Abstract
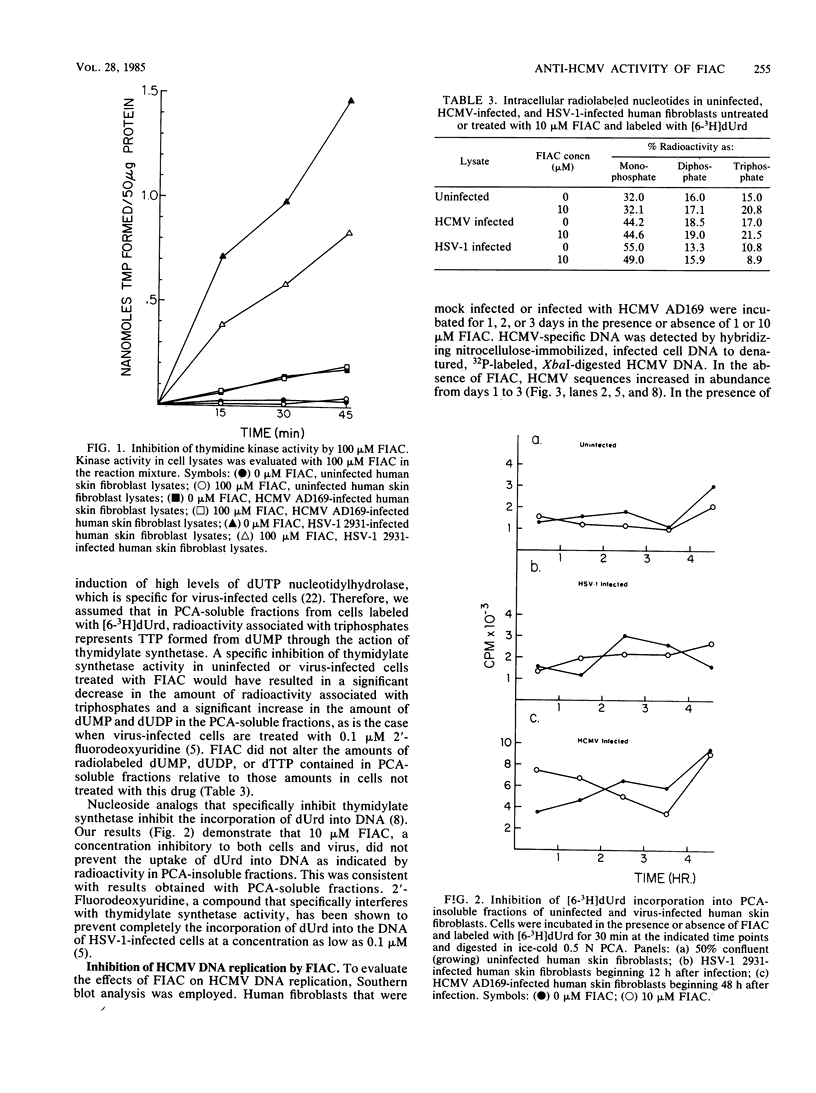
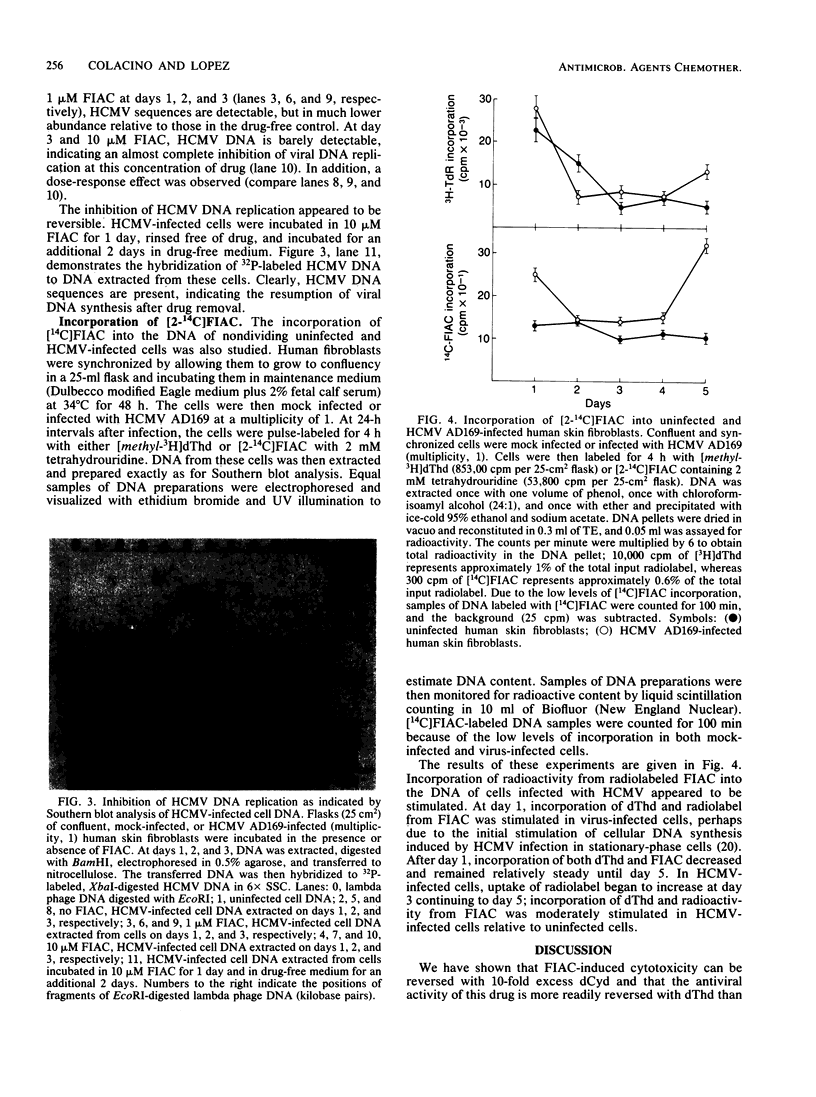
2'-Deoxy-2'-fluoro-beta-D-arabinofuranosyl-5-iodocytosine (FIAC) was shown to be a selective anti-human cytomegalovirus agent in vitro with a 50% antiviral effective dose of 0.6 microM (J. M. Colacino and C. Lopez, Antimicrob. Agents Chemother. 26:505-508, 1983) and a 50% cell growth inhibitory dose of 8 microM. Antiviral activity was more readily reversed with 10-fold excess thymidine, whereby the 50% effective dose was increased to 11.3 microM. FIAC-induced cytotoxicity was more readily reversed with 10-fold excess of deoxycytidine, whereby the 50% inhibitory dose was increased to greater than 100 microM. Thymidine was unable to reverse completely the antiviral activity of FIAC. Although, the extent of phosphorylation of thymidine, deoxycytidine, and deoxyuridine was 6-, 4-, and 4-fold greater, respectively, in human cytomegalovirus-infected cell lysates than in uninfected cell lysates, the extent of phosphorylation of FIAC was only 1.3-fold greater in human cytomegalovirus-infected cell lysates than in uninfected cell lysates. By comparison, the extent of FIAC phosphorylation was 500 times greater in herpes simplex virus type 1-infected cells than in uninfected cell lysates. Methotrexate was 400 times more effective against human cytomegalovirus replication than it was against herpes simplex virus type 1 replication, indicating that thymidylate synthetase may be important for human cytomegalovirus replication. However, 10 microM FIAC did not inhibit thymidylate synthetase activity in uninfected or virus-infected cells as determined by their metabolism of [6-3H]deoxyuridine in the presence or absence of drug. FIAC at 1 microM suppresses and FIAC at 10 microM completely inhibits human cytomegalovirus DNA replication as indicated by Southern blot analysis. This inhibition was reversible. FIAC incorporation into the DNA of human cytomegalovirus strain AD169-infected cells was stimulated relative to that in nondividing, uninfected cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allaudeen H. S., Descamps J., Sehgal R. K., Fox J. J. Selective inhibition of DNA replication in herpes simplex virus infected cells by 1-(2'-deoxy-2'-fluoro-beta-D-arabinofuranosyl)-5-iodocytosine. J Biol Chem. 1982 Oct 25;257(20):11879–11882. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cheng Y. C., Dutschman G., Fox J. J., Watanabe K. A., Machida H. Differential activity of potential antiviral nucleoside analogs on herpes simplex virus-induced and human cellular thymidine kinases. Antimicrob Agents Chemother. 1981 Sep;20(3):420–423. doi: 10.1128/aac.20.3.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. C., Hoffmann P. J., Ostrander M., Grill S., Caradonna S., Tsou J., Chen J. Y., Gallagher M. R., Flanagan T. D. Properties of herpesvirus-specific thymidine kinase, DNA polymerase and DNase and their implication in the development of specific antiherpes agents. Adv Ophthalmol. 1979;38:173–186. [PubMed] [Google Scholar]
- Cheng Y. C., Nakayama K., Grill S. P. Roles of thymidylate synthetase activity in herpes simplex virus-infected HeLa cells. Biochem Pharmacol. 1983 Apr 15;32(8):1407–1410. doi: 10.1016/0006-2952(83)90454-9. [DOI] [PubMed] [Google Scholar]
- Colacino J. M., Lopez C. Efficacy and selectivity of some nucleoside analogs as anti-human cytomegalovirus agents. Antimicrob Agents Chemother. 1983 Oct;24(4):505–508. doi: 10.1128/aac.24.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E., Balzarini J., Torrence P. F., Mertes M. P., Schmidt C. L., Shugar D., Barr P. J., Jones A. S., Verhelst G., Walker R. T. Thymidylate synthetase as target enzyme for the inhibitory activity of 5-substituted 2'-deoxyuridines on mouse leukemia L1210 cell growth. Mol Pharmacol. 1981 Mar;19(2):321–330. [PubMed] [Google Scholar]
- De Clercq E., Descamps J., Huang G. F., Torrence P. F. 5-Nitro-2'-deoxyuridine and 5-nitro-2'-deoxyuridine 5'-monophosphate: antiviral activity and inhibition of thymidylate synthetase in vivo. Mol Pharmacol. 1978 May;14(3):422–430. [PubMed] [Google Scholar]
- Estes J. E., Huang E. S. Stimulation of cellular thymidine kinases by human cytomegalovirus. J Virol. 1977 Oct;24(1):13–21. doi: 10.1128/jvi.24.1.13-21.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman P. A., de Miranda P., St Clair M. H., Elion G. B. Metabolism of acyclovir in virus-infected and uninfected cells. Antimicrob Agents Chemother. 1981 Oct;20(4):518–524. doi: 10.1128/aac.20.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E. S. Human cytomegalovirus. III. Virus-induced DNA polymerase. J Virol. 1975 Aug;16(2):298–310. doi: 10.1128/jvi.16.2.298-310.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeor S. C., Albrecht T. B., Funk F. D., Rapp F. Stimulation of cellular DNA synthesis by human cytomegalovirus. J Virol. 1974 Feb;13(2):353–362. doi: 10.1128/jvi.13.2.353-362.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., Leung W. C., Trkula D., Jorgensen G. Gel electrophoresis and isoelectric focusing of mitochondrial and viral-induced thymidine kinases. Int J Cancer. 1974 Feb 15;13(2):203–218. doi: 10.1002/ijc.2910130208. [DOI] [PubMed] [Google Scholar]
- Kreis W., Damin L., Colacino J., Lopez C. In vitro metabolism of 1-beta-D-arabinofuranosylcytosine and 1-beta-2'-fluoroarabino-5-iodocytosine in normal and herpes simplex type 1 virus-infected cells. Biochem Pharmacol. 1982 Mar 1;31(5):767–773. doi: 10.1016/0006-2952(82)90461-0. [DOI] [PubMed] [Google Scholar]
- Mar E. C., Patel P. C., Cheng Y. C., Fox J. J., Watanabe K. A., Huang E. S. Effects of certain nucleoside analogues on human cytomegalovirus replication in vitro. J Gen Virol. 1984 Jan;65(Pt 1):47–53. doi: 10.1099/0022-1317-65-1-47. [DOI] [PubMed] [Google Scholar]
- McBurney M. W., Whitmore G. F. Mechanism of growth inhibition by methotrexate. Cancer Res. 1975 Mar;35(3):586–590. [PubMed] [Google Scholar]
- Reichman U., Watanabe K. A., Fox J. J. A practical synthesis of 2-deoxy-2-fluoro-D-arabinofuranose derivatives. Carbohydr Res. 1975 Jul;42(2):233–240. doi: 10.1016/s0008-6215(00)84265-2. [DOI] [PubMed] [Google Scholar]
- Smee D. F., Martin J. C., Verheyden J. P., Matthews T. R. Anti-herpesvirus activity of the acyclic nucleoside 9-(1,3-dihydroxy-2-propoxymethyl)guanine. Antimicrob Agents Chemother. 1983 May;23(5):676–682. doi: 10.1128/aac.23.5.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Watanabe K. A., Reichman U., Hirota K., Lopez C., Fox J. J. Nucleosides. 110. Synthesis and antiherpes virus activity of some 2'-fluoro-2'-deoxyarabinofuranosylpyrimidine nucleosides. J Med Chem. 1979 Jan;22(1):21–24. doi: 10.1021/jm00187a005. [DOI] [PubMed] [Google Scholar]
- Wohlrab F., Francke B. Deoxyribopyrimidine triphosphatase activity specific for cells infected with herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1872–1876. doi: 10.1073/pnas.77.4.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Závada V., Erban V., Rezácová D., Vonka V. Thymidine-kinase in cytomegalovirus infected cells. Arch Virol. 1976;52(4):333–339. doi: 10.1007/BF01315622. [DOI] [PubMed] [Google Scholar]