Abstract
Normal turnover of body tissues yields apoptotic cells while infections cause tissue injuries and cell necrosis. The interaction of these dying cells with dendritic cells (DCs) may provide immunological instructions leading to either immune tolerance or activation. We hypothesize that neonatal and adult DCs differ in their responses to dying cells, thereby contributing to the observed differences in immune responses between neonates and adults. We compare the outcome of interaction of cord and adult blood-derived DCs with dying Epstein–Barr-virus-transformed lymphoblastoid cells (LCLs) and the responsiveness to lipopolysaccharide. While cord DCs were able to phagocytose both apoptotic and necrotic LCLs, the subsequent responses differed significantly from those of adult DCs. Interaction of adult DCs with necrotic but not early apoptotic LCLs resulted in high expression of DC costimulatory molecules (CD80/CD86) and activation markers (CD83), production of both proinflammatory and anti-inflammatory cytokines (tumour necrosis factor-α, interleukin-10), and strong T-cell-stimulating activities. In contrast, in response to either necrotic or apoptotic LCLs, cord DCs had minimal up-regulation of those DC functional markers, little cytokine production and poor stimulation on T-cell proliferation. In response to lipopolysaccharide, however, both adult and cord DCs produced comparable levels of tumour necrosis factor-α and interleukin-10, but only adult DCs produced interleukin-12(p70). Taken together, these results suggest that neonatal DCs generally favour immune tolerance with minimal activation and cytokine production, except in extremely dangerous situations, such as bacterial sepsis, when neonatal DCs may produce certain types of cytokines and stimulate T-cell proliferation.
Keywords: apoptosis, cord blood, cytokines, dendritic cell, necrosis, lymphoblastoid cells
Introduction
The higher susceptibility of neonates to infectious agents might be partly the result of the lack of immunological memory and the small number of immune cells in the peripheral lymphoid tissues.1 Many studies have demonstrated the functional deficiencies of B cells, T cells and antigen-presenting cells in neonates. Recent studies2–4 however, showed that neonates are competent, under certain circumstances, to develop fully mature adaptive immune responses in vivo. It is becoming clear that neonatal immunity can demonstrate considerable plasticity, ranging from low or deviant response, as compared to adult immunity, to mature adult-like response, depending on the conditions of antigen exposure and the level of danger encountered.1
Dendritic cells (DCs) are the professional antigen-presenting cells that initiate immune responses by priming naïve T cells5 as well as inducing tolerance to self-antigens.6 Several studies demonstrated that neonatal DCs are functionally immature with defective expression of costimulatory molecules and deficiency in interleukin-12 (IL-12) production, and are less effective than adult DCs in stimulating adult or cord blood T-cell proliferation.7–12 However, other studies demonstrated that cord blood-derived DCs can produce adult-like levels of IL-1213 and can efficiently prime an antigen-specific cytotoxic T lymphocyte response.14 It is likely that the DC is a key player in shaping the neonatal immune response. DCs take up dying cells as a source of antigens and present these antigens to the adaptive immune system15,16 while dying cells contain important messages that may direct DCs to induce immune activation or tolerance.17 DCs that have captured apoptotic cells may induce tolerance18 while necrotic cells release endogenous ‘danger signals’ that induce DC activation and maturation.19,20 We hypothesize that neonatal and adult DCs differ in their responses to dying cells. To test the hypothesis, we compared the outcome of interaction of cord blood and adult blood-derived DCs with dying lymphoblastoid cells (LCLs) and the responsiveness of these DCs to lipopolysaccharide (LPS). The outcome of interaction was examined in terms of phagocytic function, phenotypic changes, T-cell stimulatory capacity and cytokine production under the same experimental conditions.
Materials and methods
Study samples
Human umbilical cord blood samples were collected from the placentae of normal full-term Chinese neonates after written informed consent was obtained from the mothers. The study was approved by the Institution Review Board of the University of Hong Kong. Adult peripheral blood samples were obtained using buffy coats of normal blood donors of the Hong Kong Red Cross.
Generation of DCs
Cord blood (CBMCs) or adult peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll-Paque Plus (Amersham Pharmacia Biotech, Piscataway, NJ) density gradient centrifugation. To generate immature DCs, positive selection for CD14+ CBMCs or PBMCs was performed using anti-CD14 magnetic antibody cell sorting (MACS) magnetic beads in the MACS purification system (Miltenyi Biotech, Bergisch Gladbach, Germany), according to the manufacturer's protocol. Cells from the positively selected fraction were collected and cultured in RPMI-1640 medium supplemented with 10% heat-inactivated fetal calf serum, 100 units/ml penicillin and 100 μg/ml streptomycin (all components from Invitrogen Life Technologies, Grand Island, NY; hereafter this is called complete medium), 10 ng/ml human recombinant IL-4 (R & D Systems, Minneapolis, MN) and 50 ng/ml human recombinant granulocyte–macrophage colony-stimulating factor (GM-CSF; R & D Systems) at a density of 1 × 106/ml for 6 days at 37° in 5% CO2. On day 6, >95% of the cells were CD14_ CD11c+ DCs, as determined by immunophenotypic analysis using flow cytometry performed on an EPICS Elite ESP (Coulter, Miami, FL). Day 6 immature DCs were used in all subsequent experiments.
LPS treatment
Lipopolysaccharide (10 μg/ml; Escherichia coli 026:B6, Sigma-Aldrich, St Louis, MO) was added to the immature DCs for 36 hr.
Establishment of Epstein–Barr virus (EBV) transformed lymphoblastoid cells (LCLs)
LCLs were established by infection of CBMCs or PBMCs with culture supernatant obtained from the B95-8 EBV producer cell line (a kind gift from Dr K.H. Chan, The University of Hong Kong). They were cultured in complete medium supplemented with 15% fetal calf serum at 37° in 5% CO2. Cyclosporin A (0·5 μg/ml; Sigma-Aldrich) was added to the culture for adult PBMCs.
Induction and detection of early apoptotic and necrotic LCLs
LCLs were induced to undergo early apoptosis by UV-irradiation. They were serum-starved for 24 hr before induction of apoptosis by UV-irradiation at a dosage of 20 mJ/cm2 in a UV Crosslinker (Spectrolinker, Westbury, NY). Cell death was evaluated using the Annexin V–fluorescein isothiocyanante (FITC) Kit (Immunotech, Marseilles, France). Cells were stained with Annexin V–FITC (AV) and propidium iodide (PI) according to the manufacturer's protocol and early apoptosis was detected by flow cytometry. The kinetics of death was carefully worked out to ensure that the LCLs were undergoing early apoptosis. Following UV-irradiation and incubation at 37° for 8 hr, >70% of the cells were early apoptotic (AV+ PI_) while at 48 hr after irradiation, >90% of the cells were late apoptotic (AV+ PI+). Hence, LCLs at 8 hr post-UV treatment were used as early apoptotic LCLs. Necrotic LCLs were obtained by four freeze–thaw cycles that resulted in complete disruption of the cells into fragments.
Phagocytosis assay
Phagocytosis assay was performed to compare the ability of cord and adult DCs to take up dying cells. LCLs were labelled with red-fluorescent dye PKH26 and immature DCs were labelled with green-fluorescent dye PKH67, according to the manufacturer's protocol (SigmaAldrich). Labelled DCs were then cocultured with apoptotic or necrotic LCLs at cell ratio of 1 : 2 in complete medium for 3, 12, or 24 hr at 37° or 4°. Phagocytosis of apoptotic or necrotic LCLs by DCs was defined by the percentage of double positive fluorescent signals analysed by flow cytometry.
Confocal microscopy
Cells were prepared by cytospin after coculturing cord and adult DCs with early apoptotic or necrotic LCLs for 12 hr. Cell smears were fixed in 4% paraformaldehyde in phosphate-buffered saline for 10 min. All images were captured using a confocal microscope (Bio-Rad Laboratories, Hercules, CA).
Immunophenotyping
The surface marker expression of cord and adult DCs after treatment with apoptotic or necrotic LCLs or LPS for 36 hr was determined by flow cytometry. DCs were washed and resuspended in phosphate-buffered saline supplemented with 0·5% bovine serum albumin (Sigma-Aldrich) and 0·01% sodium azide (Sigma-Aldrich) and incubated for 30 min at 4° with one of the following fluorochrome-conjugated monoclonal antibodies: CD11c (clone B-ly6), CD14 (clone M5E2), CD40 (clone 5C3), CD80 (clone L307.4), CD83 (clone HB15e), CD86 (clone FUN-1), major histocompatibility complex (MHC) class II (clone G46-6) (BD-Pharmingen, San Diego, CA). The isotype controls for the above fluorochrome-conjugated monoclonal antibodies were either IgG1 or IgG2a (BD-Pharmingen). The flow cytometry data were analysed by winmdi 2·8 software (The Scripps Research Institute, La Jolla, CA) and expressed as the mean fluorescence intensity (MFI). The MFI for the isotype controls was within the first decade and did not change significantly with the different DC treatment.
Cytokine determination
Cytokines in the supernatants of cocultures of cord and adult DCs and apoptotic or necrotic LCLs were measured by enzyme-linked immunosorbent assay (ELISA) using a DuoSet ELISA Development System (R & D Systems) according to the manufacturer's protocol. Day 6 immature DCs (1 × 106) were cocultured with apoptotic or necrotic LCLs (2 × 106) in complete medium for 36 hr at 37° in 5% CO2. The supernatants were collected, kept frozen at −80° and assayed for the release of IL-12, tumour necrosis factor-α (TNF-α) and IL-10. Positive or negative control supernatants were obtained by culturing DCs with LPS or in medium in the same conditions, respectively. Optical density was determined by a microtitre plate reader (Model 550, Bio-Rad).
T-cell proliferation assay
After coculture of cord and adult DCs with early apoptotic or necrotic LCLs for 36 hr, the cells were irradiated at 30 Gy before the addition of allogeneic adult CD3+ T cells. Positive selection for CD3+ adult T cells was performed using anti-CD3 MACS magnetic beads in the MACS purification system (Miltenyi Biotech). They were cultured in triplicate in a 96-well, flat-bottom microplate (Corning Costar, Rochester, NY) at different DC : T-cell ratios ranging from 1 : 10 to 1 : 320. Cultures were maintained for 5 days at 37° in 5% CO2. Bromodeoxyuridine (BrdU; 100 μm, Roche, Mannheim, Germany) were added 16 hr before the end of the 5-day culture. BrdU incorporation was measured using a BrdU Cell Proliferation Kit (Roche) according to the manufacturer's protocol. The data are expressed as absorbance at a wavelength of 450 nm (A450) using the microtitre plate reader.
Statistical analysis
All experimental data are expressed as the mean ± standard error of means (SEM). Differences in phenotypic expression and cytokine production between cord and adult DCs were tested by Mann–Whitney test while differences in T-cell stimulatory capacity were tested by analysis of variance (anova) at each DC : T-cell ratio. A value of P < 0·05 was considered significant.
Results
Phenotypic changes of cord and adult DCs after phagocytosing apoptotic and necrotic LCLs
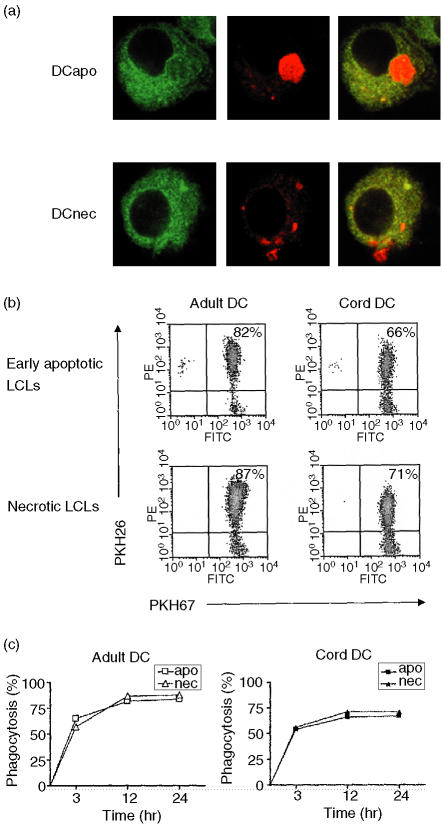
Immature DCs were generated from freshly isolated cord or adult peripheral blood CD14+ monocytes in the presence of GM-CSF and IL-4 for 6 days. On day 6, >95% of cells showed the immunophenotype of CD14_ CD11c+ and DC morphology. To determine the ability of these cells to phagocytose dying cells, PKH67-labelled cord and adult DCs were cocultured with PKH26-labelled dying LCLs at a ratio of 1 : 2 at 37° in 5% CO2. Phagocytosis of dying LCLs by cord and adult DCs was visualized using confocal microscopy (Fig. 1a) and assayed by flow cytometry (Fig. 1b). Using the confocal microscopy, we observed that the amount of phagocytosed dying LCLs and the distribution of the phagocytosed materials in both cord and adult DCs were similar. Kinetics of phagocytosis was also carried out to determine the efficiency of the two types of DCs in taking up the dying LCLs (Fig. 1c). As early as 3 hr after cell coculturing, more than 50% of both cord and adult DCs were found to have taken up either early apoptotic or necrotic LCLs. At 12 hr, >60% cord DCs and >80% adult DCs had taken up the dying LCLs. The uptake of dying cells did not appear to increase further with longer incubation time up to 24 hr. The uptake of both apoptotic and necrotic cells appeared higher in adult than cord DCs. The phagocytosis of dying cells was <5% when the cells were incubated at 4° (data not shown).
Figure 1.
Phagocyosis of early apoptotic or necrotic lymphoblastoid cells (LCLs) by cord and adult dendritic cells (DCs). PKH67-labelled DCs were cocultured with PKH26-labelled dying LCLs for 3, 12, or 24 hr. Confocal micrographs showing DCs (green) and the internalized early apoptotic (DCapo, whole cell) or necrotic (DCnec, cellular fragments) LCLs (red) (a). The efficiency of DC uptake of dying cells was analysed by flow cytometry after coincubation of cord and adult DCs with early apoptotic or necrotic LCLs for 12 hr (b). Kinetics of phagocytosis showed that DC uptake of dying cells peaked at 12 hr after coculture and there was no further increase in uptake with longer incubation time (c). The results shown are representative of three different experiments.
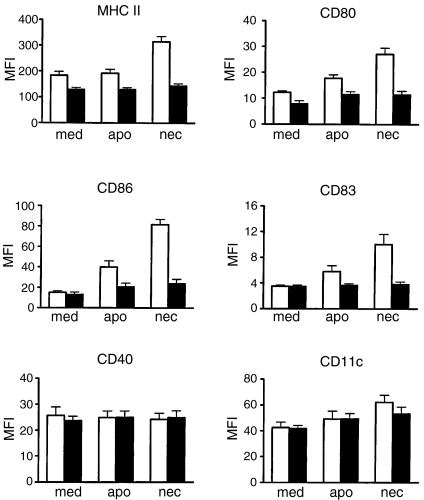
The effect of dying cells on the maturation of cord and adult DCs was evaluated by the expression of surface markers after coculturing of DCs with early apoptotic or necrotic LCLs for 36 hr. Minimal up-regulation of surface markers in cord DCs was observed after phagocytosis of either apoptotic or necrotic LCLs (Fig. 2). In contrast, significantly up-regulated expression of MHC class II, CD80, CD86 and CD83 was observed after exposure of the adult DCs to necrotic, but not apoptotic, LCLs (P < 0·01) (Fig. 2). No significant changes were observed on the expression of CD40 or CD11c on cord and adult DCs after coculturing with either apoptotic or necrotic LCLs.
Figure 2.
Comparison of surface marker expression of cord and adult dendritic cells (DCs) 36 hr after exposure to culture medium control (med), early apoptotic (apo) or necrotic (nec) LCLs. The expression of DC phenotypic and functional markers (as indicated) was analysed by flow cytometry. Isotype controls were either mouse immunoglobulin G1 or immunoglobulin G2a and the mean fluorescence intensity (MFI) for the isotype controls was within the first decade and did not change significantly with the different DC treatment. The data are presented as MFI ± SEM of six independent experiments. Cord DCs, solid bars; adult DCs, open bars.
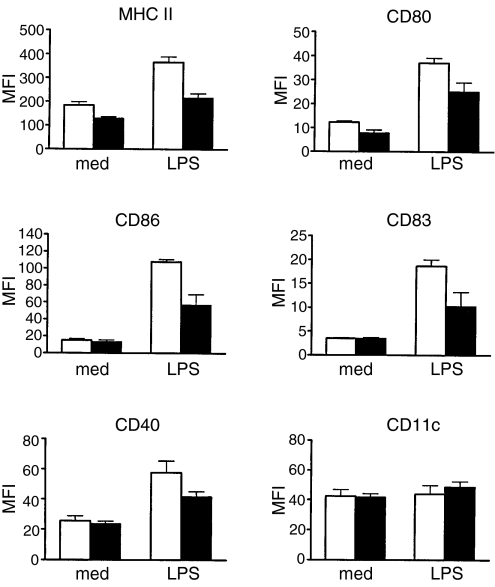
The responsiveness of cord and adult DCs to LPS was also compared. As shown in Fig. 3, immature DCs derived from cord and adult CD14+ monocytes showed similarly low levels of MHC class II, costimulatory molecules CD80 and CD86, CD83 and CD40. Upon LPS stimulation, expression of these important DC functional markers was significantly up-regulated in both cord and adult DCs (P < 0·01). However, the level of expression of these surface markers was significantly lower on the cord DCs compared to adult DCs (P < 0·05). No significant changes were observed on the expression of CD11c on cord and adult DCs after LPS treatment.
Figure 3.
Phenotypic changes of cord and adult dendritic cells (DCs) in response to lipopolysaccharide (LPS) stimulation. Day 6 cord and adult DCs were cultured for 36 hr in the presence or absence of LPS (10 μg/ml). The expression of DC phenotypic and functional markers (as indicated) was analysed by flow cytometry. Isotype controls were either mouse immunoglobulin G1 or immunoglobulin G2a and the mean fluorescence intensity (MFI) for the isotype controls was within the first decade and did not change significantly with LPS treatment. The data are presented as MFI ± SEM of six independent experiments. Cord DCs, solid bars; adult DCs, open bars.
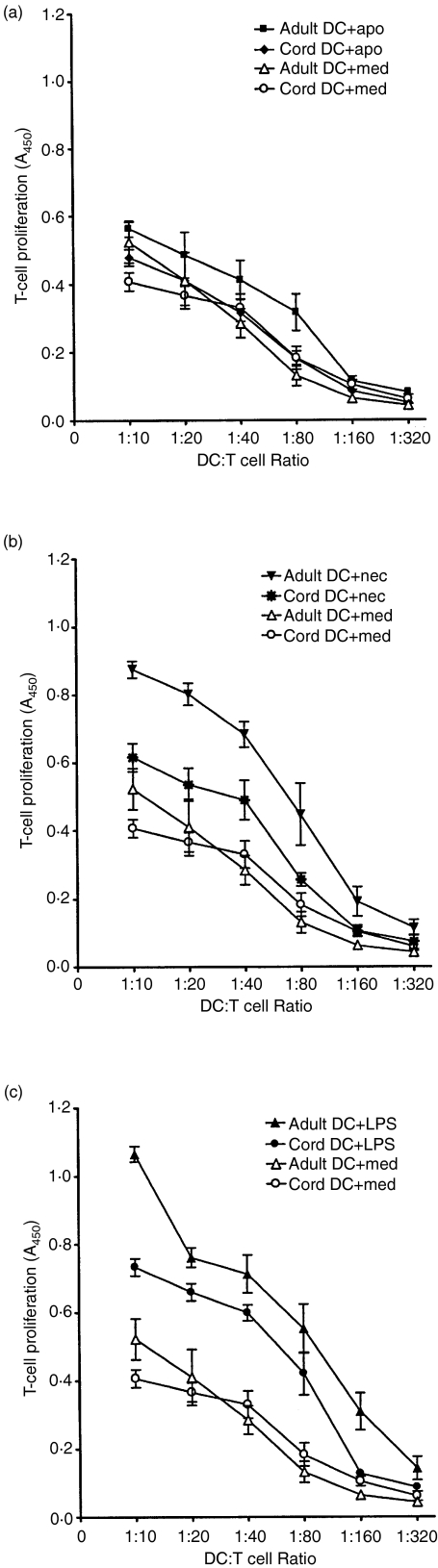
T-cell stimulatory capacity of cord and adult DCs following interaction with dying LCLs
Mature DCs are potent stimulators of T cells. We therefore examined the capacity of both cord and adult DCs in stimulating allogeneic T-cell proliferation after exposure to early apoptotic LCLs, necrotic LCLs and LPS. After 36-hr coincubation with either early apoptotic or necrotic LCLs, LPS or culture medium control, DCs were added in graded doses to 1 × 105 allogeneic CD3+ T cells. As shown in Fig. 4, a clear difference was seen in the T-cell stimulatory capacity between cord and adult DCs although it did not reach statistical significance by anova test. Interaction of cord and adult DCs with apoptotic LCLs resulted in minimal up-regulation of T-cell stimulatory capacity (Fig. 4a) while that with necrotic LCLs (Fig. 4b) and LPS (Fig. 4c) resulted in intermediate and strong up-regulation of T-cell stimulatory activities, respectively. Furthermore, adult DCs showed consistently higher T-cell stimulatory capacity than cord DCs in all three experimental treatments. The T-cell stimulatory capacity of cord and adult DCs correlated with their levels of phenotypic marker expression (illustrated in Figs 2 and 3).
Figure 4.
Comparison of the T-cell stimulatory capacity of cord and adult dendritic cells (DCs) in medium alone (med) or after stimulation by LPS, early apoptotic (apo) or necrotic (nec) lymphoblastoid cells (LCLs) for 36 hr. The cord and adult DCs were irradiated before coculturing with allogeneic adult T cells at different DC : T cell ratios as indicated. T-cell proliferation was measured by BrdU incorporation and the data are expressed as mean absorbance at A450 ± SEM of three independent experiments.
Cytokine expression profiles of cord and adult DCs after stimulation with LPS and dying LCLs
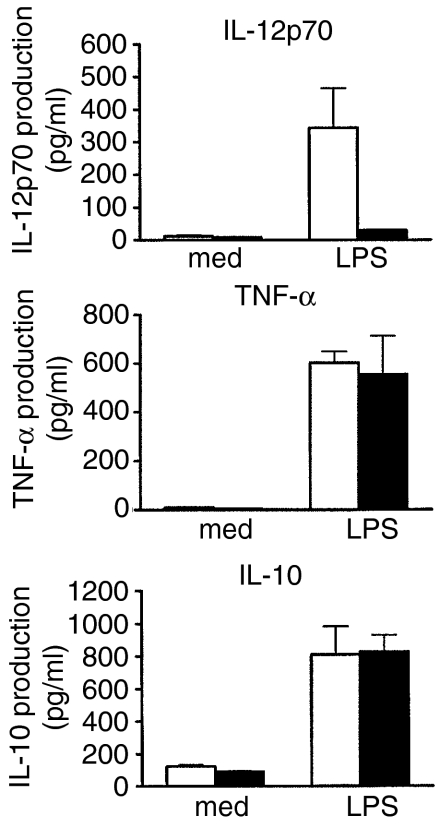
To study the mechanism underlying the defective functions of cord DCs, we next analysed their ability to produce several cytokines which are known to be important in mediating the functions of DCs. IL-12, TNF-α and IL-10 are closely related to the ability of DCs to stimulate and regulate the activation of naive T cells. We first evaluated the production of these cytokines by cord and adult DCs after LPS stimulation. Culture supernatant was collected and cytokine production was assayed by ELISA after culturing DCs in the presence or absence of LPS for 36 hr. Both cord and adult DCs produced large and comparable amount of TNF-α and IL-10 in response to LPS stimulation (Fig. 5). While adult DCs also produced high levels of IL-12p70, cord DCs failed to produce this cytokine after LPS stimulation (Fig. 5).
Figure 5.
Cytokine expression profile of cord and adult dendritic cells (DCs) upon lipopolysaccharide (LPS) stimulation. Both adult and cord DCs were incubated with medium alone (med) or medium containing 10 μg/ml LPS for 36 hr. The release of interleukin (IL)-12p70, tumour necrosis factor-α (TNF-α) and IL-10 in the culture supernatants was determined by ELISA. The data shown represent the mean concentration ± SEM of five independent experiments. Cord DCs, solid bars; adult DCs, open bars.
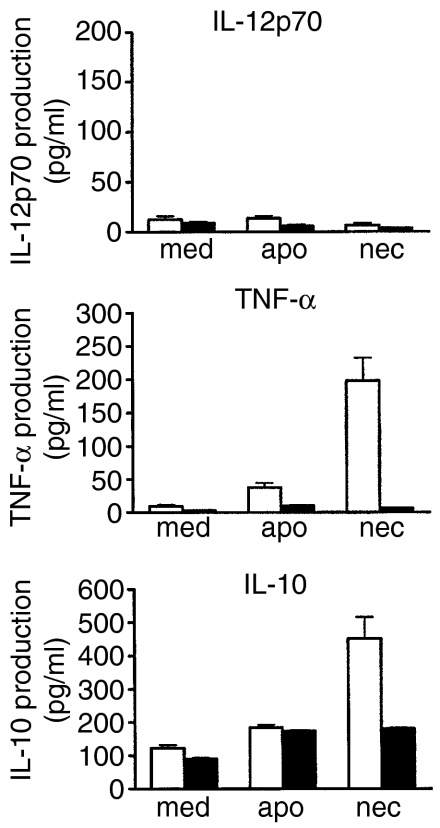
We next evaluated the cytokine production of cord and adult DCs upon ingestion of early apoptotic or necrotic LCLs. Clear differences were detected between cord and adult DCs upon interaction with two different types of dying cells. After phagocytosis of necrotic, but not apoptotic, LCLs, adult DCs were able to produce TNF-α and IL-10, but not IL-12p70. In contrast, cord DCs produced none, or only minimal levels, of these cytokines after phagocytosis of either apoptotic or necrotic LCLs (Fig. 6).
Figure 6.
Cytokine expression profile of cord and adult dendritic cells (DCs) following interaction with dying lymphoblastoid cells (LCLs). Adult and cord DCs were incubated with early apoptotic (apo) or necrotic (nec) LCLs, or medium alone (med) for 36 hr. The release of interleukin (IL)-12p70, tumour necrosis factor-α TNF-α) and IL-10 in the culture supernatants was determined by ELISA. The data shown are mean concentration ± SEM of five independent experiments. Cord DCs, solid bars; adult DCs, open bars.
Discussion
In this study, we examined systematically the responses of neonatal DCs to two physiologically very different types of dying cells, and to the bacteria-derived potent immune modulator LPS. We analysed the phagocytic function, phenotypic changes, T-cell stimulatory capacity and cytokine production of the cord DCs in comparison with that of adult DCs under the same experimental conditions. While neonatal DCs were capable of rapidly phagocytosing apoptotic and necrotic LCLs, they assumed a non-mature phenotype expressing lower levels of activation markers, produced no or less IL-12p70, TNF-α and IL-10, and demonstrated weaker capacity to stimulate allogeneic T-cell responses, when compared to adult DCs. In response to LPS, however, neonatal DCs were capable of up-regulating activation markers, producing comparable levels of TNF-α and IL-10 but not IL-12p70, and stimulating allogeneic T cells. These results support the emerging concept that the neonatal immune system can have considerable plasticity favouring immune tolerance in general, but mounting mature protective responses in the face of danger.
Cord blood-derived DCs were reported to have a reduced endocytotic ability in the uptake of dextran, which might be the result of the reduced expression of surface mannose receptor.9 However, little information is available on the phagocytic ability of dying cells by cord DCs, although many previous studies have demonstrated that adult DCs can phagocytose efficiently different types of dying cells.20,21 We showed here that cord DCs may phagocytose rapidly apoptotic and necrotic LCLs, but the relative efficiency was still lower compared to that of their adult counterparts. Phagocytosis of dying cells is known to be associated with receptors such as integrins αvβ3,22αvβ5 and CD36,23 phosphatidylserine receptor and Fc receptors.17 It remains to be determined whether a lack of any of these receptors could be responsible at least in part for the functional deficiency of cord DCs observed in the present study.
Apoptosis is a programmed form of cell death which may exert anti-inflammatory and immunosuppressive effects on the immune system24 while necrosis is an accidental cell death with disruption and release of cellular contents that may exert pro-inflammatory effects.17 The interaction of DCs with these two physiologically very different types of dying cells may modulate immune responses differentially by inducing tolerance or activation. We showed here that upon interaction with necrotic but not apoptotic LCLs, adult DCs acquired a mature immunogenic phenotype. Interestingly, cord DCs, upon exposure to either apoptotic or necrotic LCLs maintained an immature phenotype with minimal up-regulation of surface markers, little cytokine production and weak capacity to stimulate T-cell proliferation. This suggests that the neonatal DCs are intrinsically programmed to achieve low responsiveness under non-threatening conditions. In normal cell turnover and even in certain inflammatory situations (represented by interaction with early apoptotic and necrotic LCLs, respectively), the lack of response of the neonatal DCs may favour immune tolerance. This unique property of the neonatal immunity is thought to be important in the early development of the organism when the immune cells must establish a tolerant state to the large number of environmental and self antigens encountered in the early postnatal life.1
While adult DCs resembled the cord DCs in maintaining an immature phenotype upon exposure to early apoptotic LCLs, they were able to up-regulate activation markers, produce cytokines and stimulate T-cell proliferation robustly upon exposure to necrotic LCLs. The endogenous ‘danger signals’ released by necrotic cells activate DCs.19,20 Heat-shock proteins,25,26 intracellular nucleotides such as ATP and UTP,27 uric acid,28 and high mobility group box 129 released by the necrotic cells have been shown to deliver such maturation signals to DCs through the NF-κB pathway.
To examine whether neonatal immunity can respond to a more extreme situation, such as bacterial sepsis, cord DCs were treated with LPS. Cord DCs showed significant up-regulation of activation markers, were able to produce large amounts of TNF-α and IL-10, at levels similar to those produced by adult DCs, and to stimulate T-cell proliferation. Unlike the adult DCs, however, cord DCs were still unable to produce IL-12p70 in response to LPS, confirming findings from other studies.7,8,12 These findings support the notion that the neonatal immunity tends to deviate from the T-helper type 1 responses. The high level of TNF-α production may be important in initiating inflammatory responses by recruiting DC precursors to respond to dangerous situations. It may also be possible that autocrine TNF-α mediates the maturation and activation of DCs.30 It is known that different subsets of DCs can secrete different cytokines upon activation. Activated CD14+ cell-derived DCs may secrete IL-10.31 In the present study, both cord and adult DCs were generated from CD14+ monocytes, and they produced IL-10 after LPS stimulation and after phagocytosis of necrotic LCLs. Autocrine IL-10 can limit the maturation of DCs and their capacity to initiate T helper type 1 responses.32 The production of IL-10 by CD14+ cell-derived DCs may suggest its role in the control of T-cell activation.33
In conclusion, our results support the concept that neonatal DCs are flexible in their response depending on the signals encountered. In general, neonatal DCs favour immune tolerance with minimal activation and cytokine production, but they are capable of producing certain mature immune responses in dangerous situations such as bacterial sepsis.
Acknowledgments
We would like to thank all the midwives of the labour ward in Queen Mary Hospital and the mothers who donated the cord blood samples. This work forms part of the MPhil thesis of O.H. Wong at the University of Hong Kong; her MPhil study is supported by the Edward Sai-Kim Hotung Paediatric Education and Research Fund (HT045) and by the University of Hong Kong postgraduate studentship. This work is supported by a research grant to A.K.S. Chiang (HKU CRCG grant # 10203873).
References
- 1.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–64. doi: 10.1038/nri1394. 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 2.Ridge JP, Fuchs EJ, Matzinger P. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science. 1996;271:1723–6. doi: 10.1126/science.271.5256.1723. [DOI] [PubMed] [Google Scholar]
- 3.Sarzotti M, Robbins DS, Hoffman PM. Induction of protective CTL responses in newborn mice by a murine retrovirus. Science. 1996;271:1726–8. doi: 10.1126/science.271.5256.1726. [DOI] [PubMed] [Google Scholar]
- 4.Forsthuber T, Yip HC, Lehmann PV. Induction of TH1 and TH2 immunity in neonatal mice. Science. 1996;271:1728–30. doi: 10.1126/science.271.5256.1728. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 6.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 7.Goriely S, Vincart B, Stordeur P, Vekemans J, Willems F, Goldman M, De Wit D. Deficient IL-12 (p35) gene expression by dendritic cells derived from neonatal monocytes. J Immunol. 2001;166:2141–6. doi: 10.4049/jimmunol.166.3.2141. [DOI] [PubMed] [Google Scholar]
- 8.Langrish CL, Buddle JC, Thrasher AJ, Goldblatt D. Neonatal dendritic cells are intrinsically biased against Th-1 immune responses. Clin Exp Immunol. 2002;128:118–23. doi: 10.1046/j.1365-2249.2002.01817.x. 10.1046/j.1365-2249.2002.01817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu E, Tu W, Law HK, Lau YL. Decreased yield, phenotypic expression and function of immature monocyte-derived dendritic cells in cord blood. Br J Haematol. 2001;113:240–6. doi: 10.1046/j.1365-2141.2001.02720.x. 10.1046/j.1365-2141.2001.02720.x. [DOI] [PubMed] [Google Scholar]
- 10.Petty RE, Hunt DW. Neonatal dendritic cells. Vaccine. 1998;16:1378–82. doi: 10.1016/s0264-410x(98)00095-4. 10.1016/S0264-410X(98)00095-4. [DOI] [PubMed] [Google Scholar]
- 11.Hunt DW, Huppertz HI, Jiang HJ, Petty RE. Studies of human cord blood dendritic cells: evidence for functional immaturity. Blood. 1994;84:4333–43. [PubMed] [Google Scholar]
- 12.De Wit D, Tonon S, Olislagers V, Goriely S, Boutriaux M, Goldman M, Willems F. Impaired responses to toll-like receptor 4 and toll-like receptor 3 ligands in human cord blood. J Autoimmun. 2003;21:277–81. doi: 10.1016/j.jaut.2003.08.003. 10.1016/j.jaut.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Karlsson H, Hessle C, Rudin A. Innate immune responses of human neonatal cells to bacteria from the normal gastrointestinal flora. Infect Immun. 2002;70:6688–96. doi: 10.1128/IAI.70.12.6688-6696.2002. 10.1128/IAI.70.12.6688-6696.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salio M, Dulphy N, Renneson J, Herbert M, McMichael A, Marchant A, Cerundolo V. Efficient priming of antigen-specific cytotoxic T lymphocytes by human cord blood dendritic cells. Int Immunol. 2003;15:1265–73. doi: 10.1093/intimm/dxg123. 10.1093/intimm/dxg123. [DOI] [PubMed] [Google Scholar]
- 15.Larsson M, Fonteneau JF, Somersan S, et al. Efficiency of cross presentation of vaccinia virus-derived antigens by human dendritic cells. Eur J Immunol. 2001;31:3432–42. doi: 10.1002/1521-4141(200112)31:12<3432::aid-immu3432>3.0.co;2-r. 10.1002/1521-4141(200112)31:12<3432::AID-IMMU3432>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 16.Fonteneau JF, Kavanagh DG, Lirvall M, Sanders C, Cover TL, Bhardwaj N, Larsson M. Characterization of the MHC class I cross-presentation pathway for cell-associated antigens by human dendritic cells. Blood. 2003;102:4448–55. doi: 10.1182/blood-2003-06-1801. [DOI] [PubMed] [Google Scholar]
- 17.Albert ML. Death-defying immunity. do apoptotic cells influence antigen processing and presentation? Nat Rev Immunol. 2004;4:223–31. doi: 10.1038/nri11308. 10.1038/nri11308. [DOI] [PubMed] [Google Scholar]
- 18.Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med. 2000;191:411–16. doi: 10.1084/jem.191.3.411. 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–55. doi: 10.1038/15200. 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 20.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–34. doi: 10.1084/jem.191.3.423. 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaif-Muthana M, McIntyre C, Sisley K, Rennie I, Murray A. Dead or alive: immunogenicity of human melanoma cells when presented by dendritic cells. Cancer Res. 2000;60:6441–7. [PubMed] [Google Scholar]
- 22.Rubartelli A, Poggi A, Zocchi MR. The selective engulfment of apoptotic bodies by dendritic cells is mediated by the alpha (v) beta3 integrin and requires intracellular and extracellular calcium. Eur J Immunol. 1997;27:1893–900. doi: 10.1002/eji.1830270812. [DOI] [PubMed] [Google Scholar]
- 23.Albert ML, Pearce SF, Francisco LM, Sauter B, Roy P, Silverstein RL, Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–68. doi: 10.1084/jem.188.7.1359. 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000;407:784–8. doi: 10.1038/35037722. 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- 25.Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol. 2000;12:1539–46. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- 26.Somersan S, Larsson M, Fonteneau JF, Basu S, Srivastava P, Bhardwaj N. Primary tumor tissue lysates are enriched in heat shock proteins and induce the maturation of human dendritic cells. J Immunol. 2001;167:4844–52. doi: 10.4049/jimmunol.167.9.4844. [DOI] [PubMed] [Google Scholar]
- 27.Schnurr M, Then F, Galambos P, Scholz C, Siegmund B, Endres S, Eigler A. Extracellular ATP and TNF-alpha synergize in the activation and maturation of human dendritic cells. J Immunol. 2000;165:4704–9. doi: 10.4049/jimmunol.165.8.4704. [DOI] [PubMed] [Google Scholar]
- 28.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–21. doi: 10.1038/nature01991. 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 29.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–5. doi: 10.1038/nature00858. 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 30.Granucci F, Vizzardelli C, Virzi E, Rescigno M, Ricciardi-Castagnoli P. Transcriptional reprogramming of dendritic cells by differentiation stimuli. Eur J Immunol. 2001;31:2539–46. doi: 10.1002/1521-4141(200109)31:9<2539::aid-immu2539>3.0.co;2-9. 10.1002/1521-4141(200109)31:9<2539::AID-IMMU2539>3.3.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 31.de Saint-Vis B, Fugier-Vivier I, Massacrier C, et al. The cytokine profile expressed by human dendritic cells is dependent on cell subtype and mode of activation. J Immunol. 1998;160:1666–76. [PubMed] [Google Scholar]
- 32.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 33.Caux C, Massacrier C, Vanbervliet B, Barthelemy C, Liu YJ, Banchereau J. Interleukin 10 inhibits T cell alloreaction induced by human dendritic cells. Int Immunol. 1994;6:1177–85. doi: 10.1093/intimm/6.8.1177. [DOI] [PubMed] [Google Scholar]