Abstract
Activation of B cells occurring in hosts infected with protozoan parasites has been implicated either in protective or parasite-evasion immune-mediated mechanisms. Intraperitoneal inoculation of Neospora caninum tachyzoites into BALB/c mice induces an acute response characterized by a rapid increase in the numbers of CD69-expressing peritoneal and splenic B cells. This early B-cell stimulatory effect preceded an increase in the numbers of total and immunoglobulin-secreting splenic B cells and a rise in serum levels of N. caninum-specific immunoglobulins, predominantly of the immunoglobulin G2a (IgG2a) and IgM isotypes. Increased numbers of B cells expressing the costimulatory molecules CD80 and CD86 were also observed in the N. caninum-infected mice. The B-cell stimulatory effect observed in mice challenged with N. caninum tachyzoites was reduced in mice challenged with γ-irradiated parasites. Contrasting with the peripheral B-cell expansion, a depletion of B-lineage cells was observed in the bone-marrow of the N. caninum-infected mice. Intradermal immunization of BALB/c mice with diverse N. caninum antigenic preparations although inducing the production of parasite-specific antibodies nevertheless impaired interferon-γ (IFN-γ) mRNA expression and caused lethal susceptibility to infection in mice inoculated with a non-lethal parasitic inoculum. This increased susceptibility to N. caninum was not observed in naïve mice passively transferred with anti-N. caninum antibodies. Taken together, these results show that N. caninum induces in BALB/c mice a parasite-specific, non-polyclonal, B-cell response, reinforce previous observations made by others showing that immunization with N. caninum whole structural antigens increases susceptibility to murine neosporosis and further stress the role of IFN-γ in the host protective immune mechanisms against this parasite.
Keywords: Neospora caninum, B cells, antibodies
Introduction
Neospora caninum is a cyst-forming coccidian parasite first identified as the causative agent of a fatal disease in dogs.1 Further reports showed that clinical N. caninum infection was also detected naturally in cattle, horse, sheep, goat, deer and rhinoceros2,3 and experimentally induced in animal models.4–7 In cattle, N. caninum is now known to be responsible for abortion or stillbirths worldwide2 with a major economic impact on the dairy industry.8
The murine model of N. caninum infection has been extensively used for the study of immune responses elicited in the host by this parasite.9–17 These studies have shown that both innate and acquired immune responses mediate resistance to neosporosis.5,10,12,14–17 In particular, T cells have been demonstrated to play a major role in the murine host response against N. caninum.17 There is now ample evidence that in the murine model a T helper 1 (Th1)-type immune response involving interferon-γ (IFN-γ) and interleukin-12 (IL-12) mediates protection to acute N. caninum infection9,11,13–16 whereas a Th2-type immune response is correlated with increased susceptibility.12–14 Additionally, parasite-specific CD4+ cytotoxic T cells were reported to be involved in the bovine host immune response against this parasite.18
Comparatively to the T-cell mediated immune response, the B-cell response elicited in the course of murine N. caninum infection has been less characterized. A host protective role of this lymphocyte population has been, however, described in a murine model of neosporosis10 and production of N. caninum-specific antibodies in mice infected with this parasite has been extensively reported.9,12,19 In addition, B cells and/or antibodies have also been implicated in the murine host resistance to infection with the related apicomplexan parasite Toxoplasma gondii.20,21 On the other hand B cells, and polyclonal B-lymphocyte stimulation in particular, have also been shown to mediate immune mechanisms of parasite evasion.22 In the present report we therefore aimed at better characterizing the B-cell immune response elicited in the murine BALB/c mice model of N. caninum infection and, in particular, to investigate whether a polyclonal immune response is induced by this parasite in the murine host.
Materials and methods
Mice
Male BALB/c mice (6–8 weeks old) were purchased from the Gulbenkian Institute of Science (Oeiras, Portugal). Animals were kept at the animal facilities of the Institute Abel Salazar during the time of the experiments. All procedures involving mice were performed according to the European Convention for the Protection of Vertebrate Animals used for Experimenttal and Other Scientific Purposes (ETS 123) and 86/609/EEC Directive and Portuguese rules (DL 129/92).
Neospora caninum
Neospora caninum tachyzoites (NC-1 isolate) were cultured and serially passaged in VERO cells maintained at 37° in minimum essential medium (MEM) supplemented with 10% fetal calf serum, Earle's salts, L-glutamine, penicillin (100 IU/ml) and streptomycin (50 µg/ml) (all from Sigma, St Louis, MO) in a humidified atmosphere of 5% CO2 in air. To obtain the free parasitic forms N. caninum-infected VERO cells were cultured until the host cell monolayer was completely destroyed and culture supernatants were collected. Cell debris were removed by three serial centrifugations at 100 g for 10 min in phosphate-buffered saline (PBS) and the supernatants thus obtained were then passed through a PD-10 column (Amersham Biosciences Europe GmbH, Freiburg, Germany) to improve the tachyzoite purification. The filtered tachyzoite suspensions were then washed in PBS and the concentration of parasites determined with a hemocytometer using trypan blue to exclude dead cells.
Challenge infections
N. caninum infections were performed by intraperitoneal (i.p.) inoculation of 0·5 ml PBS containing 5 × 105 or 5 × 106 tachyzoites. Alternatively, mice were similarly inoculated with 0·5 ml of PBS (control) or with 0·5 ml PBS containing 5 × 105 tachyzoites γ-irradiated with 200 Gy in a Gammacell1000Elite irradiator (Nordion International, Inc., Ottawa, Canada).
Pathologic examination and immunohistochemistry
The brains of N. caninum-infected mice and controls were collected and fixed in 10% formalin and four sagittal sections with 3 mm gap of each brain were obtained. These were dehydrated, embedded in paraffin wax and four serial sections were cut from each block. One section was stained with haematoxylin–eosin and the others were used for immunohistochemistry, performed according to the modified avidin–biotin–peroxidase complex (ABC) method.23 Briefly, sections were dewaxed, rehydrated and immersed in 10% target retrieval solution (Dako, Carpenteria, CA) and incubated in a water bath at 100° for 20 min. Endogenous peroxidase activity was blocked by treatment with 0·3% hydrogen peroxide in methanol (Merck, Darmstadt, Germany) for 10 min. Sections were then incubated in a moist chamber for 20 min with normal pig serum (Dako, Glostrup, Denmark) diluted 1 : 5 in 10% bovine serum albumin (Sigma), to eliminate non-specific staining. Excess serum was removed and the sections were incubated overnight at 4°, with an anti-N. caninum rabbit antiserum24 diluted 1 : 5000. Subsequently, slides were incubated for 30 min with a 1 : 200 dilution of biotin-labelled anti-rabbit secondary antibody (Dako) and then with the avidin-biotin-peroxidase complex (Dako), for further 30 min. The colour was developed by incubation with diaminobenzidine (Dako) for 7 min. After counterstaining tissue sections with haematoxylin, slides were mounted in Entellan (Merck). A positive reaction was indicated by the presence of brown cytoplasmic staining and formalin-fixed N. caninum tachyzoites (NcT) were used as positive controls.
Preparation of micro-organism sonicates
N. caninum tachyzoites obtained from in vitro cultures as described above were disrupted by freeze-thawing twice followed by sonication (10 cycles of 30 s at 100 W) with a Branson cell disrupter, model W 185 D, on ice. The N. caninum sonicates (NcS) were successively filtered through 0·45 and 0·2 µm pore-size filters (Schleicher & Schuell) and stored in small aliquots at −80°. Sonicates of Candida albicans yeast or Streptoccus agalactiae cells were similarly prepared.
In vitro mononuclear cell cultures
Spleen cells were obtained by gently teasing the organ in RPMI-1640 medium (Sigma) supplemented with penicillin (100 IU/ml), streptomycin (50 µg/ml), 2-mercaptoethanol (0·05 m) and 10% of fetal bovine serum (all from Sigma) (RPMI). Cell suspensions were layered onto 2·5 ml of a polysucrose/sodium ditrizoate solution (histopaque-1083, Sigma) and centrifuged at 650 g for 20 min at room temperature. Cells collected from the medium/histopaque interface, were washed with RPMI, distributed in 96-well plates (5 × 105 cells/well) and cultured for 6 h at 37° in a humidified atmosphere containing 5% CO2 in air. Plated cells were stimulated with medium alone or with 2·5 µg of LPS (as positive control) or with serial 10-fold amounts (102 to 105) of NcT per ml of culture medium.
Flow cytometric analysis
For cytometric analysis spleen, peritoneal exudate or bone marrow cells from BALB/c mice, the latter collected by flushing the femoral shaft with 5 ml of balanced salt solution (BSS) using a syringe fitted with a 25G needle, were resuspended in BSS, supplemented with 10 mm of sodium azide and 1% BSA. The following monoclonal antibodies were used on previously titrated dilutions for immunofluorescence cytometric analysis in a FACScan (Becton Dickinson, San Jose, CA) with the CellQuest software (Becton Dickinson): goat anti-mouse IgM fluoroscein isothiocyanate (FITC) conjugate (Southern Biotechnology Associates, Birmingham, AL); hamster anti-mouse CD3 FITC conjugate (Southern Biotechnology Associates); rat anti-mouse B220 phycoerythrin (PE) conjugate (PharMingen, San Diego, CA); hamster anti-mouse CD80 FITC conjugate (PharMingen) rat anti-mouse CD86 FITC conjugate (PharMingen); hamster anti-mouse CD69 PE conjugate (PharMingen) and rat anti-mouse major histocompatibility complex (MHC) Class II biotin-conjugate (Southern Biotechnology Associates) revealed with Cy-Chrome-conjugated streptavidin (PharMingen). Peanut-agglutinin (PNA) FITC-conjugate (Sigma) was used for the detection of germinal centre spleen cells. A number of 5 × 105 mononuclear cells were stained per sample. Dead cells were excluded by propidium iodide incorporation. For the detection of apoptotic cells the TACS™ Annexin V–FITC apoptosis detection kit (R & D systems, Minneapolis, MN) was used according to manufacturer's instructions.
Immunizations
Different groups of mice were injected intradermally (i.d.) twice at 3-week intervals, with 100 µl of the following preparations: 10 µg of NcS in a 1 : 1 PBS/alum (aluminium hydroxide Gel, Brenntag, Frederikssund, Denmark, a kind gift of Dr Erik Lindblad, Biosector, Frederikssund, Denmark); 10 µg of NcS in a 1 : 1 PBS/Freund's adjuvant (Sigma), complete in the first and incomplete in the second immunizations; 5 × 105γ-irradiated N. caninum tachyzoites in PBS. Control animals received 100 µl of a 1 : 1 PBS/alum suspension, a 1 : 1 PBS Freund's adjuvant suspension or PBS, respectively. Passive immunization was performed by i.p. administration of 800 µg of immunoglobulin G (IgG) antibodies purified from pooled sera of mice NcS-immunized as described above or 800 µg of IgG purified from pooled sera of sham-immunized mice, both prepared as described below, 6 hr prior to parasite i.p. challenge. Survival of the NcT-inoculated animals was monitored daily.
Antibody detection
Total antibodies or specific for NcS, Candida albicans extracellular proteins (CaEP), C. albicans sonicates (CaS), Streptococcus agalactiae extracellular proteins (SaEP) or Streptococcus agalactiae sonicates (SaS) antibodies in mice sera, collected by retro-orbital bleeding, were quantified by ELISA. Briefly, polystyrene microtitre plates (Nunc, Roskilde, Denmark) were coated with 5 µg/ml of goat anti-mouse immunoglobulin (Southern Biotechnology Associates) or with 10 µg/ml of NcS, CaEP, CaS, SaEP or SaS and incubated overnight at 4°. Wells were then saturated with 1% BSA in PBS for 1 hr at room temperature. Serial dilutions of the serum samples were then plated and incubated for 2 hr at room temperature. After washing, bound antibodies were detected by addition of alkaline phosphatase-coupled monoclonal goat anti-mouse-IgG isotypes (IgG1, IgG2a, IgG2b, IgG3) or goat anti-mouse–IgM antibody (all from Southern Biotechnology Associates) for 30 min at room temperature. Substrate solution containing p-nitrophenyl phosphate (Sigma) was then added after washing and the reaction was stopped by addition of 0·1 m ethylenediaminetetraacetic acid (EDTA) pH 8·0. The absorbance was measured at 405 nm. The enzyme-linked immunosorbent assay (ELISA) antibody titres were expressed as the reciprocal of the highest dilution giving an absorbance of 0·1 above that of the control (no serum added).
Purification of serum IgG antibodies
Sera of mice immunized i.d. with NcS plus adjuvant or sham-immunized with adjuvant alone, twice at a 3-week interval, as described above, were collected and pooled 20 days after the second i.d. inoculation with any of the two preparations. Purification of IgG antibodies from these sera was then performed as follows: pooled serum samples were equilibrated in binding buffer (20 mm sodium phosphate, pH 7·0) by overnight dialysis and 3 ml of these preparations were applied to a PROTEIN G HP affinity column (HiTrap, Amersham Biosciences UK Limited, Little Chalfont, UK) for each separation. Bound antibodies were eluted with glycine-HCl buffer, pH 2·7 and recovered in 50 µl of 1 m Tris-HCl pH 9·0 per ml of eluent, according to the manufacturer's instructions. Recovered IgG antibody fractions were further equilibrated in PBS in a VIVAPORE concentrator with a 7·5 000 MW cutoff membrane (Vivascience, Hanover, Germany) and stored at −80° in frozen aliquots. The purified IgG antibody fractions obtained from sera of PBS/alum or PBS/alum/NcS inoculated mice were designated IgG-alum and IgG-alumNcS, respectively.
ELISA-spot assays
The numbers of splenic immunoglobulin-producing cells were assessed by an ELISA-spot assay as described elsewhere.25 Briefly, polystyrene microtitre plates (Nunc) were coated overnight at 4° with 10 µg/ml of goat anti-mouse immunoglobulin (Southern Biotechnology Associates) or with 20 µg/ml of NcS prepared as described above. The wells were then saturated for 30 min with 2% bovine serum albumin in PBS at 37°. Appropriate serial suspensions of spleen cells in RPMI-1640 (Sigma) supplemented with 3% calf serum (Gibco Biocult, Irvine, UK) were incubated in the plates for 6 hr at 37° in a humidified atmosphere of 5% CO2 in air. The plates were then rinsed with 0·075% Tween-20 in water (Sigma) and washed four times with PBS containing 0·075% Tween-20. Antigen-specific or total antibody-secreting cells were revealed by the addition of alkaline phosphatase-coupled monoclonal goat anti-mouse–IgG isotypes (IgG1, IgG2a, IgG2b, IgG3) or goat anti-mouse-IgM antibodies (all from Southern Biotechnology Associates) overnight at 4°. After washing, 5-bromo 4-chloro 3-indolyl phosphate (Sigma) in 2-amino 2-methyl 1-propanol (Sigma) buffer was used as substrate for 2 hr at 37°. After washing four times with distilled water, the number of spots was quantified in triplicate wells with a dissecting microscope.
RNA isolation and quantitative reverse transcription–polymerase chain reaction (RT–PCR) analysis
Total RNA was extracted from 1 × 106 spleen mononuclear cells of BALB/c mice of the different experimental groups using TriPure™ Isolation Reagent (Roche Diagnostics, Indianapolis, IN) according to the manufacturer's instructions. All RNA samples were recovered in 50 µl of nuclease-free H2O and spectrophotometrically analysed for quantity and quality. Synthesis of cDNA was then performed from 200 µg of total RNA prepared as described above in a 20 µl final volume using 200 U of Superscript™ RNAse H Reverse Transcriptase (Invitrogen, Carlsbad, CA) according to manufacturer's instructions. The PCR programme run (42°, 60 min; 70°, 15 min; 4° infinite) was performed in a Bio-Rad iCycler Thermal Cycler (Bio-Rad, Hercules, CA). Real time PCR was then used for the quantification of hypoxanthine phosphonibosyl transferase (HRPT) or cytokine mRNA expression levels with the LightCycler FastStart DNA Master Hybridization Probes kit (Roche) in a LightCycler device (Roche). For the quantification of HPRT expression levels, the reaction was performed in a final volume of 10 µl containing 5·0 mm MgCl2, 1·0 µm of each primer (sense: 5′-GCT GGT GAA AAG GAC CTC T, antisense: 5′-CAC AGG ACT AGA ACA CCT GC), 0·2 µm of each probe (3′-fluorescein-labelled [FL] 5′-AAA GCC TAA GAT GAG CGC AAG TTG A – FL, 5′-LC-red 640-labelled [LC] 5′-TCT GCA AAT ACG AGG AGT CCT GTT G – PH) and 1 × Master Mix plus 1 µl of the synthesized cDNA. The PCR programme run was as follows: (1) denaturation at 95°, 10 min; (2) amplification in 40 cycles 95°, 10 s; 60°, 10 s; 72°, 11 s; (3) cooling 40°, 2 min. The temperature transition rate was 20°/s in all steps. Quantification of IFN-γ expression levels was similarly performed with specific primers (sense: 5′-TGG CAA AAG GAT GGT GAC ATG, antisense: 3′-GAC TCC TTT TCC GCT TCC TGA) (TibMol, Berlin, Germany), and probes (FL TGC CAA GTT TGA GGT CAA CAA CCC ACA – FL; LC TCC AGC GCC AAG CAT TCA ATG AGC – PH) (TibMol) in MgCl2 4·0 mm and an annealing temperature of 52°, and extension at 72°, 12 s. At the end of each annealing phase fluorescence was measured in a ‘single’ acquisition mode with the channel setting F2/F1. Quantitative evaluation of fluorescence signals from the PCR products was performed with the LightCycler software (version 3.5) and was determined by plotting the fluorescence signals versus the cycle numbers at which the signals crossed the baseline. The baseline adjustment was performed in ‘minimize error’ mode. Samples containing 108−102 cDNA molecules of the respective gene were included as external standards. The correlation coefficient among the standard reactions assured linearity.
IFN-γ serum measurements
Serum IFN-γ titres in NcT-inoculated mice and controls were quantified with the Quantikine® M Murine IFN-γ ELISA kit (R & D Systems) according to manufacturer's instructions.
Statistical analysis
Unless otherwise indicated, the level of significance of the results obtained in parasite-inoculated mice versus respective control groups was determined using analysis of variance (anova) single factor, calculated with Microsoft Excel 2000 software. On results described in Fig. 8 the Kaplan–Meier method was used to estimate survival time and the median survival was calculated and respective 95% CI. For comparing distribution of survival in experimental and control groups the log rank test was used.
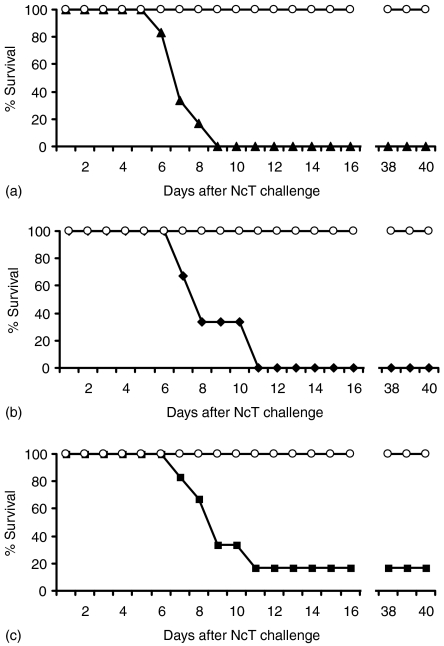
Figure 8.
Increased susceptibility to neosporosis in mice immunized with N. caninum antigens. Survival rates of mice infected i.p. with 5 × 106 NcT 30 days after the second i.d. inoculation at a 3-week interval of (a) alum (open circles) or 10 µg of NcS in alum adjuvant (closed triangles), (b) Freund's adjuvant (open circles) or 10 µg of NcS in Freund's adjuvant (closed lozenges), (c) PBS (open circles) or 5 × 105 gamma-irradiated NcT in PBS (closed squares). The survival rate of mice sham-immunized was significantly different from that of immunized mice immunized in all the groups (median survival time in days (MST) = 7 (95% CI: 6; 8) chi-square = 12·1, d.f. = 1, P < 0·0006; MST = 8 (95% CI: 7; 9) chi-square = 11·4, d.f. = 1, P < 0·0008; MST = 9 (95% CI: 8; 10) chi-square = 8·1, d.f. = 1, P < 0·005, for results shown in panels A, B and C, respectively). Six mice per group were used. This is one representative result of two independent experiments.
Results
Expression of CD69 on the surface of B cells induced in vivo and in vitro by N. caninum
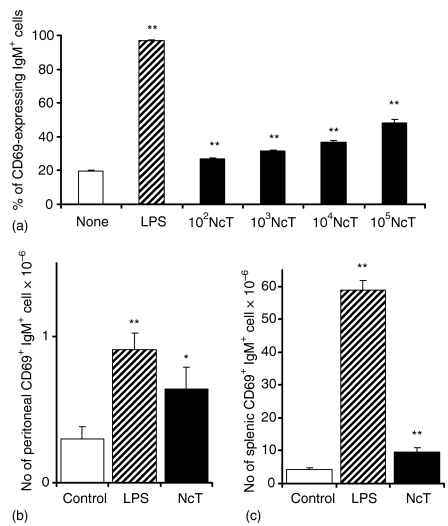
Polyclonal B-cell activation is a common feauture of several parasitic infections that has been considered as a mechanism of immune evasion.26 In order to determine the ability of NcT to induce the stimulation of B cells, the expression of the early activation marker CD69 on the surface of IgM+ splenocytes upon in vitro or in vivo stimulation with this parasite was quantified by flow cytometric analysis. Stimulation of splenocytes in vitro with different numbers of NcT induced an increased expression of the early activation marker CD69 on a sizeable population of B lymphocytes in a dose-dependent manner (Fig. 1a). Similarly, BALB/c mice that received 5 × 105 parasites i.p. showed increased numbers of peritoneal and splenic CD69-expressing B cells when compared to control animals, observed 14 hr after the parasitic challenge (Figs 1b,c). The stimulatory effect of NcT was however, less marked than the one obtained in vitro or in vivo with the B-cell polyclonal activator lipoloysaccharide (LPS), used as a positive control (Fig. 1). These results indicate that NcT have a B-cell stimulatory effect, already noticeable at very early stages of infection.
Figure 1.
Early B-cell activation induced in vitro and in vivo by N. caninum. (a) Expression of CD69 on the surface of BALB/c mice splenic IgM+ cells as evaluated by flow cytometric analysis 14 hr after in vitro stimulation with medium alone (None), with 5 µg/ml of LPS (LPS) as a positive control or with 102−105N. caninum tachyzoites/ml (NcT) as indicated. Bars represent means plus 1 SD of triplicated well samples for each indicated group. This is one representative result of three independent experiments. (b and c) Numbers of BALB/c mice peritoneal (b) or splenic (c) CD69+ IgM+ cells, 14 hr after i.p. treatment with PBS (Control), 12·5 µg of LPS (LPS) or 5 × 105 NcT. Bars represent the mean plus one SD of three mice per control groups and four mice per N. caninum-treated groups. This is one representative result of three independent experiments. In this and in the following figures statistically significant differences between control and N. caninum-stimulated groups were indicated (*P < 0·05; **P < 0·01).
B-cell population kinetics in the spleen of N. caninum-challenged mice
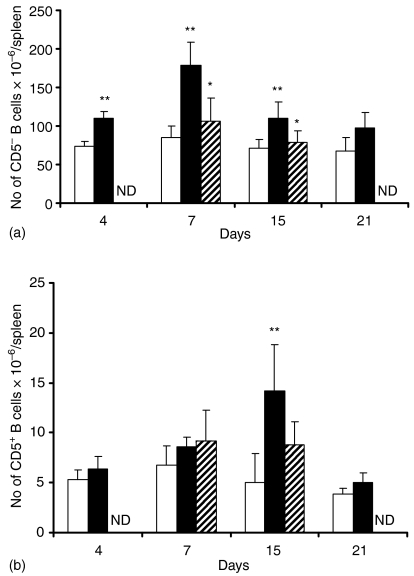
Because NcT induced in vivo an early stimulation of splenic B cells we determined to what extent this early stimulatory effect induced a later expansion of this lymphocyte population at different time-points of infection. As shown in Fig. 2 there was an increase in the numbers of B cells in the spleen of mice infected i.p. with N. caninum as compared to PBS-inoculated controls. This increase was statistically significant for CD5– B cells already at day 4 after the parasitic inoculation, reached an observed maximum at day 7 and was still noticeable 15 days after the parasitic challenge (Fig. 2a). At day 21 postinoculation (p.i) the increase in B-cell numbers observed in infected mice had already subsided (Fig. 2a). Spleen CD5+ B cells also increased in number in N. caninum-infected animals. However, this increase was only statistically significant at day 15 p.i. as compared to PBS-inoculated controls thus occurring later than the one detected for CD5– B cells (Fig. 2b). Apicomplexan parasites attenuated by γ-irradiation were previously used in immunoprotection studies.27,28 Therefore we also quantitated the effect of the i.p. inoculation of irradiated NcT in the numbers of splenic B cells, regarding their potential usage as antigen targets in immunoprotective studies. As shown in Fig. 2 irradiated parasites did not induce an increase in the numbers of the CD5+ B cells and caused a reduced response in the CD5– population.
Figure 2.
B-cell population kinetics in the spleen. Numbers of IgM+ CD5– (a) and of IgM+ CD5+ (b) B cells in the spleen of BALB/c mice evaluated by flow cytometric analysis on the indicated days after i.p. injection with PBS (open bars), 5 × 105N. caninum tachyzoites (closed bars) or 5 × 105N. caninumγ-irradiated tachyzoites (dashed bars). Bars represent the mean plus 1 SD of three mice per control groups and four mice per N. caninum-treated groups. This is one representative result of three independent experiments. ND, not done.
These results show that the early stimulatory effect on B cells observed in mice challenged with N. caninum is followed by the expansion of this cell population in the spleen.
Effect of N. caninum challenge on B-cell maturation in the bone marrow
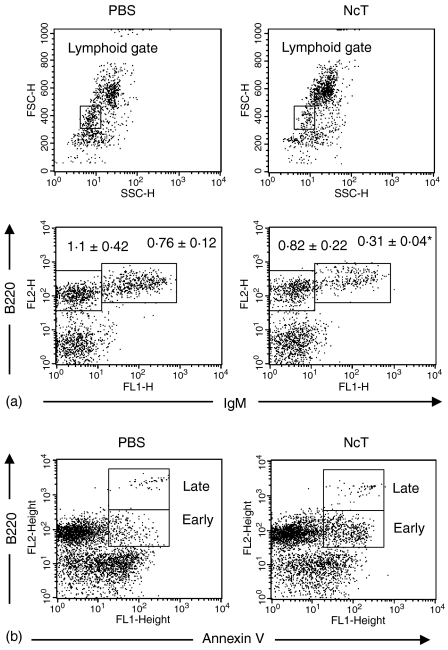
Because B-cell activation and expansion was observed in the spleen of NcT-infected mice we studied the effect of N. caninum infection on the maturation of B cells in the bone-marrow. In contrast and prior to the peripheral expansion observed in the B-cell compartment, a reduction in the numbers of B220+ IgM+ cells was observed in the bone marrow of NcT-infected mice at day 3 p.i. (Fig. 3a). As shown in Fig. 3(b), this reduction was preceded by an increase in the numbers of cells displaying the apoptotic marker annexin V in the membrane, observed 24 hr after the parasitic challenge. These results indicate that the observed reduction in bone-marrow B220+ IgM+ cells in mice infected with N. caninum can therefore be a consequence of an apoptotic effect of the NcT infection on B-cell precursors rather than of an accelerated recruitment into peripheral lymphoid organs induced by this parasite.
Figure 3.
Effect of N. caninum challenge on B-cell maturation in the bone-marrow. Quantification of B-cell precursors (a) in the bone marrow of BALB/c mice 3 days after injection i.p. with PBS or with 5 × 105N. caninum tachyzoites (NcT). Figures shown in the lower dot-plots above the respective regions represent numbers in millions of B220+ IgM– and B220+ IgM+ bone-marrow cells displaying lymphoid forward and side scatter parameters (gated as shown in the upper dot-plots) observed in PBS- or NcT-infected mice as indicated. Numbers represent the mean ± one SD of three mice in the control group and four mice on the N. caninum-infected group. This is one representative result of three independent experiments. Quantification of apoptotic B-lineage (B220+) cells (b) gated as described above, observed in the bone marrow of BALB/c mice 24 hr after PBS- or NcT-inoculation as indicated. Regions on the figure identify early apoptotic (annexin V+) B220+ cells (Early) and late apoptotic/necrotic (propidium iodide+) cells (Late). Represented is one typical result from each mice group of an experiment repeated three times. The mean numbers ± 1 SD of early apoptotic cells were of 92 × 103 ± 3·5 × 103 and 234 × 103 ± 76 × 103 for PBS- or NcT-inoculated mice, respectively (n = 4 on each group, P < 0·05). No significant difference in the numbers of late apoptotic/necrotic cells between these two mice groups was observed.
Production of antibodies in N. caninum-challenged mice
Because we observed an increase in the numbers of splenic B cells in N. caninum-infected mice we quantitated by an ELISA-spot assay the numbers of antibody-secreting cells in the spleen of mice inoculated i.p. with 5 × 105 NcT or irradiated NcT, or with PBS (controls). As shown in Table 1, an increase in the numbers of immunoglobulin-secreting cells, comparatively to PBS-inoculated controls, was observed in BALB/c mice 7 days after the NcT infection. This increase was statistically significant for IgG2a-, IgG2b-, IgG3- and IgM-secreting cells, but not for IgG1-secreting cells. Increased numbers of antibody-secreting cells, were also observed 7 days after inoculation of irradiated NcT. However this increase was only statistically significant for IgG2a- and IgM-secreting cells as compared to control mice and less marked than that observed in NcT-challenged mice for the cells producing immunoglobulins of these isotypes.
Table 1.
Numbers of immunoglobulin-secreting cells of the indicated isotypes detected in the spleen of BALB/c mice 7 days after i.p. injection with PBS, or 5 × 105 N. caninum tachyzoites (NcT) or with the same number of irradiated NcT (Irr-NcT)
| Injection i.p. | IgG1 | IgG2a | IgG2b | IgG3 | IgM |
|---|---|---|---|---|---|
| PBS | 52 034 ± 29081 | 47 214 ± 19 605 | 758 ± 59 | 2645 ± 694 | 698 226 ± 206 261 |
| NcT | 62 901 ± 5442 | 111 852 ± 2816** | 1823 ± 431** | 5621 ± 702* | 1 634 721 ± 311 355* |
| Irr-NcT | 66 492 ± 13 186 | 80 554 ± 19 537* | 1240 ± 591 | 3808 ± 1365 | 1 266 213 ± 184 402* |
Values are means ± 1 SD of immunoglobulin-secreting cells of four mice per group and are one representative result of three independent experiments. Significance of the results between control (PBS) and NcT or Irr-NcT groups determined using anova is indicated in the table
P < 0·05
P < 0·01.
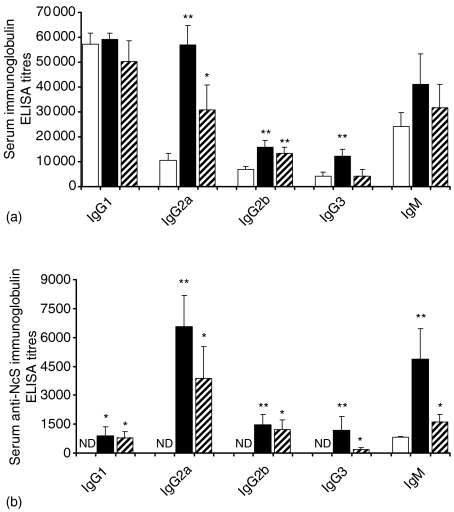
This B-cell stimulatory effect detected in the spleen of NcT-inoculated BALB/c mice led to an increase in the serum levels of immunoglobulins of all IgG isotypes but IgG1 and of IgM, comparatively to those of PBS-inoculated controls, as determined by ELISA 15 days after the i.p. NcT inoculation (Fig. 4a). In mice inoculated with irradiated NcT only IgG2a and IgG2b serum levels were significantly above those of controls, 15 days after the i.p. challenge (Fig. 4a). This increase in IgG2a and IgG2b serum levels was however, much lower than that detected for these two isotypes in mice inoculated with non-irradiated parasites.
Figure 4.
Increased serum antibody titres in NcT-infected mice. Serum ELISA titres of total (a) or NcS-specific (b) antibodies of the indicated isotypes detected in mice sera collected 15 days after i.p. inoculation with PBS (open bars), 5 × 105N. caninum tachyzoites (closed bars) or 5 × 105N. caninumγ-irradiated tachyzoites (dashed bars). Bars represent the mean plus 1 SD of four mice per control groups and six mice per N. caninum-infected groups. This is one representative result of three independent experiments. ND, not detected.
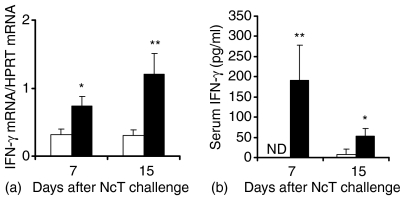
The levels of N. caninum-specific serum antibodies of all tested isotypes were also increased in mice challenged with NcT or irradiated NcT as compared to those of PBS-inoculated controls as detected by ELISA 15 days after the N. caninum i.p inoculation. This increase in N. caninum-specific antibodies was higher for IgG2a and IgM than for the other studied isotypes (Fig. 4b). The production of antigen-specific IgG2a antibodies is consistent with a significant increase in the levels of IFN-γ m-RNA detected by RT–PCR in spleen cells of NcT-infected mice, comparatively to controls, at days 7 and 15 after the parasitic challenge (Fig. 5). As also shown in Fig. 5, serum levels of IFN-γ were also increased in the NcT infected mice, comparatively to non-infected controls, as observed at days 7 and 15 after the parasitic challenge. No spleen IFN-γ mRNA or serum IFN-γ were detected above that of controls at the same days postchallenge in mice inoculated with irradiated-NcT (data not shown). However, at 60 days p.i. higher levels of N. caninum-specific IgG1 antibodies than those of IgG2a antibodies with the same specificity, 5711 ± 830 and 2713 ± 798 serum ELISA titres, respectively (n = 4, P < 0·05), were detected in NcT challenged mice, as compared with non-infected controls where no detectable levels of anti-N. caninum antibodies were found. The preferential production of parasite-specific IgG1 antibodies over that of IgG2a antibodies with that specificity was also detected in mice 15 days after challenging with a higher parasitic inoculum consisting of 5 × 106 NcT (3820 ± 1853 and 1971 ± 142, respectively, n = 6, P < 0·05).
Figure 5.
Increased IFN-γ production in BALB/c mice challenged with N. caninum tachyzoites. Levels of IFN-γ mRNA expression, normalized to HPRT mRNA (a) detected by real time RT–PCR in the spleen of BALB/c mice inoculated i.p. with PBS (controls, open bars) or 5 × 105N. caninum tachyzoites (closed bars), 7 and 15 days after challenge. Bars represent the mean plus 1 SD of four mice per group. This is one representative result of two independent experiments. Serum ELISA titres of IFN-γ (b) detected in mice sera collected 7 and 15 days after i.p. inoculation with PBS (open bars) or 5 × 105N. caninum tachyzoites (closed bars). Bars represent the mean plus 1 SD of four mice per group. This is one representative result of three independent experiments. ND, not detected.
Inoculation of irradiated parasites also increased the serum levels of parasite-specific antibodies of all tested isotypes, although to a lesser level than the one reached when the challenge was performed with non-irradiated parasites (Fig. 4b).
In order to investigate whether the increase in serum immunoglobulins observed in N. caninum infected mice was antigen driven or polyclonal we also evaluated by ELISA the reactivity of IgG2a and IgM antibodies against secreted or constitutive fungal or bacterial antigens. As shown in Table 2, the ELISA serum titres of IgG2a antibodies specific for bacterial or fungal structural or secreted antigens were very low or not detectable in the sera of N. caninum-inoculated mice. In contrast, serum IgG2a anti-NcS titres were clearly increased in the NcT- or irradiated-NcT-inoculated mice, comparatively to that observed in PBS inoculated controls (Table 2). Because polyclonal B-cell activation is a characteristic previously observed in mice infected with Leishmania spp.29–31 we compared the pattern of reactivity of antibodies in the sera of mice infected with L. infantum (a kind gift of Dr Ana Tomás, Institute for Molecular and Cellular Biology, Porto, Portugal) with that observed for serum antibodies of NcT-infected mice. As shown in Table 2, in L. infantum-infected mice the serum IgG2a antibody levels against yeast (Candida albicans) and bacterial (Streptococcus agalactiae) antigens were higher than those observed in the serum of NcT-infected mice (Table 2). As also shown in Table 2, the levels of serum IgM antibodies specific for NcS, but not of those specific for C. albicans or S. agalactiae antigens, were significantly increased in the NcT or irradiated NcT inoculated mice as compared to PBS-inoculated controls. The titres of N. caninum non-specific IgM serum antibody levels observed in NcT-inoculated mice groups and those of N. caninum-specific antibodies detected in PBS-treated controls probably reflect the binding of polyreactive natural antibodies.32 The serum IgM antibody titres against C. albicans or anti-S. agalactiae structural or secreted antigens detected in L. infantum-infected mice were significantly higher than those observed in PBS-inoculated controls (Table 2). Together, the above results indicate that the B-cell stimulatory effect observed in N. caninum-infected BALB/c mice is parasite-specific rather than polyclonal. This stimulatory effect elicits the preferential production of IgG2a and IgM antibodies.
Table 2.
ELISA titres of IgG2a and IgM antibodies anti-N. caninum sonicates (NcS), C. albicans extracellular proteins (CaEP), C. albicans sonicates (CaS), Streptococcus agalactiae extracellular proteins (SaEP) or S. agalactiae sonicates (SaS), detected in BALB/c mice sera collected 15 days after i.p. injection with PBS, 106Leishmania infantum promastigotes, 5 × 105N. caninum tachyzoites (NcT) or with the same number of irradiated NcT (Irr-NcT)
| Mice groups | NcS | CaS | CaEP | SaS | SaEP |
|---|---|---|---|---|---|
| IgG2a | |||||
| PBS | ND1 | ND | ND | 13 ± 152 | 10 ± 17 |
| L. infantum | 70 ± 34** | 20 ± 12* | 5 ± 7 | 108 ± 10** | 98 ± 42* |
| NcT | 6075 ± 4670** | ND | ND | 7 ± 11 | 32 ± 21 |
| Irr-NcT | 2145 ± 1275** | ND | ND | ND | 5 ± 6 |
| IgM | |||||
| PBS | 811 ± 46 | 150 ± 104 | 396 ± 228 | 1953 ± 1022 | 1134 ± 428 |
| L. infantum | 864 ± 93 | 1242 ± 187** | 1224 ± 82** | 4212 ± 1122* | 4724 ± 306** |
| NcT | 3564 ± 1223* | 241 ± 51 | 540 ± 270 | 3454 ± 1686 | 1134 ± 290 |
| Irr-NcT | 1674 ± 247** | 244 ± 96 | 630 ± 311 | 2943 ± 1032 | 1458 ± 730 |
ND, not detected.
Numbers represent means ± 1SD of three serum samples on the PBS group and of four samples in the L. infantum, NcT or Irr-NcT groups. This is one representative result of three independent experiments. Significance of the results between control (PBS) and NcT or Irr-NcT groups determined using anova is indicated in the table
P < 0·05
P < 0·01.
Expression of T-cell costimulatory molecules on the surface of B-cells induced in mice challenged with NcT
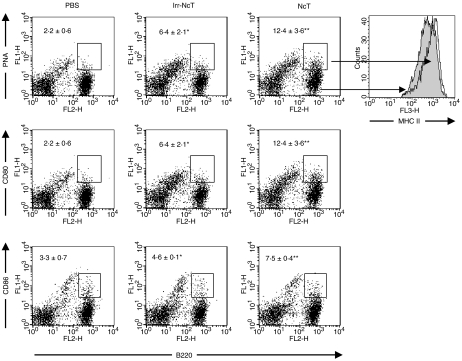
Because a parasite-specific B-cell immune response was elicited in N. caninum-infected BALB/c mice we evaluated by flow cytometric analysis the expression in this lymphocyte cell population of the costimulatory molecules CD80 and CD86. As shown in Fig. 6, an increase in the numbers of B cells expressing CD80 and, to a lesser extent, CD86 was observed in NcT and less markedly in irradiated NcT-inoculated mice, 15 days p.i. Also, increased numbers of PNAhigh B cells, characteristically germinal centre B cells, were observed in the spleen of NcT-inoculated mice at the same time p.i. (Fig. 6). An up-regulation of class II molecules was observed on the surface of PNAhigh cells comparatively to that observed on PNAlow B-cell population, as shown in Fig. 6. These results suggest that B-cells could participate in the activation of T-cells or in T-cell mediated responses elicited by N. caninum in BALB/c mice.
Figure 6.
Expression of costimulatory molecules on B cells is increased in NcT-infected mice. Flow cytometric analysis of PNA binding and of CD80 or CD86 expression on the surface of splenic cells from BALB/c mice 15 days after i.p. inoculation with PBS (PBS), 5 × 105γ-irradiated NcT (Irr-NcT) or with 5 × 105 NcT (NcT). Figures shown inside the dot plots are means ± 1 SD of the numbers (in millions) of PNA+, CD80+ or CD86+ B cells (B220+ cells) as indicated, on each mouse group. Four mice per group were used and the results are representative of one experiment repeated three times. Also shown on the far right is a representative example of MHC class II expression observed on the surface of PNAlow (grey histogram) and PNAhigh (overlay open histogram) B220+ cells in NcT-infected mice.
Pathologic examination and immunohistochemistry of N. caninum-challenged mice brains
Because brain lesions were previously reported to occur in N. caninum-infected mice we analysed histopathologically and immunohistochemically serial sections of BALB/c mice (four animals per group) brains collected 3 and 10 weeks after i.p. infection with 5 × 105 NcT or inoculation with PBS (controls). No evidence of inflammatory lesions in any of the analysed tissue samples was detected. Moreover, no NcT or cysts were observed in any of the brain sections of the NcT-infected mice upon specific staining with a polyclonal rabbit anti-N. caninum antiserum.
Increased susceptibility to N. caninum infection in BALB/c mice immunized with NcT structural antigens or irradiated parasites
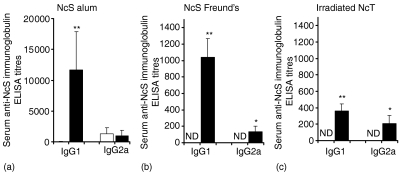
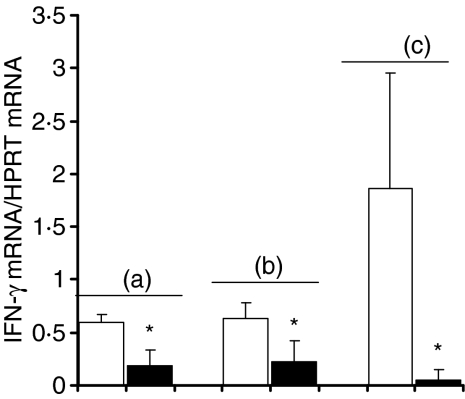
Because a specific antibody response against N. caninum structural antigens was elicited in mice infected with this parasite, we investigated the possibility that an immune response involving the production of parasite-specific antibodies could have a protective effect against the infection of BALB/c mice by N. caninum. We thus immunized mice with two i.d. inoculations at 3-week intervals with 10 µg of NcS using alum or Freund's as adjuvants. Sham-immunized controls were inoculated i.d. with the respective adjuvants alone. A third group of mice were immunized i.d. with 5 × 105 irradiated-NcT in PBS or sham-immunized with PBS alone as controls. All mice were challenged i.p. 30 days after the second immunogenic i.d. inoculation with 5 × 106 NcT, an inoculum we previously determined as inducing the appearance of N. caninum cysts in the brain of BALB/c mice, detectable by immunofluorescence already at 21 days after challenge (data not shown). As shown in Fig. 7, mice immunized with NcS in alum or Freund's adjuvant induced a predominant production of serum IgG1 comparatively to IgG2a anti-NcS antibodies whereas low levels or absence of antibodies with this specificity were detected in sham-immunized controls, as determined by ELISA on the day of the parasitic challenge. Low titres of anti-NcS antibodies of the other isotypes were detected on the same day in immunized mice and sham-immunized controls (data not shown). In spite of the N. caninum-specific antibody response elicited in the NcS-immunized mice, all of them eventually died between day 6 and day 9 after the i.p. parasitic challenge whereas mice of alum or Freund's control groups survived for at least 40 days after parasitic challenge (Fig. 8a, b). Interestingly, as shown in Fig. 8(c) the immunization of BALB/c mice i.d. with irradiated NcT in the absence of adjuvant, however, inducing lower levels of N. caninum-specific antibodies than those detected for mice immunized with NcS plus adjuvant (Fig. 7), also lead to an increased susceptibility to infection by this parasite. As shown in Fig. 9, immunization with N. caninum antigens by any of the three different protocols used here lead to a diminished IFN-γ mRNA expression in the spleen of the immunized mice, comparatively to the respective sham-immunized controls, observed 6 days after i.p. challenge with 5 × 106 NcT.
Figure 7.
Anti-NcS antibody titres in NcS- or irradiated-NcT immunized BALB/c mice. Immunoglobulin ELISA titres of anti-NcS antibodies of the indicated isotypes detected in mice sera 30 days after the second i.d. inoculation with the different immunogenic preparations (right closed bars on each panel), from left to right, 10 µg of NcS in alum adjuvant, 10 µg of NcS in Freund's adjuvant, 5 × 105γ-irradiated NcT or with control preparations (left open bars): alum adjuvant, Freund's adjuvant, PBS, respectively. Bars represent the mean plus 1 SD of six mice per group. This is one representative result of two independent experiments. ND not detected.
Figure 9.
Decreased IFN-γ mRNA expression, comparatively to sham-immunized controls, in BALB/c mice immunized with N. caninum antigens and challenged with N. caninum tachyzoites. Levels of IFN-γ mRNA expression, normalized to HPRT mRNA, detected by real time RT–PCR, 6 days after challenge i.p. with 5 × 106N. caninum tachyzoites, in the spleen of mice (a) sham-immunized with alum (open bars) or immunized with NcS in alum adjuvant (closed bars); (b) sham-immunized with Freund's adjuvant (open bars) or immunized with NcS in Freund's adjuvant (closed bars); (c) sham-immunized with PBS (open bars) or immunized with irradiated NcT in PBS (closed bars). Bars represent the mean plus 1 SD of three mice per group. This is one representative result of two independent experiments.
To test the hypothesis that the production of antibodies specific for N. caninum whole structural antigens could actually be responsible for the increased susceptibility to neosporosis of the immunized mice, BALB/c mice were passively transferred with preparations of IgG antibodies purified from pooled sera of mice immunized with NcS in alum adjuvant, the immunization procedure eliciting a greater N. caninum-specific antibody production, or sham-immunized with adjuvant alone (IgG-NcS/alum or IgG-alum, respectively) and challenged i.p. with 5 × 106 NcT. No enhanced susceptibility to this challenge infection was observed in the mice treated with the antibody preparations since all were alive for at least 40 days p.i.
The results show that immunization of BALB/c mice with N. caninum structural antigens in alum or Freund's adjuvant leads to an increased susceptibility to this parasite. This increased susceptibility was also observed in mice immunized with irradiated NcT in the absence of adjuvant and correlates with a decrease in IFN-γ mRNA expression observed in the immunized mice upon NcT challenge.
Discussion
Polyclonal B-cell activation has been described in a vast number of infections caused by different micro-organisms including viruses, bacteria, fungi and protozoa.26 The induction of lymphocyte polyclonal activation of the host elicited by infectious agents has been pointed out as a major mechanism of parasite-induced host susceptibility to infection.22 We therefore studied here the possibility that N. caninum could induce a polyclonal B-lymphocyte activation in BALB/c mice, a murine model previously used for the study of neosporosis.9,12,13,17 Our results show that NcT stimulate BALB/c mice B cells. This stimulatory effect was already noticeable early after infection as evidenced by a rapid increase in surface expression of the activation marker CD69 observed on B-cells after in vivo or in vitro stimulation with this parasite. Stimulation of the peripheral B-cell compartment was also evident at later times p.i. where an increase in the numbers of total and immunoglobulin-secreting B cells was also observed. However, the numbers of activated (CD69-expressing) B cells at early times after infection were markedly lower than those observed upon stimulation with the B-cell polyclonal activator LPS or than those induced by other B-cell mitogens of microbial origin33 suggesting that a specific rather than a parasite non-specific B-cell activation was elicited by N. caninum. This is further indicated by a rise in N. caninum-specific serum antibodies detected in NcT-infected mice and not in antibodies specific for Neospora-unrelated antigens. Moreover, in mice infected with Leishmania, higher serum antifungal or antibacterial antibody titres were detected than those observed in N. caninum-infected mice. Together these observations indicate the parasite-specific character of the N. caninum-elicited B-cell response.
Interestingly, and in contrast to peripheral B-cell expansion, a depletion of B220+ IgM+ cells was observed in the bone marrow of NcT-infected mice. Depletion of B-cell precursors in the bone marrow was previously described in other conditions characterized by extensive peripheral B-cell activation34,35 and has been interpreted as an inhibitory ‘feed back’ on B lymphopoiesis caused by peripheral immunoglobulin production.35 However, as the lymphoid depletion observed in the bone-marrow of parasite-infected mice was already noticeable prior to the observed peripheral B-cell expansion, it is unlikely that it could result from a so sudden increase in serum immunoglobulin concentrations. Tumour necrosis factor-α and lymphotoxin-α have been shown to mediate B-cell precursor depletion by an apoptotic mechanism in a murine model of influenza virus infection.36 Therefore, the depletion of B-cell precursors observed at early times after the murine N. caninum infection can thus be explained by a rapid parasite-induced innate response. This is also suggested by the increased number of B-lineage apoptotic cells observed in the bone marrow of the NcT-infected mice 24 hr after the parasitic challenge.
In agreement with previous reports showing that in BALB/c mice a bias towards a Th1-type immune response was observed in the initial weeks after N. caninum infection9,37 we observed a rise in spleen IFN-γ mRNA expression and in IFN-γ serum levels as well as parasite-specific IgG2a antibodies in mice of the same strain infected with 5 × 105 NcT. The Th1 bias is however, apparently abrogated at later times p.i., as higher titres of N. caninum-specific IgG1 than those of IgG2a antibodies with that specificity were detected in mice sera collected at later times p.i.. This result is in agreement with other reports showing the predominance of an IgG1 N caninum-specific antibody response in mice infected with this parasite.9,19 Moreover, the challenging antigen dose could also determine the isotypic profile of the NcT-elicited antibody response since higher serum levels of IgG1 than those of IgG2a parasite-specific antibodies, Th2- and Th1-associated isotypes, respectively, were detected at 15 days p.i. when BALB/c mice were challenged with a 5 × 106 parasite inoculum. Thus in contrast with the preferential IgG2a response elicited by a 10-fold lower inoculum. The polarization of the immune responses towards a Th2 phenotype by high antigen doses has been previously described in other in vivo and in vitro systems.38–40 Antigen-specific IgG immune responses have been shown to depend of costimulatory signals provided by CD80 or CD86 molecules.41 Both these molecules were up-regulated on the surface of splenic B cells on NcT-infected mice and therefore the participation of B cells on the antigen-driven T-cell activation and differentiation in these mice can be hypothesized. This is also suggested by an increase in the numbers of PNAhigh B cells with up-regulated surface expression of MHC class II molecules, a phenotype characteristic of germinal centre B cells42,43 observed in the spleen of NcT-infected mice.
Lack of detected brain lesions in NcT-infected mice could suggest that in the NcT-infected BALB/c mice some degree of protection against this parasite was achieved. However, persistence of N. caninum and latent chronic infection in experimentally infected BALB/c mice has previously been described by others7 and cannot be excluded by histopathological or immunohistochemical examination. The presence of high titres of N. caninum-specific IgG1 antibodies detected in the sera of NcT-infected mice 60 days after the parasitic challenge, a distinct isotype than that predominant at earlier p.i. times, could also reflect parasitic-antigen persistence. Therefore, the lack of easily detectable brain lesions or parasites could merely result from the number of parasites used in the inoculum, too low regarding this aspect. Absence of brain lesions was also reported by others in BALB/c mice infected with 2 × 105 of NcT.37
Previous results showing that B-cell deficient mice are more susceptible to N. caninum infection than normal wild-type counterparts indicate that B cells could have an active role in the protective immune response against neosporosis.10 Our finding as well as those previously reported by others19,44 showing that mice immunized with N. caninum structural antigens are more susceptible to Neospora infection, despite possessing high titres of parasite-specific IgG antibodies, could suggest that a protective role of B cells in murine neosporosis is not antibody mediated. However, it cannot be excluded that an antibody-mediated immune response targeting particular NcT antigens, instead of whole NcT sonicates, may have a protective role against neosporosis. The production of antibodies specific for particular N. caninum antigens has been previously associated with immunoprotection against neosporosis in other immunization studies.45,46 Moreover, because the immunization procedures described here elicited the predominant production of the Th2-associated IgG1 isotype, it could be hypothesized that a Th2 cell-mediated immune response strongly associated with host susceptibility to neosporosis19 could overcame a putative protective role of antibodies. This is further suggested by a decrease in the levels of IFN-γ mRNA expression elicited in the immunized mice challenged with N. caninum comparatively to that detected on sham-immunized controls. A protective role of B cells can however, be explained by stimulation or costimulation of T cells through CD80 or CD86. The interaction of these molecules with CD28 on the surface of T cells has previously been shown to be crucial in the host resistance to Trypanosoma cruzi infection.47 A possible role in host protective mechanisms could also be played by B-1 cells that are transiently expanded in NcT-infected mice. Primed B cells of this lymphocyte population have been shown to promote protective immunity against the apicomplexan parasite Toxoplasma gondii.48 Also, and similarly to our finding in NcT-infected mice, an expansion of splenic B-1 cells, associated with host protection, has previously been described in Plasmodium chabaudi-infected mice.49
On the other hand, it has been reported in several experimental infections that pathogen-specific antibodies could facilitate host colonization thus implying a deleterious effect of B cells in host immunity to several micro-organisms.50–52 Our results suggest that such a mechanism is unlikely to increase the susceptibility of the murine host to N. caninum infection as mice passively transferred with N. caninum-specific antibodies survived a parasite inoculum that was lethal for NcS-imunized mice. However, administration of different antibody preparations containing higher parasite-specific immunoglobulin concentrations or enriched in other IgG isotypes should also be attempted to better evaluate the role played by antibodies in N. caninum infection.
The B-cell stimulatory effect was markedly decreased when BALB/c mice were treated with gamma-irradiated NcT, as shown by a decrease in the numbers of total, immunoglobulin-secreting or costimulatory molecule-expressing splenic B cells observed in irradiated NcT-treated mice as compared to that of mice inoculated with non-irradited NcT. This milder B-cell stimulatory effect of irradiated NcT was however, not qualitatively different from that induced by non-irradiated NcT because no modification on the isotypic profile of the N. caninum-specific antibodies produced in the challenged mice was observed. It can thus be hypothesized that the lower B-cell stimulation obtained when mice were challenged with irradiated NcT can be the result of a lower antigenic load thus indicating that non-irradiated NcT are able to multiply inside BALB/c mice. On the other hand, since IgG2a production was elicited in this host by i.p. inoculation with irradiated NcT this raises the possibility that a Th1-type protective immune response can in this way be elicited in the host by irradiated parasites. Previous reports have shown that γ-irradiated tachyzoites28 or oocysts27 of the apicomplexan parasite Toxoplasma gondii have been used to elicit protective immune responses against murine toxoplasmosis. However, i.d inoculation of irradiated NcT in the absence of adjuvant, as used here for immunization purposes, actually induced lethal susceptibility to non-irradiated NcT challenge in the immunized mice, as well as a reduction in the levels of spleen IFN-γ mRNA expression, comparatively to sham-immunized mice. Impairment of host protective immune mechanisms against S. mansoni by previous immunization has been also reported and shown to depend on the antigen administration route.53 Moreover, the increased susceptibility to N. caninum observed in mice immunized i.d. with irradiated NcT, is in apparent agreement with a previous report showing that immunization of mice with N. caninum lysate, also without adjuvant, elicited an immune response more skewed towards the Th2 type44 associated with susceptibility to this parasite.12–14 However, since no clear switch towards the preferential production of IgG1 over that of IgG2a antibodies was induced by the i.d. immunization with irradiated NcT, other mechanisms, than just a mere Th2 switch, could be hypothesized. Immunization-induced transforming growth factor-β production has been previously implicated in the suppression of protective immune responses to S. mansoni.53 It will thus be worth investigate the possibility that specific N. caninum antigens could directly promote the induction of host immune responses ultimately involved in successful host colonization.
Acknowledgments
The authors are indebted to Dr Rui Appelberg for critically reviewing this manuscript, to Fátima Faria, Célia Lopes and Alexandra Rema for their excellent technical assistance in the immunohistochemistry procedures and to Dr Gil Castro and Dr Helena Sofia Domingues for their help and supervision on quantitative PCR experiments. This work and Carla Sofia Meireles fellowship were supported by Fundação para a Ciência e a Tecnologia (FCT), grant n° POCTI/CVT/38791/MGI/2001 and FEDER. Luzia Teixeira is financed by FCT fellowship SFRH/BD/12983/2003.
References
- 1.Bjerkås I, Mohn SF, Presthus J. Unidentified cyst-forming sporozoan causing encephalomyelitis and myositis in dogs. Z Parasitenkd. 1984;70:271–4. doi: 10.1007/BF00942230. 10.1007/BF00942230. [DOI] [PubMed] [Google Scholar]
- 2.Dubey JP. Review of Neospora caninum and neosporosis in animals. Korean J Parasitol. 2003;41:1–16. doi: 10.3347/kjp.2003.41.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hemphill A. The host–parasite relationship in neosporosis. Adv Parasitol. 1999;43:47–104. doi: 10.1016/s0065-308x(08)60241-9. [DOI] [PubMed] [Google Scholar]
- 4.Barr BC, Rowe JD, Sverlow KW, BonDurant RH, Ardans AA, Oliver MN, Conrad PA. Experimental reproduction of bovine fetal Neospora infection and death with a bovine Neospora isolate. J Vet Diagn Invest. 1994;6:207–15. doi: 10.1177/104063879400600212. [DOI] [PubMed] [Google Scholar]
- 5.Dubey JP, Lindsay DS. Gerbils (Meriones unguiculatus) are highly susceptible to oral infection with Neospora caninum oocysts. Parasitol Res. 2000;86:165–8. doi: 10.1007/s004360050027. 10.1007/s004360050027. [DOI] [PubMed] [Google Scholar]
- 6.Lindsay DS, Dubey JP. Neospora caninum (Protozoa: apicomplexa) infections in mice. J Parasitol. 1989;75:772–9. [PubMed] [Google Scholar]
- 7.Lindsay DS, Lenz SD, Cole RA, Dubey JP, Blagburn BL. Mouse model for central nervous system Neospora caninum infections. J Parasitol. 1995;81:313–5. 10.1006/expr.1995.1122. [PubMed] [Google Scholar]
- 8.Trees AJ, Davison HC, Innes EA, Wastling JM. Towards evaluating the economic impact of bovine neosporosis. Int J Parasitol. 1999;29:1195–200. doi: 10.1016/s0020-7519(99)00093-4. 10.1016/S0020-7519(99)00093-4. [DOI] [PubMed] [Google Scholar]
- 9.Baszler TV, Long MT, McElwain TF, Mathison BA. Interferon-γ and interleukin-12 mediate protection to acute Neospora caninum infection in BALB/c mice. Int J Parasitol. 1999;29:1635–46. doi: 10.1016/s0020-7519(99)00141-1. [DOI] [PubMed] [Google Scholar]
- 10.Eperon S, Bronnimann K, Hemphill A, Gottstein B. Susceptibility of B-cell deficient C57BL/6 (microMT) mice to Neospora caninum infection. Parasite Immunol. 1999;21:225–36. doi: 10.1046/j.1365-3024.1999.00223.x. 10.1046/j.1365-3024.1999.00223.x. [DOI] [PubMed] [Google Scholar]
- 11.Khan IA, Schwartzman JD, Fonseka S, Kasper LH. Neospora caninum: role for immune cytokines in host immunity. Exp Parasitol. 1997;85:24–34. doi: 10.1006/expr.1996.4110. 10.1006/expr.1996.4110. [DOI] [PubMed] [Google Scholar]
- 12.Long MT, Baszler TV. Neutralization of maternal IL-4 modulates congenital protozoal transmission: comparison of innate versus acquired immune responses. J Immunol. 2000;164:4768–74. doi: 10.4049/jimmunol.164.9.4768. [DOI] [PubMed] [Google Scholar]
- 13.Long MT, Baszler TV, Mathison BA. Comparison of intracerebral parasite load, lesion development, and systemic cytokines in mouse strains infected with Neospora caninum. J Parasitol. 1998;84:316–20. [PubMed] [Google Scholar]
- 14.Nishikawa Y, Inoue N, Makala L, Nagasawa H. A role for balance of interferon-gamma and interleukin-4 production in protective immunity against Neospora caninum infection. Vet Parasitol. 2003;116:175–84. doi: 10.1016/j.vetpar.2003.07.001. 10.1016/j.vetpar.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Nishikawa Y, Tragoolpua K, Inoue N, Makala L, Nagasawa H, Otsuka H, Mikami T. In the absence of endogenous gamma interferon, mice acutely infected with Neospora caninum succumb to a lethal immune response characterized by inactivation of peritoneal macrophages. Clin Diagn Lab Immunol. 2001;8:811–6. doi: 10.1128/CDLI.8.4.811-817.2001. 10.1128/CDLI.8.4.811-817.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ritter DM, Kerlin R, Sibert G, Brake D. Immune factors influencing the course of infection with Neospora caninum in the murine host. J Parasitol. 2002;88:271–80. doi: 10.1645/0022-3395(2002)088[0271:IFITCO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka T, Hamada T, Inoue N, Nagasawa H, Fujisaki K, Suzuki N, Mikami T. The role of CD4 (+) or CD8 (+) T cells in the protective immune response of BALB/c mice to Neospora caninum infection. Vet Parasitol. 2000;90:183–91. doi: 10.1016/s0304-4017(00)00238-7. 10.1016/S0304-4017(00)00238-7. [DOI] [PubMed] [Google Scholar]
- 18.Staska LM, McGuire TC, Davies CJ, Lewin HA, Baszler TV. Neospora caninum-infected cattle develop parasite-specific CD4+ cytotoxic T lymphocytes. Infect Immun. 2003;71:3272–9. doi: 10.1128/IAI.71.6.3272-3279.2003. 10.1128/IAI.71.6.3272-3279.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baszler TV, McElwain TF, Mathison BA. Immunization of BALB/c mice with killed Neospora caninum tachyzoite antigen induces a type 2 immune response and exacerbates encephalitis and neurological disease. Clin Diagn Lab Immunol. 2000;7:893–8. doi: 10.1128/cdli.7.6.893-898.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang H, Remington JS, Suzuki Y. Decreased resistance of B cell-deficient mice to infection with Toxoplasma gondii despite unimpaired expression of IFN-gamma, TNF-alpha, and inducible nitric oxide synthase. J Immunol. 2000;164:2629–34. doi: 10.4049/jimmunol.164.5.2629. [DOI] [PubMed] [Google Scholar]
- 21.Sayles PC, Gibson GW, Johnson LL. B cells are essential for vaccination-induced resistance to virulent Toxoplasma gondii. Infect Immun. 2000;68:1026–33. doi: 10.1128/iai.68.3.1026-1033.2000. 10.1128/IAI.68.3.1026-1033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minoprio P. Parasite polyclonal activators: new targets for vaccination approaches? Int J Parasitol. 2001;31:588–91. doi: 10.1016/s0020-7519(01)00171-0. [DOI] [PubMed] [Google Scholar]
- 23.Hsu SM, Raine L, Fanger H. The use of antividin antibody and avidin–biotin–peroxidase complex in immunoperoxidase techniques. Am J Clin Pathol. 1981;75:816–21. doi: 10.1093/ajcp/75.6.816. [DOI] [PubMed] [Google Scholar]
- 24.Lindsay DS, Dubey JP. Immunohistochemical diagnosis of Neospora caninum in tissue sections. Am J Vet Res. 1989;50:1981–3. [PubMed] [Google Scholar]
- 25.Czerkinsky CC, Nilsson LA, Nygren H, Ouchterlony O, Tarkowski A. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J Immunol Meth. 1983;65:109–21. doi: 10.1016/0022-1759(83)90308-3. [DOI] [PubMed] [Google Scholar]
- 26.Reina-San-Martin B, Cosson A, Minoprio P. Lymphocyte polyclonal activation: a pitfall for vaccine design against infectious agents. Parasitol Today. 2000;16:62–7. doi: 10.1016/s0169-4758(99)01591-4. 10.1016/S0169-4758(99)01591-4. [DOI] [PubMed] [Google Scholar]
- 27.Dubey JP, Jenkins MC, Thayer DW, Kwok OC, Shen SK. Killing of Toxoplasma gondii oocysts by irradiation and protective immunity induced by vaccination with irradiated oocysts. J Parasitol. 1996;82:724–7. [PubMed] [Google Scholar]
- 28.Hiramoto RM, Galisteo AJ, do Nascimento N, de Andrade HF., Jr 200 Gy sterilised Toxoplasma gondii tachyzoites maintain metabolic functions and mammalian cell invasion, eliciting cellular immunity and cytokine response similar to natural infection in mice. Vaccine. 2002;20:2072–81. doi: 10.1016/s0264-410x(02)00054-3. 10.1016/S0264-410X(02)00054-3. [DOI] [PubMed] [Google Scholar]
- 29.Colle JH, Truffa-Bachi P, Chedid L, Modabber F. Lack of general immunosuppression during visceral Leishmania tropica infection in BALB/c mice: augmented antibody response to thymus-independent antigens and polyclonal activation. J Immunol. 1983;131:1492–5. [PubMed] [Google Scholar]
- 30.Cordeiro-Da-Silva A, Borges MC, Guilvard E, Ouaissi A. Dual role of the Leishmania major ribosomal protein S3a homologue in regulation of T- and B-cell activation. Infect Immun. 2001;69:6588–96. doi: 10.1128/IAI.69.11.6588-6596.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lohoff M, Matzner C, Rollinghoff M. Polyclonal B-cell stimulation by L3T4+ T cells in experimental leishmaniasis. Infect Immun. 1988;56:2120–4. doi: 10.1128/iai.56.8.2120-2124.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klinman DM. Cross-reactivity of IgM-secreting B cells from normal BALB/c mice. J Immunol. 1992;149:3569–73. [PubMed] [Google Scholar]
- 33.Vilanova M, Tavares D, Ferreira P, Oliveira L, Nóbrega A, Appelberg R, Arala-Chaves M. Role of monocytes in the up-regulation of the early activation marker CD69 on B and T murine lymphocytes induced by microbial mitogens. Scand J Immunol. 1996;43:155–63. doi: 10.1046/j.1365-3083.1996.d01-25.x. 10.1046/j.1365-3083.1996.d01-25.x. [DOI] [PubMed] [Google Scholar]
- 34.Lima M, Portnoi D, Bandeira A, Arala-Chaves M. Peripheral lymphoid hyperplasia and central lymphoid depletion in mice treated with a bacterial B-cell mitogen (F3′EP-Si/p90) Scand J Immunol. 1993;37:605–14. doi: 10.1111/j.1365-3083.1993.tb02579.x. [DOI] [PubMed] [Google Scholar]
- 35.Sundblad A, Coutinho A. B-lineage cell deficits in bone marrow of lpr/lpr mice. Int Immunol. 1996;8:247–54. doi: 10.1093/intimm/8.2.247. [DOI] [PubMed] [Google Scholar]
- 36.Sedger LM, Hou S, Osvath SR, Glaccum MB, Peschon JJ, van Rooijen N, Hyland L. Bone marrow B cell apoptosis during in vivo influenza virus infection requires TNF-alpha and lymphotoxin-alpha. J Immunol. 2002;169:6193–201. doi: 10.4049/jimmunol.169.11.6193. [DOI] [PubMed] [Google Scholar]
- 37.Shibahara T, Kokuho T, Eto M, Haritani M, Hamaoka T, Shimura K, Nakamura K, Yokomizo Y, Yamane I. Pathological and immunological findings of athymic nude and congenic wild type BALB/c mice experimentally infected with Neospora caninum. Vet Pathol. 1999;36:321–7. doi: 10.1354/vp.36-4-321. 10.1354/vp.36-4-321. [DOI] [PubMed] [Google Scholar]
- 38.Bretscher PA, Wei G, Menon JN, Bielefeldt-Ohmann H. Establishment of stable, cell-mediated immunity that makes ‘susceptible’ mice resistant to Leishmania major. Science. 1992;257:539–42. doi: 10.1126/science.1636090. [DOI] [PubMed] [Google Scholar]
- 39.Hosken NA, Shibuya K, Heath AW, Murphy KM, O'Garra A. The effect of antigen dose on CD4+ T helper cell phenotype development in a T cell receptor-β-transgenic model. J Exp Med. 1995;182:1579–84. doi: 10.1084/jem.182.5.1579. 10.1084/jem.182.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parish CR. The relationship between humoral and cell-mediated immunity. Transplant Rev. 1972;13:35–66. doi: 10.1111/j.1600-065x.1972.tb00059.x. [DOI] [PubMed] [Google Scholar]
- 41.Borriello F, Sethna MP, Boyd SD, et al. B7–1 and B7–2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity. 1997;6:303–13. doi: 10.1016/s1074-7613(00)80333-7. [DOI] [PubMed] [Google Scholar]
- 42.Butcher EC, Rouse RV, Coffman RL, Nottenburg CN, Hardy RR, Weissman IL. Surface phenotype of Peyer's patch germinal center cells: implications for the role of germinal centers in B cell differentiation. J Immunol. 1982;129:2698–707. [PubMed] [Google Scholar]
- 43.Kosco-Vilbois MH, Gray D, Scheidegger D, Julius M. Follicular dendritic cells help resting B cells to become effective antigen-presenting cells. induction of B7/BB1 and upregulation of major histocompatibility complex class II molecules. J Exp Med. 1993;178:2055–66. doi: 10.1084/jem.178.6.2055. 10.1084/jem.178.6.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lunden A, Wright S, Allen JE, Buxton D. Immunisation of mice against neosporosis. Int J Parasitol. 2002;32:867–76. doi: 10.1016/s0020-7519(02)00024-3. 10.1016/S0020-7519(02)00024-3. [DOI] [PubMed] [Google Scholar]
- 45.Cannas A, Naguleswaran A, Muller N, Gottstein B, Hemphill A. Reduced cerebral infection of Neospora caninum-infected mice after vaccination with recombinant microneme protein NcMIC3 and ribi adjuvant. J Parasitol. 2003;89:44–50. doi: 10.1645/0022-3395(2003)089[0044:RCIONC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 46.Nishikawa Y, Xuan X, Nagasawa H, Igarashi I, Fujisaki K, Otsuka H, Mikami T. Prevention of vertical transmission of Neospora caninum in BALB/C mice by recombinant vaccinia virus carrying Ncsrs2 gene. Vaccine. 2001;19:1710–6. doi: 10.1016/s0264-410x(00)00407-2. 10.1016/S0264-410X(00)00407-2. [DOI] [PubMed] [Google Scholar]
- 47.Miyahira Y, Katae M, Kobayashi S, Takeuchi T, Fukuchi Y, Abe R, Okumura K, Yagita H, Aoki T. Critical contribution of CD28-CD80/CD86 costimulatory pathway to protection from Trypanosoma cruzi infection. Infect Immun. 2003;71:3131–7. doi: 10.1128/IAI.71.6.3131-3137.2003. 10.1128/IAI.71.6.3131-3137.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen M, Mun HS, Piao LX, et al. Induction of protective immunity by primed B-1 cells in Toxoplasma gondii-infected B cell-deficient mice. Microbiol Immunol. 2003;47:997–1003. doi: 10.1111/j.1348-0421.2003.tb03460.x. [DOI] [PubMed] [Google Scholar]
- 49.Yoder BJ, Goodrum KJ. Plasmodium chabaudi chabaudi B-1 cell expansion correlates with semiresistance in BALB/Cj mice. Exp Parasitol. 2001;98:71–82. doi: 10.1006/expr.2001.4622. 10.1006/expr.2001.4622. [DOI] [PubMed] [Google Scholar]
- 50.Casadevall A. Antibody immunity and invasive fungal infections. Infect Immun. 1995;63:4211–8. doi: 10.1128/iai.63.11.4211-4218.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mahalingam S, Lidbury BA. Antibody-dependent enhancement of infection: bacteria do it too. Trends Immunol. 2003;24:465–7. doi: 10.1016/s1471-4906(03)00210-2. 10.1016/S1471-4906(03)00210-2. [DOI] [PubMed] [Google Scholar]
- 52.Suhrbier A, La Linn M. Suppression of antiviral responses by antibody-dependent enhancement of macrophage infection. Trends Immunol. 2003;24:165–8. doi: 10.1016/s1471-4906(03)00065-6. 10.1016/S1471-4906(03)00065-6. [DOI] [PubMed] [Google Scholar]
- 53.Williams ME, Caspar P, Oswald I, Sharma HK, Pankewycz O, Sher A, James SL. Vaccination routes that fail to elicit protective immunity against Schistosoma mansoni induce the production of TGF-beta which down-regulates macrophage antiparasitic activity. J Immunol. 1995;154:4693–700. [PubMed] [Google Scholar]