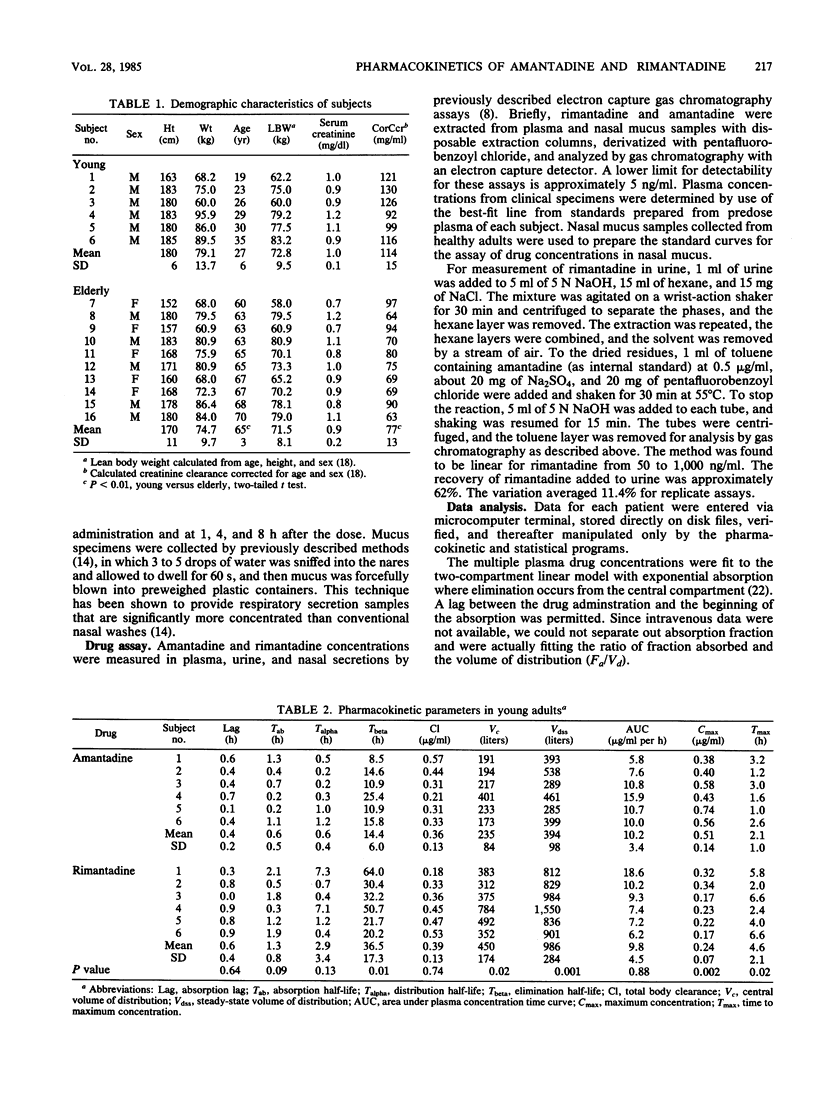
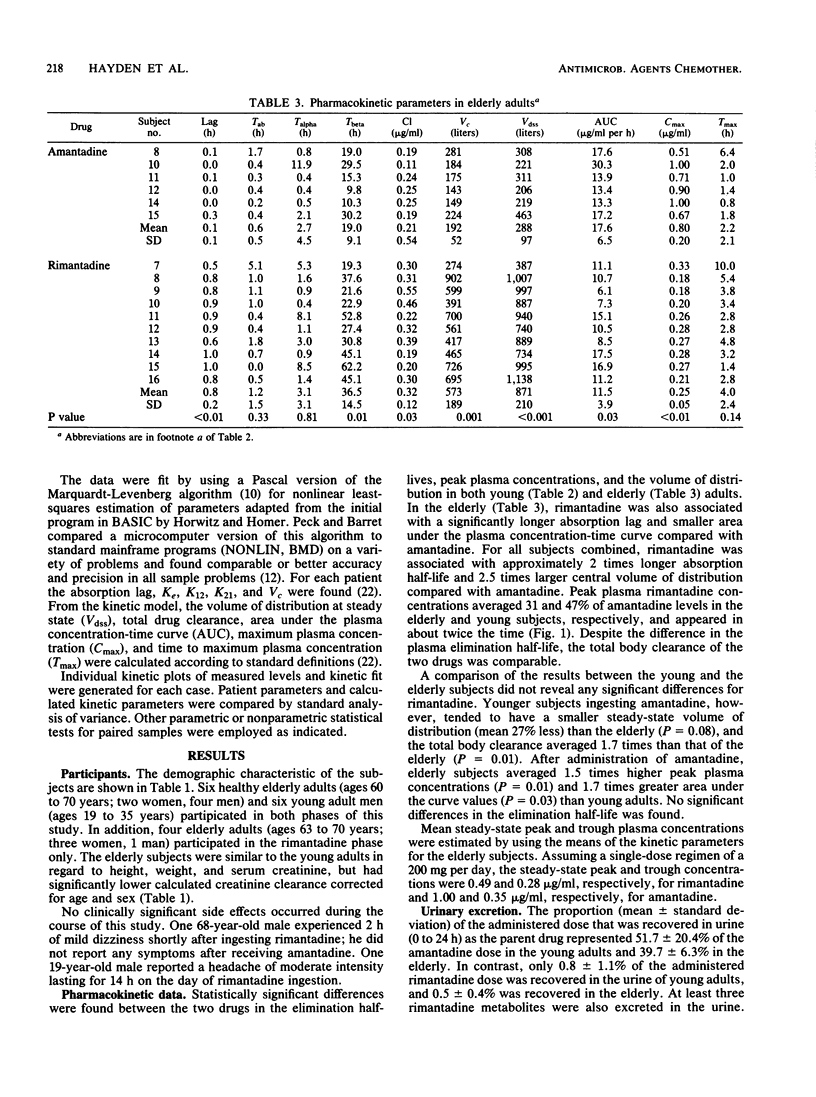
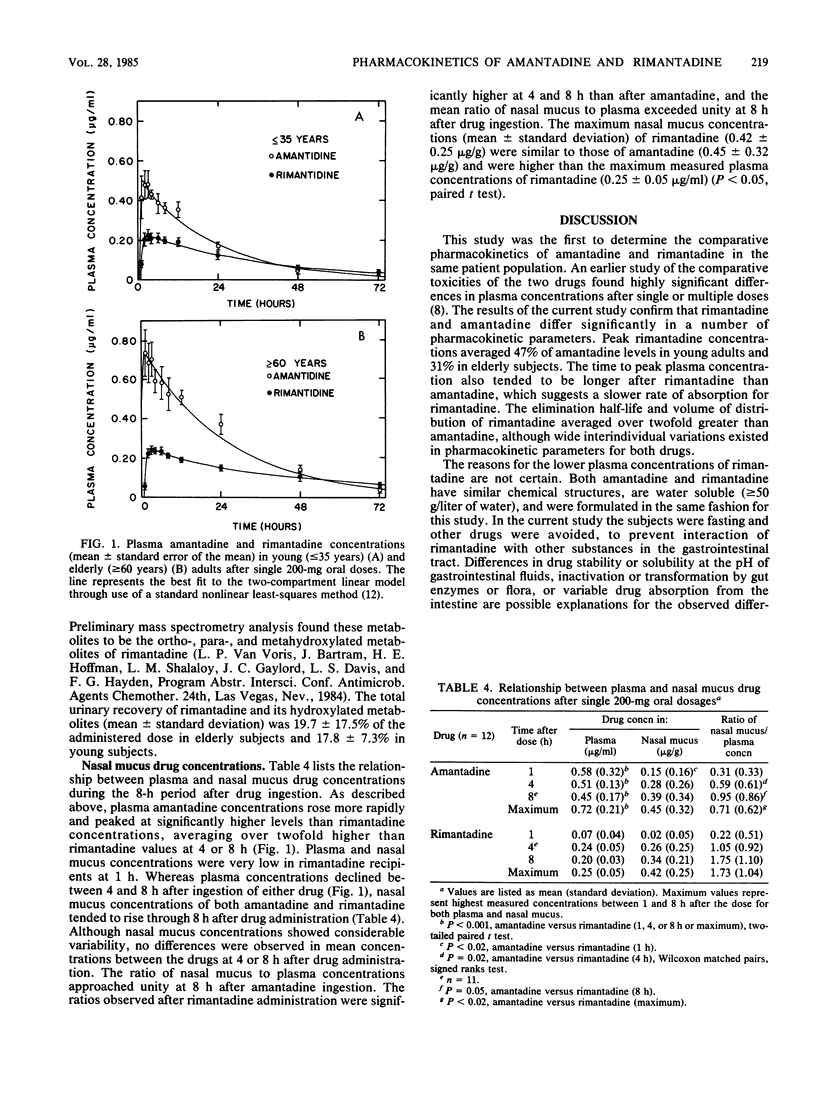
The single-dose pharmacokinetics of amantadine hydrochloride and rimantadine hydrochloride were compared in a randomized, two-period, crossover study involving six young (less than or equal to 35 years) and six elderly (less than or equal to 60 years) adults. Subjects ingested single 200-mg oral doses after an overnight fast, and serial plasma (0 to 96 h), nasal mucus (0 to 8 h), and urine (0 to 24 h) samples were collected for assay of drug concentration by electron capture gas chromatography. For both groups combined, rimantadine differed significantly from amantadine in peak plasma concentration (mean +/- standard deviation, 0.25 +/- 0.06 versus 0.65 +/- 0.22 micrograms/ml), plasma elimination half-life (36.5 +/- 15 versus 16.7 +/- 7.7 h), and percentage of administered dose excreted unchanged in urine (0.6 +/- 0.8 versus 45.7 +/- 15.7%). No significant age-related differences were noted for rimantadine. Urinary excretion (0 to 24 h) of rimantadine and its hydroxylated metabolites averaged 19% of the administered dose. The maximum nasal mucus drug concentration was similar for both drugs (0.42 +/- 0.25 versus 0.45 +/- 0.32 micrograms/g), and the ratio of maximum nasal mucus to plasma concentration was over twofold higher after rimantadine than after amantadine. These findings may in part explain the clinical effectiveness of rimantadine in influenza A virus infections at dosages that have lower toxicity than those of amantadine.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki F. Y., Sitar D. S. Amantadine kinetics in healthy elderly men: implications for influenza prevention. Clin Pharmacol Ther. 1985 Feb;37(2):137–144. doi: 10.1038/clpt.1985.25. [DOI] [PubMed] [Google Scholar]
- Aoki F. Y., Sitar D. S., Ogilvie R. I. Amantadine kinetics in healthy young subjects after long-term dosing. Clin Pharmacol Ther. 1979 Dec;26(6):729–736. doi: 10.1002/cpt1979266729. [DOI] [PubMed] [Google Scholar]
- Aoki F. Y., Stiver H. G., Sitar D. S., Boudreault A., Ogilvie R. I. Prophylactic amantadine dose and plasma concentration-effect relationships in healthy adults. Clin Pharmacol Ther. 1985 Feb;37(2):128–136. doi: 10.1038/clpt.1985.24. [DOI] [PubMed] [Google Scholar]
- Bleidner W. E., Harmon J. B., Hewes W. E., Lynes T. E., Hermann E. C. Absorption, distribution and excretion of amantadine hydrochloride. J Pharmacol Exp Ther. 1965 Dec;150(3):484–490. [PubMed] [Google Scholar]
- Dolin R., Reichman R. C., Madore H. P., Maynard R., Linton P. N., Webber-Jones J. A controlled trial of amantadine and rimantadine in the prophylaxis of influenza A infection. N Engl J Med. 1982 Sep 2;307(10):580–584. doi: 10.1056/NEJM198209023071002. [DOI] [PubMed] [Google Scholar]
- Fishaut M., Mostow S. R. Amantadine for severe influenza A pneumonia in infancy. Am J Dis Child. 1980 Mar;134(3):321–323. doi: 10.1001/archpedi.1980.02130150075022. [DOI] [PubMed] [Google Scholar]
- Hayden F. G., Cote K. M., Douglas R. G., Jr Plaque inhibition assay for drug susceptibility testing of influenza viruses. Antimicrob Agents Chemother. 1980 May;17(5):865–870. doi: 10.1128/aac.17.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden F. G., Hoffman H. E., Spyker D. A. Differences in side effects of amantadine hydrochloride and rimantadine hydrochloride relate to differences in pharmacokinetics. Antimicrob Agents Chemother. 1983 Mar;23(3):458–464. doi: 10.1128/aac.23.3.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horadam V. W., Sharp J. G., Smilack J. D., McAnalley B. H., Garriott J. C., Stephens M. K., Prati R. C., Brater D. C. Pharmacokinetics of amantadine hydrochloride in subjects with normal and impaired renal function. Ann Intern Med. 1981 Apr;94(4 Pt 1):454–458. doi: 10.7326/0003-4819-94-4-454. [DOI] [PubMed] [Google Scholar]
- Patriarca P. A., Kater N. A., Kendal A. P., Bregman D. J., Smith J. D., Sikes R. K. Safety of prolonged administration of rimantadine hydrochloride in the prophylaxis of influenza A virus infections in nursing homes. Antimicrob Agents Chemother. 1984 Jul;26(1):101–103. doi: 10.1128/aac.26.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck C. C., Barrett B. B. Nonlinear least-squares regression programs for microcomputers. J Pharmacokinet Biopharm. 1979 Oct;7(5):537–541. doi: 10.1007/BF01062394. [DOI] [PubMed] [Google Scholar]
- Powell K. R., Shorr R., Cherry J. D., Hendley J. O. Improved method for collection of nasal mucus. J Infect Dis. 1977 Jul;136(1):109–111. doi: 10.1093/infdis/136.1.109. [DOI] [PubMed] [Google Scholar]
- Smith G. B., Purcell R. H., Chanock R. M. Effect of amantadine hydrochloride on parainfluenza type 1 virus infections in adult volunteers. Am Rev Respir Dis. 1967 Apr;95(4):689–690. doi: 10.1164/arrd.1967.95.4.689. [DOI] [PubMed] [Google Scholar]
- Soung L. S., Ing T. S., Daugirdas J. T., Wu M. J., Gandhi V. C., Ivanovich P. T., Hano J. E., Viol G. W. Amantadine hydrochloride pharmacokinetics in hemodialysis patients. Ann Intern Med. 1980 Jul;93(1):46–49. doi: 10.7326/0003-4819-93-1-46. [DOI] [PubMed] [Google Scholar]
- Van Voris L. P., Betts R. F., Hayden F. G., Christmas W. A., Douglas R. G., Jr Successful treatment of naturally occurring influenza A/USSR/77 H1N1. JAMA. 1981 Mar 20;245(11):1128–1131. doi: 10.1001/jama.245.11.1128. [DOI] [PubMed] [Google Scholar]



