Abstract
Chemokines are a large family of small, generally secreted polypeptides which guide lymphocyte movement throughout the body by controlling integrin avidity and inducing migration. Here, we look at recent, exciting findings on chemokine function throughout lymphocyte development and co-ordinated T and B cell migration during immune responses. Finally, we will review data on the regional control of immunity by tissue-specific chemokine receptors on effector/memory lymphocytes.
Keywords: chemokines, integrin activation, lymphocytes, migration, secondary lymphoid organs
Lymphocyte trafficking and chemokines
Lymphocytes are the mammalian cell type migrating by far the largest distances in their considerable life span.1,2 After undergoing a selection process in primary lymphoid organs, naive lymphocytes continually home from blood to secondary lymphoid organs (SLO), such as peripheral and mesenteric lymph nodes (PLN and MLN, respectively), spleen and gut-associated lymphoid tissue including Peyer's patches (PP). Inside SLO, T and B cells localize in T cell area and B cell follicles, respectively, where they screen antigen (Ag)-presenting cells for specific surface Ag complexes. Upon activation with cognate Ag in presence of costimulatory molecules, T and B cells undergo specific changes in microenvironmental positioning. These changes allow T–B cell interactions at the T cell area–B cell follicle border and in germinal centre (GC) light zones to occur.3–6 Activated T and B cells eventually leave SLO to accumulate at sites of inflammation or other effector sites.4,7 Activation of naive T cells in PP results in selective imprinting of gut tropism of effector cells, whereas activation in PLN gives rise to skin homing T cells.8 After immune responses, various long-lived memory T cell subsets patrol the body showing tissue-selective recirculation pathways through gut, skin or SLO.8,9
Chemokines constitute a large family of small (8–12 kDa), structurally related polypeptides which, together with adhesion receptors, orchestrate all lymphocyte migration described above. Chemokines can be considered organizing factors of the immune system and co-ordinate microenvironmental architecture of primary and SLO during physiological and pathological conditions. Chemokines exert their functions by binding specific Gαi-protein coupled chemokine receptors (CKR) on the lymphocyte surface (Table 1). The extraordinary diversity of chemokine function is due principally to two effects triggered by chemokine binding to its cognate receptor. First, chemokines induce directed migration of lmphocytes in subset-specific manner in in vitro migration assays. Within tissue, chemokines induce T and B lymphocyte separation into physically distinct microenvironments such as T and B cell areas where subset-attracting chemokines are expressed.3 It is currently unclear whether chemokines induce separation along a chemotactic (i.e. soluble) or haptotactic (i.e. surface-bound) gradient, or whether chemokines promote mainly random migration (chemokinesis) and retention of cells in their specific microenvironment.10,11 In support of the latter, multiphoton intravital images of mouse lymph nodes revealed apparently random migration patterns of lymphocytes within their specific microenvironments.11,12 In addition, chemokine gradients between adjacent microenvironments have thus far not been well characterized.11 Future experiments including a careful quantitative analysis of chemokine distribution in tissue sections and multiphoton observation of Ag-specific cell migration at the T cell area–B cell follicle border (see below) should shed light on this important issue.
Table 1.
Mouse chemokines and their receptors. Chemokines can be classified into two major subfamilies differing in the position of two of four highly conserved cysteines. In CXC chemokines, the two N-terminal cysteines are separated by a single amino acid, whereas in CC chemokines these two cysteines are not separated. Exceptions to this classification are chemokines that lack two of the four mentioned cysteines (XC chemokines) and CX3CL1, in which three amino acids separate both cysteines. Alternatively, chemokines can be divided according to their expression patterns. Homeostatic chemokines, for example CCL21 and CXCL13, are expressed constitutively in secondary lymphoid organs, whereas inflammatory chemokines, such as CXCL9/10, are often associated with inflamed tissue.86 No mouse homologues have been found so far for the human chemokines CCL13, CCL14, CCL15, CCL18, CCL23, CXCL8 and the receptors CXCR1 and CXCR3b. Interactions between chemokines and their CKR which are inferred from the human system but not tested directly in the murine system are not shown in this table
| Chemokine | Receptor |
|---|---|
| CC chemokine/receptor family | |
| CCL1 | CCR8 |
| CCL2 | CCR2 |
| CCL3 | CCR1, CCR5 |
| CCL4 | CCR5 |
| CCL5 | CCR1, CCR3, CCR5 |
| CCL61 | CCR1? |
| CCL7 | CCR1 |
| CCL8 | CCR3 |
| CCL91 | CCR1 |
| CCL101 | Unknown |
| CCL11 | CCR3 |
| CCL121 | CCR2 |
| CCL16 | CCR1 |
| CCL17 | CCR4 |
| CCL19 | CCR7 |
| CCL20 | CCR6 |
| CCL21 | CCR7 |
| CCL22 | CCR4 |
| CCL24 | CCR3 |
| CCL25 | CCR9 |
| CCL26 | CCR3 |
| CCL27 | CCR10 |
| CCL28 | CCR3, CCR10 |
| CXC chemokine/receptor family | |
| CXCL1 | CXCR2 |
| CXCL2 | CXCR2 |
| CXCL3 | CXCR2 |
| CXCL4 | Unknown |
| CXCL5 | CXCR2 |
| CXCL6 | CXCR2 |
| CXCL7 | CXCR2 |
| CXCL9 | CXCR3 |
| CXCL10 | CXCR3 |
| CXCL11 | CXCR3 |
| CXCL12 | CXCR4 |
| CXCL13 | CXCR5 |
| CXCL14 | Unknown |
| CXCL151 | Unknown |
| CXCL16 | CXCR6 |
| XC chemokine/receptor family | |
| XCL1 | XCR1 |
| XCL2 | XCR1 |
| CX3C Chemokine/receptor family | |
| CX3CL1 | CX3CR1 |
Mouse chemokines with no known human homologues.
Secondly, in addition to migration, chemokines rapidly increase binding avidity of integrin adhesion receptors by inside-out signalling pathways.13,14 Integrin activation usually implies conformational changes, which increase binding avidity to members of the Ig superfamily receptors.15 This function of chemokines is absolutely required to arrest blood-borne lymphocytes inside postcapillary venules of SLO or non-lymphoid tissue. Under physiological conditions, integrin activation appears restricted to selected chemokines presented on the luminal side of blood vessels.16 Lymphocytes express mainly members of the α4 and β2 integrin families. Simplistically, the α4β7 integrin mediates gut tropism by binding MAdCAM-1, whereas the β2 integrin LFA-1 plays a more universal role for cell adhesion. The α4β1, or VLA-4, integrin, is required for adhesion to bone marrow (BM) vessels.4,17 In some cases, migration and integrin activation are interdependent events. As an example, integrins are required for interstitial B cell migration within spleen,18 although in in vitro assays lymphocyte migration can occurr in absence of integrin ligands.
In this review, we will focus on chemokines controlling lymphocyte migration and integrin activation in primary and SLO. Furthermore, we will discuss chemokine regulation of lymphocyte migration within SLO during immune responses, and towards extralymphoid sites during the effector phase. Finally, exciting recent findings of specialization of memory lymphocyte migration will be revised. Due to space constraints, this review can none the less provide only a limited overview over the numerous pathways by which chemokines organize the adaptive immune system.
Migration within primary lymphoid organs
Primary lymphoid organs − fetal liver during development versus thymus and BM in adult animals − are the sites of lymphocyte production. CKR expression on lymphocyte precursors within thymus and BM correlates with chemokine expression patterns and, especially in thymus, clearly defined microenvironments.19 Here, we review the evidence for chemokine regulation of lymphocyte development.
T cell migration into, within and out of thymus: potential roles for CXCR4, CCR7 and CCR9
During embryogenesis, blood-borne prethymocytes derived from hematopoietic stem cells first colonize the thymus primordium at embryonic day 11·5 (E11·5) in mice and after 7–8 weeks of gestation in humans.20,21 Prethymocytes colonize fetal thymus prior to organ vascularization (which takes place between E13·5 and E15·521,22), possibly via blood vessels adjacent to the thymus anlage. In plt/plt mice, a naturally occurring mutant mouse strain lacking expression of CCL19 and a SLO-expressed isoform of CCL21, and in CCR7–/– mice, fetal thymus colonization at early embryonic stages is delayed as reflected in a decrease of total thymocyte numbers.21 CCR9-deficient mice display a similar delay in thymic cell numbers on E13·5–E18·5.22,23 Of note, mice lacking CCR9 do not have a defect in precursor cell numbers at E11·5, the earliest time-point these cells can be detected in fetal thymi.22 This is consistent with the observation that thymic expression of the CCR9 ligand CCL25 is detected only from day E12·5 onwards.21 It is currently unclear whether the approximately 10-fold increase of precursors between E11·5 and 12·5 is due to massive proliferation of precursors that homed to thymus on E11·5, or a second wave of immigrant precursors on E12·5 mediated by CCL25, or both.21–23 In any case, at later time-points (approximately E18·5 onwards), thymic cellularity in absence of CCR7 or CCR9 is again comparable to control thymi, due perhaps to increased proliferation. Fetal thymocytes also migrate in vitro to CXCL12;24 nevertheless, CXCL12 and CXCR4 are not needed for thymic colonization.25
Interstitial thymocyte migration in adult animals
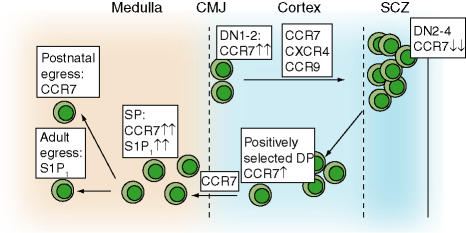
In adult animals, BM-derived prethymocytes enter thymus through blood vessels located at the cortical–medullary junction (CMJ). The factors inducing thymic recruitment of these rare blood-borne precursors are not well defined but may include CCR9 ligands.26 From the CMJ, precursors migrate into the subcortical zone (SCZ) for T cell receptor (TCR)β chain rearrangement (Fig. 1). Recent data has shown that CCR7 is required for efficient accumulation of recent thymic immigrants in the SCZ.27 CCR7 is highly expressed in early thymocytes displaying an intermediate phenotype between CD4/8-double negative (DN) 1 and DN2, termed DN1-2.27 In plt/plt or CCR7-deficient mice, DN1-2 cells accumulate at the CMJ while DN3/4 cellularity is decreased.27
Figure 1.
CKR expression during postnatal thymocyte maturation. Blood-borne immature thymocyte precursors enter thymus by poorly defined adhesion pathways in blood vessels at the corticomedullar junction (CMJ). At least three CKR (CCR7, CXCR4, CCR9) were described independently to mediate migration and/or retention in the subcortical zone (SCZ), where massive proliferation takes place. Positively selected DP cells increase CCR7 expression, which allows translocation to the cortex. SP thymocytes exit thymus via CCR7- or S1P1-dependent pathways to join the peripheral T cell pool. Despite multiple chemokine–CKR interactions during thymocyte development, no single CKR has been found thus far to be indispensable for T cell generation. Thymocytes and mature T cells are labelled green in this and subsequent figures. Please refer to text for more details. Adapted from Witt and Robey.30 DN: double negative cells; DP: double positive cells; SP: single positive cells.
Positively selected DP thymocytes reinduce CCR7 expression, which is required for efficient migration into thymic medulla. Overexpression of CCR7 induces premature migration of DP cells into medulla28 and CCR7-deficient single positive (SP) thymocytes appear inside cortex instead of medulla.29 A point deserving further investigation is how the same CKR, CCR7, mediates migration in two opposite directions, from SCZ to CMJ and from cortex to medulla, at different stages of thymocyte development. CCL21 was suggested to have a chemorepulsive, rather than chemotactic, effect on DN1-2 thymocytes;30 however, this needs to be addressed experimentally.
In mice reconstituted with CXCR4-deficient fetal liver cells containing thymic precursors, thymocyte development is not strongly impaired.31 In postnatal thymocyte precursors carrying a thymocyte-specific deletion of CXCR4, however, progenitors do not develop further than the DN1 stage and accumulate at the CMJ.32 The authors linked the failure of CXCR4–/– thymocytes to progress beyond DN1 stage to the failed migration to the SCZ, where niches may provide survival factors, such as IL-7, to early progenitors.32 CCR9-deficient immature thymocytes, on the other hand, do not accumulate at the SCZ but rather distribute randomly across the thymic cortex.22 Despite impaired SCZ localization, thymocyte proliferation and maturation into SP cells appeared largely intact in these mice. CCR9 thus acts as an attraction/retention factor for SCZ localization whereas homing to this microenvironment is not a prerequisite for thymic development.22 It is possible that the impaired maturation of CXCR4–/– postnatal thymocytes is due to lack of survival signals transmitted via this CKR, as observed for human T cell precursors.33
Positively selected SP thymocytes exit thymus to join the peripheral naive T cell pool. Recent studies identified two receptors involved in thymocyte emigration into periphery. In newborn mice, CCL19–CCR7 interactions are involved in thymocyte egress.34 The sphingosine-1-phosphate (S1P) receptor 1 (S1P1), a Gαi-protein-coupled receptor highly expressed in SP thymocytes and naive lymphocytes, is playing an essential role for egress in adult animals.35 S1P1 is also central to lymphocyte egress from SLO into efferent lymphatic vessels, highlighting a double role for this receptor for efficient T cell exit from thymus and SLO.
In summary, chemokines clearly shape thymocyte localization and three-dimensional tissue architecture. Overall effects on thymocyte development in absence of single chemokines or their receptors are none the less rather mild in most cases. Mice deficient in multiple chemokines or their receptors should uncover potential redundancy in chemokine usage during thymic T cell development.
Chemokines during B cell development: a central role for CXCR4
During embryogenesis, B cell development takes place in fetal liver. B cell production in postnatal mice is confined to BM where pre–pro B cells develop into pro-, pre- and immature B cells, which are released into the bloodstream. CXCR4 plays a central role during B cell development as lack of this receptor or its ligand CXCL12 severely blocks B lymphopoiesis.36–38 Subsequent studies identified a requirement for CXCL12 for the development of the earliest identifiable B cell precursors in fetal liver and BM.39
In addition, CXCR4-mediated signals prevent premature release of myeloid and B cell precursors from BM microenvironment. As a consequence, CXCR4–/– precursors are found in increased numbers in blood.31 CXCR4 thus translates a retention signal to early precursors while immature B cells somehow lose this signal despite similar CXCR4 expression. Silberstein and colleagues showed that B cell precursors translate CXCR4 signals differently to integrins compared to immature and naive B cells.40 Whereas chemokine-induced integrin activation in the latter is short-lived and decreases after few minutes, pre- and pro-B cells activate α4 integrins for a much longer time.40 These studies thus provide an elegant explanation for the long-term retention of precursors in BM, and identify a change in molecular wiring of CXCR4 signal transduction during transition from sessile precursors to recirculating B cells.
B cell precursors are characterized, furthermore, by defined patterns of CKR expression and chemokine responsiveness.41 Pre–pro B cells migrate uniquely to CCL25.41 Although CCR9-deficient mice show a reduction in the number of this subtype in BM, overall B cell lymphopoiesis is only mildly affected in these animals, excluding a central role for CCL25–CCR9 during B cell development.23 Responsiveness for SLO-associated chemokines (CCL19, CCL21, CXCL13) increases during B cell maturation from pro- to pre- to immature B cells, with CCR7 ligand responsiveness appearing prior to CXCR5 ligand migration.41
Recent BM emigrants home to spleen where they accumulate at the T–B cell border. Reduced CXCR5 expression in immature B cells is likely to be the reason for their accumulation at the edge of spleen B cell follicles.41 After receiving appropriate survival signals required for entry into the follicular B cell pool,42 these cells increase CXCR5 expression which allows their migration into CXCL13-expressing B cell follicles.41
Chemokines during naive lymphocyte trafficking: CXCL12, CCL19/21 and CXCL13
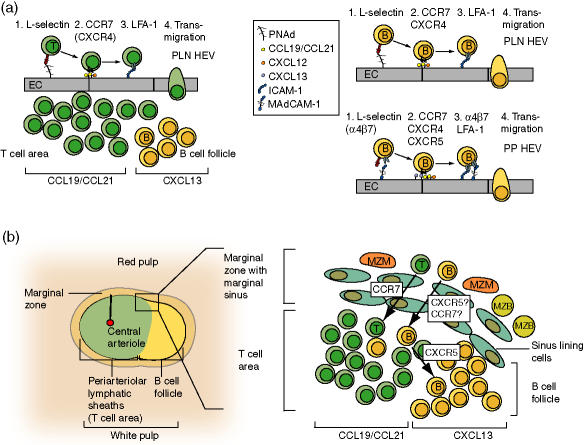
Positively selected naive T and B cells home specifically to SLO. B cells generally recirculate less avidly through SLO, although their homing to gut-associated lymphoid tissue is still relatively efficient.17 The principal port of entry of blood-borne lymphocytes to PLN, MLN and PP (but not spleen) are specialized postcapillary venules, the so-called high endothelial venules (HEV).4,17 Lymphocyte adhesion follows a multistep ‘cross-talk’ with counter-receptors on high endothelial cells (Fig. 2a). First, cells tether and roll before they become firmly adherent prior to transmigration. l-Selectin and β2/α4 integrins on lymphocytes are key adhesion receptors mediating lymphocyte adhesion in HEV. Because the majority of leucocytes shares these receptors but does not usually enter lymphoid organs, lymphocyte tropism depends on endothelium-presented lymphocyte-specific chemokines, which are causing selective integrin activation of lymphocytes but not other leucocytes inside HEV. For T cells, the CCR7 ligands CCL21 and, to a minor extent, CCL19 are the principal integrin activating chemokines found on the luminal side of SLO venules (Fig. 2a).4 CXCL12 also participates to a small extent during T cell adhesion to SLO venules.43 CXCL12–CXCR4 interactions might be especially relevant for memory T cells, some of which retain a certain capacity to recirculate through SLO.44
Figure 2.
Chemokines during physiological lymphocyte homing to secondary lymphoid organ. (a) Multistep adhesion pathways of T and B cells in the microcirculation of peripheral lymph nodes and Peyer's patches microcirculation. After l-selectin-mediated tethering and rolling of blood-borne T cells along peripheral node addressin (PNAd), endothelium-presented CCL21 and CCL19 bind CCR7 on rolling cells and rapidly trigger signals that are transmitted to integrins such as LFA-1. CKR-mediated signals induce conformational changes on integrins, resulting in affinity up-regulation and firm adhesion to ICAM-1 and-2. Adherent T cells transmigrate into the surrounding lymphoid tissue and accumulate in the T cell area, attracted by CCR7 ligands expressed in this microenvironment. B cell (labelled yellow in this and subsequent figures) adhesion in PLN venules additionally relies on CXCR4- and potentially CXCR5-dependent signals. Firm lymphocyte arrest in PP venules involves α4β7 integrin–MAdCAM−1 interactions during rolling and firm arrest, and in the case of B cells, CXCR5. After transmigration, B cells accumulate in CXCL13-expressing B cell follicles. (b) Lymphocyte homing to spleen white pulp. T and B cells are released in sinuses located in the marginal zone and red pulp, from where they are attracted via G-protein-dependent signals towards the T cell area. In the case of T cells, this signal probably derives from CCR7 ligands, whereas in the case of B cells, both CCR7 and CXCR5 ligands might contribute to this migration. After a short delay, B cells continue their migration into CXCL13-expressing B cell follicles, whereas T cells are held back in the T cell area. Please refer to text for more details. EC: endothelial cells; LFA-1: lymphocyte function-associated molecule-1, αLβ2; PNAd: peripheral node addressin; ICAM-1: intracellular adhesion molecule-1; MAdCAM-1: mucosal adhesion cellular adhesion molecule-1; MZM: marginal zone macrophages; MZB: marginal zone B cells.
Chemokine requirements for B cell entry into SLO differ slightly from T cells. B cell integrin activation relies on both CCR7 and CXCR4 in PLN HEV.43 CXCR5 and its ligand CXCL13 are additionally involved in integrin activation in PP HEV (Fig. 2a) and B cell homing to MLN.43,45 CXCL13 is also found by immunohistology on most PLN HEV, but its physiological significance is as yet unclear.45 Interestingly, PP HEV display subset preference during lymphocyte recruitment: whereas T cells arrest predominantly in T cell area-associated PP HEV, B cells accumulate preferentially in follicle-associated HEV.43,46
Arrested lymphocytes cross the endothelial lining and transmigrate into underlying lymphoid tissue by poorly characterized mechanisms which may depend, at least in part, on shear forces exerted by blood.12,47 CCL19 and CCL21 expressed in the T cell area attract or retain T cells in this microenvironment, whereas B cells accumulate in B cell follicles, where CXCL13 is produced by FDC (Fig. 2a).3 Importantly, a subset of CD4 cells also expresses CXCR5 which allows them to migrate towards B cell follicles and provide B cell help (see below). In PP, CXCR4–/– B cells home to B cell follicles but were also found in adjacent lamina propria, arguing for a role of CXCR4 in B cell retention inside follicles.48
In contrast to HEV-containing SLO, T and B cells entering spleen are passively released in sinuses of the marginal zone (MZ) and red pulp (Fig. 2b). From there, lymphocytes are attracted by CCR7 ligands, and possibly other CKR such as CXCR5, into splenic white pulp, usually entering at the border between T cell area and B cell follicles (Fig. 2b).3 B cell migration into white pulp is integrin-dependent, whereas integrin-independent pathways exist in T cells.18 In adoptive transfer experiments, both subsets accumulate first in the T cell area (1 hr after i.v. injection) from where B cells migrate into CXCL13-expressing B cell follicles (after 2–4 hr).49 CXCR5–/– B cells fail to form proper follicles and accumulate instead around the T cell area, probably attracted with low efficacy by CCR7 ligands.50
A role for CXCR4 during B cell homing to spleen was investigated further using B cell-restricted CXCR4 mutant mice. CXCR4-deficient B cells still accumulated in splenic B cell follicles although, unexpectedly, the authors did not observe in vitro migration towards CXCL13·48 It is currently unclear whether other CKR, such as CCR6, may mediate follicular localization of B cells under these circumstances.
Interstitial lymphocyte migration and egress
Recent studies using multiphoton intravital microscopy have revealed that far from being stationary, T and B cells move vigorously within their specific microenvironments following apparently random migration pathways.11,12 Interstitial lymphocyte migration probably increases DC screening efficiency, which may accelerate immune response initiation. As chemokines, even in the absence of a gradient, provoke random migration, CCL19/21 and CXCL12/13 may be important motility-inducing factors in vivo. Other tissue factors, such as thromboxane A2 (TXA2), may also contribute to interstitial lymphocyte motility.51 In case lymphocytes do not encounter their Ag, they emigrate via efferent lymphatic vessels back into the bloodstream using S1P-dependent pathways.35 Efferent lymphatics eventually transport T and B cells back into the bloodstream, from which they will continue homing to SLO, thus completing their recirculation.
MZ B cell and B1 migration
In spleen, immature B cells develop into follicular or MZ B cells, depending on BCR signalling strength.42 MZ B cells localize to the MZ adjacent to B cell follicles, despite similar in vitro migration to CXCL1341 and although CXCL13 is not expressed in the MZ.52 Upon stimulation with lipopolysaccharide (LPS) or other inflammatory stimuli, MZ B cells rapidly (within 3–6 hr) translocate to B cell follicles in a CXCR5–CXCL13-dependent manner.53 Recent work by Cyster and colleagues has revealed that, in the absence of inflammation, MZ B cells receive a retention signal triggered by S1P1·53 Upon pharmacological or genetic ablation of S1P1 signalling, MZ B cells migrate into B cell follicles in a CXCR5-dependent manner (Fig. 3a), even in the absence of inflammatory stimuli.53 This suggests that S1P1 signals override CXCR5-mediated migration to a potential CXCL13 gradient or, alternatively, inhibit random motility of MZ B cells and subsequent accumulation in B cell follicles. Lymphocyte egress from thymus and PLN may be similarly controlled by S1P1 signalling strength modulation, rather than decreased CKR responsiveness.54
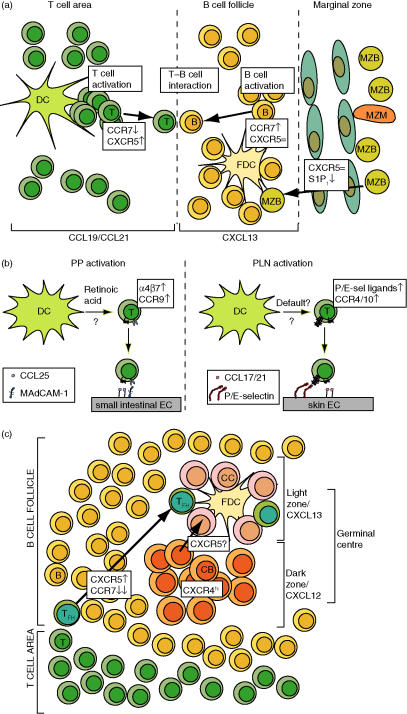
Figure 3.
Chemokines during immune responses. (a) Upon Ag encounter, T cells transiently increase CXCR5 expression while B cells increase CCR7 expression. Balanced responsiveness to CXCR5/CCR7 ligands results in Ag-specific T-B cell encounters at the T–B cell area border. In the spleen, inflammatory stimuli decrease S1P signalling in MZ B cells, allowing accumulation of MZ B cells in CXCL13-expressing B cell follicles. (b) Regional control of chemokine and adhesion receptor expression on effector T cells. Peyer's patches (PP) DC produce retinoic acid, which is required for imprinting of gut tropism via CCR9 and α4β7 up-regulation while suppressing skin-homing receptors. In contrast, peripheral lymph nodes (PLN) DC direct skin tropism on activated T cells by increasing CCR4/10 and selectin ligand expression in what appears to be at least in part a default mechanism. (c) Chemokine control of germinal centre (GC) anatomy. CXCR4 mediates centroblast accumulation in GC dark zones while CXCR5 is required for proper orientation of light–dark zones towards T cell areas. It is probable that CXCR5 is also dependent for centrocyte migration towards light zones, where follicular T helper cells participate in providing centrocyte help. Please refer to text for more details. DC: dendritic cell; FDC: follicular dendritic cell; CC: centrocyte; CB: centroblast; TFH: follicular helper CD4 T cell.
B1 cells constitute a B cell subset present in body cavities such as peritoneum. Together with MZ B cells, B1 cells are responsible for early humoral responses against bacterial pathogens.55 B1 cells chemokine responsiveness is similar to follicular and MZ B cells, although they migrate somewhat less to CCR7 ligands.41 B1 cell recruitment to peritoneum takes place in vessels present in specific lymphoid accumulations in the omentum and requires CXCL13.56 Additional selective adhesion mechanisms probably exist for the specific accumulation of B1 cells at this location, as follicular B cells do not home avidly to this microenvironment.
Balanced responsiveness to chemokines directs initial T–B cell encounters during immune responses
When confronted with cognate Ag–MHC complexes in combination with appropriate costimulatory molecules, T cells become activated, express inflammatory cytokines and divide.6 Activated T cells also change adhesion molecules surface expression levels; for instance, they down-regulate l-selectin surface levels, while increasing CD44, LFA-1 and α4 integrin levels.4 A subset of Ag-specific CD4 cells transiently increases CXCR5 expression while decreasing CCR7 ligand responsiveness, allowing them to migrate into close proximity to the B cell follicle border.3,57 Activated B cells undergo parallel changes in CKR expression: they transiently increase CCR7 levels and ligand responsiveness while maintaining CXCR5 surface expression.58 Co-ordinated regulation of CKR levels results in efficient T–B-cell encounters at the edge of follicles where B cells receive CD4 cell help via CD40L–CD40 and other costimulatory factors (Fig. 3a).6 CKR level modulation allows cells to fine-tune their response to distinct chemokine signals such that relative levels of two CKR are integrated to position cells at ‘intermediate’ points of two juxtaposed chemokine sources. Balanced responsiveness by CKR expression level modulation appears to constitute an important principle for interstitial lymphocyte positioning. Although in the set of experiments described by Cyster and colleagues58 CKR surface levels correlate with migratory capability, in other cases, CKR levels and ligand responsiveness do not necessarily correlate.59
Regional control of effector cell tropism
While some CD4 cells provide B cell help, other activated CD4 and CD8 cells leave draining PLN via efferent lymphatics and use the blood circulation to accumulate at sites of inflammation. High selectin ligand levels and expression of ‘inflammatory’ CKR, such as CXCR3, characterize these effector cells.57 The co-ordinated changes in CKR and adhesion molecule expression grant these cells access to inflamed tissue in which postcapillary venules present counter-receptors and inflammatory chemokines.4,17 Importantly, adhesion molecule and CKR expression strongly depend on whether lymphocyte activation takes place in PLN or PP. Expression of CCR4 and/or CCR10, together with increased selectin ligand expression, characterizes skin-homing effector T cells activated in PLN, whereas T cells activated in PP increase CCR9 and α4β7 expression (Fig. 3b).8 CKR patterns are paralleled by regional co-ordination of venular chemokine presentation; CCL17 and CCL25 are expressed preferentially in skin and small intestine venules, respectively.8
Recent experiments have shown that induction of skin versus gut-selective CKR expression is, at least in part, imprinted by dendritic cell (DC) origin. PP- but not PLN-derived DC induce CCR9 and α4β7 expression through pathways involving retinoic acid-mediated signalling.60,61 In the absence of retinoic acid signalling, a default skin tropism appears to prevail, although additional tissue factors present in PLN may provide further cues.62 As PLN and PP sample and present Ag from skin and gut, respectively, region-specific homing patterns have probably evolved to ensure maximal efficiency in directing effector cell migration to sites of inflammation. CKR and adhesion molecule levels are also maintained on long-lived memory T cell subsets. These cells contribute to continuous immune surveillance in extralymphoid tissue most exposed to potential pathogens, i.e. gut and skin.4 Importantly, memory T cells can reprogram their tissue tropism upon stimulation with appropriate DC, which allows for flexible redirecting of secondary immune responses. This may be important when dealing with the same pathogen at different body locations.63
CKR expression on effector and memory T cell subsets: more diverse than initially assumed
In human blood, two subsets of CD45RO+ can be distinguished on the basis of CCR7 and l-selectin expression. So-called central memory (CM) T cells are characterized by high l-selectin and CCR7 surface levels and thus maintain the ability to home to SLO.64 Upon stimulation, CM T cells were found to be poor cytokine producers but provided efficient B cell help and Ig class switching. CCR7lowL-selectinlow/med effector memory (EM) T cells efficiently produced cytokines after restimulation and expressed CKR critical for migration to sites of inflammation.64
Although CM/EM-like T cells with lymphoid and extralymphoid homing potential are clearly present in mice,65,66 subsequent studies have refined and differentiated CKR usage on CM and EM T cells.67–69 It is clear now that CCR7 on effector/memory T cells is not restricted to the CM subset, especially in the mouse system.9 Effector cells at sites of inflammation often express CCR7, although upon further differentiation in the periphery, CCR7 levels decrease.70,71 In mice, most cytokine-producing T cells in spleen are CCR7+, although their relative proportion is slightly decreased compared to the CCR7– subset. While these observations hint at a potential SLO homing capacity of effector cells, CCL19 and CCL21 might also function in effector cell recruitment to sites of inflammation. For example, CCR7 ligands are found on postcapillary venules under certain inflammatory conditions72,73 and CCR7+ effector cells are present in inflamed joints.67 CCR7 ligands can apparently act as both homeostatic and inflammatory chemokines. Under these conditions, selective recruitment of naive versus effector subsets could depend on L-selectin versus E/P-selectin ligand surface levels, rather than CKR expression. On the other hand, CCR7-independent homing of certain CM T cell subsets to SLO has been observed,44 and the most efficient B cell help-providing T cells, the follicular helper CD4 cells found in GC light zones (Fig. 3c), have completely lost CCR7 responsiveness.74,75 In summary, although CCR7+ T cells are enriched in the CM population, CCR7 alone is not a suitable marker to differentiate CM and EM T cells.
When analysing CKR expression patterns induced on Th1/Th2 cells under selected in vitro polarizing conditions, Th1 cells express predominantly CCR5 and CXCR3, whereas Th2 cells express CCR4 and CCR8·76 However, when Kim et al. isolated Th1/Th2 CD4 cells from human blood and analysed CKR expression profiles, they observed that no single CKR was expressed exclusively within a given subset.67 Although CXCR3 and CCR4 are expressed on virtually all Th1 and Th2 cells, respectively, CXCR3+CCR4+ double positive cells were found in both populations.67 Furthermore, CCR5 expression correlated only with a Th1 phenotype when coexpressed with CXCR3. In absence of CXCR3, CCR5+ memory cells are mainly non-polarized. The authors conclude that in addition to Th1 and Th2 associated CKR (CXCR3 and CCR4, respectively), a second class of CKR are enriched but not associated exclusively with effector cell subsets. A majority of Th1/Th2 effector cells also express CCR7, which may allow these cells to home to SLO, in addition to extralymphoid sites.67 Taken together, CKR expression patterns on CM/EM and Th1/Th2 T cell subsets follow more complex rules than appreciated initially, indicating that memory/effector cells can disseminate into a variety of target tissues and, at least in some cases, into both lymphoid and non-lymphoid tissue.
Regulatory T cell trafficking
Regulatory T cells (Treg) are a subset of CD4 cells, which modulate immune responses by dominantly suppressing T cell activation.77 As these cells are thought to exert their function at least in part by cell-to-cell contact during T cell activation in SLO and/or by secretion of anti-inflammatory cytokines in extralymphoid tissue, it is likely that Treg subsets home to SLO as well as to inflamed tissue.77 Recent work has indeed shown that Treg consist of at least two populations with distinct homing potential.78 Treg expressing the αE integrin (which encompass both CD25-positive and negative Treg) migrate efficiently to ligands for CXCR3, CCR4, CCR6 and CCR7, express P/E-selectin ligands and home preferentially to inflamed tissue. In contrast, CD25+αE– Treg are l-selectinhigh, migrate preferentially to CCR7 ligands and home well to SLO.78 An open question is whether αE+ Treg derive from αE–CD25+ precursors or by independent pathways. In any event, these data highlight the recurrent scheme of division of labour between memory subsets, as observed previously for CM/TM memory T cells.9 Again, CCR7 does not appear a good marker to differentiate between αE-positive and negative Treg, as both migrate efficiently to CCL19.78
Within lymphoid tissue, CCL4 produced by activated B cells and Ag-presenting cells attracts Treg.79 CCL4 depletion leads to development of autoantibodies, possibly by decreasing Treg recruitment to Ag-presenting cells. As mice deficient in the CCL4 receptor CCR5 do not develop autoimmune disease spontaneously,80 in contrast to other mouse models with impaired Treg function77 other mechanisms for Treg recruitment are likely to exist.
Chemokines during humoral immune responses
During an immune response, follicular B cells carrying appropriate BCR become activated and receive T cell help at the T–B cell border area (Fig. 3a). A subset of activated B cells differentiates directly into low-affinity IgM producing plasma B cells. Other activated B cells form GC where they start to proliferate as so-called centroblasts and where somatic hypermutation, affinity maturation and class switching occurs. High-affinity Ig producing B cells (centrocytes) are positively selected to become memory or plasma cells. Proliferation of centroblasts and positive selection of centrocytes are taking place in the dark and light zones of GC, respectively. Centroblasts that have undergone massive proliferation and somatic hypermutation migrate from the dark zone to the light zone, where high affinity Ab-producing centrocytes receive T cell help and survival signals.
Recent work has uncovered a role for chemokines during GC formation.81 CXCR4 is required for correct GC morphology by attracting or retaining centroblasts in the dark zone whereas CXCR5, albeit not required for centroblast/centrocyte zone separation, is implied in correct dark/light zone positioning relative to the T cell area (Fig. 3c). In the absence of CXCR5, GC dark zones are often not proximal to the T cell area but at apparently random positions. An interesting aspect which deserves further investigation is the lower expression of CXCL12 and CXCL13 in GC compared to the follicular B cell mantle; nevertheless, GC B cells do not migrate outside GC.81 CXCL12 and CXCL13 thus organize GC in a microenvironment-restricted manner. Several scenarios are possible to explain this observation, including expression of a GC B cell-specific chemoattractant in GC.81 Alternatively or in addition, adhesion molecules such as fibronectin or VCAM-1 might be expressed at higher levels in GC and confer superior retention of GC B cells.
Subsets of activated B cells survive long-term as memory or plasma B cells. IgA secreting plasma cells arise mainly after activation in mucosa-associated lymphoid tissue and upper aerodigestive tract. These cells disseminate subsequently to mucosal sites related to the site of induction. In addition to α4β7 and CCR9, mucosa-homing IgA plasma cells typically express CCR10, which binds epithelially presented CCL28 expressed in colon, the respiratory/urogenital tract and mammary glands.7 IgA plasma cells homing to the upper aerodigestive tract usually express CCR10 and α4β1 but are CCR9low. Conversely, IgG secreting plasma cells arise in spleen, peripheral and gut-associated lymphoid tissue, but disseminate preferentially to non-mucosal-associated tissue. IgG plasma cells express α4β1 and CXCR3, which allows them to enter non-mucosal sites of inflammation. In addition, IgG plasma cells home avidly to BM, most probably via CXCR4-dependent pathways. Of note, some IgA-secreting plasma cells also migrate to BM, as they maintain responsiveness to CXCL127.
Homeostatic chemokines and initiation of immune responses
Chemokines are clearly required for efficient effector cell migration to sites of inflammation.4 An interesting aspect is the actual importance of chemokines and, as a consequence, ordered lymphoid microenvironments, for effective immune response initiation. In analogy with thymocyte development, CKR are not always indispensable for generation of immune responses. Although CCR7 deficiency strongly reduces immune reactions which involve DC migration to draining lymph nodes, systemically administered Ag produces comparable, albeit delayed, immune reactions in these animals.49 Similar observations have been made in plt/plt mice challenged with T cell-dependent Ag82 or virus.83 Also, CXCR5 deficient mice show similar antibody production when Ag is administered systemically.50 How does this fit into the hypothesis of the importance of SLO organization? It appears that in the presence of strong inflammatory stimuli administered systemically, the role of structured lymphoid tissue for immune reaction initiation is less important. It is possible that in the absence of chemotactic cues, random motility within these organs brings immune cells into close enough contact to trigger immune responses, overriding a requirement for highly defined SLO structure. Alternatively (or in addition), as both activated DC and T cells employ CCR7 to localize in T cell areas, these two cell types may be inhibited similarly in the absence of CCR7 or its ligands and instead interact outside T cell areas.82,84 Despite these observations, it is likely that chemokine-mediated lymphocyte migration and SLO organization plays a central role in regulating immune responses under physiological conditions. Plt/plt mice show defective immune response down-regulation82 due perhaps to Treg trafficking defects. Other examples include CCR6–/– mice, where altered PP architecture correlates with impaired humoral responses to orally administered Ag85 and B cell restricted CXCR4-deficient mice, which have impaired T-independent Ag humoral responses. Finally, it is conceivable that in larger mammals, immune responses to inflammations with local character depend more strongly on efficient immune surveillance by recirculating lymphocytes.
Concluding remarks
Recent years have greatly advanced our knowledge on how chemokines regulate trafficking of lymphocyte subsets in health and inflammation/disease. Chemokines, together with adhesion receptors, are central to efficient recirculation, appropriate structuring of lymphoid tissue and cell recruitment to extralymphoid sites. Important recent results uncovered chemokines directing regional migration patterns, such as skin and gut tropism.8 Chemokine control of B cell subset positioning, such as during GC formation or B1 cell homing, constitute another area which has seen important breakthroughs.56,81 Specific, non-redundant task have now been found for many chemokines and their receptors. As the characterization of immune cell subsets, such as Tregs and effector/memory lymphocytes, is constantly evolving, CKR and adhesion receptors analysis should allow more detailed insights into trafficking, and consequently function, of these cells.
References
- 1.Sprent J, Surh CD. T cell memory. Annu Rev Immunol. 2002;20:551–79. doi: 10.1146/annurev.immunol.20.100101.151926. 10.1146/annurev.immunol.20.100101.151926. [DOI] [PubMed] [Google Scholar]
- 2.Reif K, Cyster J. The CDM protein DOCK2 in lymphocyte migration. Trends Cell Biol. 2002;12:368–73. doi: 10.1016/s0962-8924(02)02330-9. 10.1016/S0962-8924(02)02330-9. [DOI] [PubMed] [Google Scholar]
- 3.Cyster JG. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu Rev Immunol. 2005;23:127–59. doi: 10.1146/annurev.immunol.23.021704.115628. [DOI] [PubMed] [Google Scholar]
- 4.von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343:1020–34. doi: 10.1056/NEJM200010053431407. 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- 5.Cyster JG. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286:2098–102. doi: 10.1126/science.286.5447.2098. [DOI] [PubMed] [Google Scholar]
- 6.Jenkins MK, Khoruts A, Ingulli E, Mueller DL, McSorley SJ, Reinhardt RL, Itano A, Pape KA. In vivo activation of antigen-specific CD4 T cells. Annu Rev Immunol. 2001;19:23–45. doi: 10.1146/annurev.immunol.19.1.23. 10.1146/annurev.immunol.19.1.23. [DOI] [PubMed] [Google Scholar]
- 7.Kunkel EJ, Butcher EC. Plasma-cell homing. Nat Rev Immunol. 2003;3:822–9. doi: 10.1038/nri1203. 10.1038/nri1203. [DOI] [PubMed] [Google Scholar]
- 8.Kunkel EJ, Butcher EC. Chemokines and the tissue-specific migration of lymphocytes. Immunity. 2002;16:1–4. doi: 10.1016/s1074-7613(01)00261-8. 10.1016/S1074-7613(01)00261-8. [DOI] [PubMed] [Google Scholar]
- 9.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–63. doi: 10.1146/annurev.immunol.22.012703.104702. 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 10.Miyasaka M, Tanaka T. Lymphocyte trafficking across high endothelial venules: dogmas and enigmas. Nat Rev Immunol. 2004;4:360–70. doi: 10.1038/nri1354. 10.1038/nri1354. [DOI] [PubMed] [Google Scholar]
- 11.Wei SH, Parker I, Miller MJ, Cahalan MD. A stochastic view of lymphocyte motility and trafficking within the lymph node. Immunol Rev. 2003;195:136–59. doi: 10.1034/j.1600-065x.2003.00076.x. 10.1034/j.1600-065X.2003.00076.x. [DOI] [PubMed] [Google Scholar]
- 12.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–78. doi: 10.1038/nri1222. 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 13.Dustin ML, Bivona TG, Philips MR. Membranes as messengers in T cell adhesion signaling. Nat Immunol. 2004;5:363–72. doi: 10.1038/ni1057. 10.1038/ni1057. [DOI] [PubMed] [Google Scholar]
- 14.Laudanna C, Kim JY, Constantin G, Butcher E. Rapid leukocyte integrin activation by chemokines. Immunol Rev. 2002;186:37–46. doi: 10.1034/j.1600-065x.2002.18604.x. 10.1034/j.1600-065X.2002.18604.x. [DOI] [PubMed] [Google Scholar]
- 15.Kim M, Carman CV, Yang W, Salas A, Springer TA. The primacy of affinity over clustering in regulation of adhesiveness of the integrin {alpha}L{beta}2. J Cell Biol. 2004;167:1241–53. doi: 10.1083/jcb.200404160. 10.1083/jcb.200404160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Middleton J, Neil S, Wintle J, et al. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell. 1997;91:385–95. doi: 10.1016/s0092-8674(00)80422-5. [DOI] [PubMed] [Google Scholar]
- 17.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–6. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 18.Lo CG, Lu TT, Cyster JG. Integrin-dependence of lymphocyte entry into the splenic white pulp. J Exp Med. 2003;197:353–61. doi: 10.1084/jem.20021569. 10.1084/jem.20021569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bleul CC, Boehm T. Chemokines define distinct microenvironments in the developing thymus. Eur J Immunol. 2000;30:3371–9. doi: 10.1002/1521-4141(2000012)30:12<3371::AID-IMMU3371>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 20.Haynes BF, Heinly CS. Early human T cell development: analysis of the human thymus at the time of initial entry of hematopoietic stem cells into the fetal thymic microenvironment. J Exp Med. 1995;181:1445–58. doi: 10.1084/jem.181.4.1445. 10.1084/jem.181.4.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu C, Ueno T, Kuse S, et al. The role of CCL21 in recruitment of T-precursor cells to fetal thymi. Blood. 2005;105:31–9. doi: 10.1182/blood-2004-04-1369. [DOI] [PubMed] [Google Scholar]
- 22.Benz C, Heinzel K, Bleul CC. Homing of immature thymocytes to the subcapsular microenvironment within the thymus is not an absolute requirement for T cell development. Eur J Immunol. 2004;34:3652–63. doi: 10.1002/eji.200425248. [DOI] [PubMed] [Google Scholar]
- 23.Wurbel MA, Malissen M, Guy-Grand D, Meffre E, Nussenzweig MC, Richelme M, Carrier A, Malissen B. Mice lacking the CCR9 CC-chemokine receptor show a mild impairment of early T- and B-cell development and a reduction in T-cell receptor gammadelta(+) gut intraepithelial lymphocytes. Blood. 2001;98:2626–32. doi: 10.1182/blood.v98.9.2626. [DOI] [PubMed] [Google Scholar]
- 24.Onai N, Zhang Y, Yoneyama H, Kitamura T, Ishikawa S, Matsushima K. Impairment of lymphopoiesis and myelopoiesis in mice reconstituted with bone marrow–hematopoietic progenitor cells expressing SDF-1-intrakine. Blood. 2000;96:2074–80. [PubMed] [Google Scholar]
- 25.Ara T, Itoi M, Kawabata K, et al. A role of CXC chemokine ligand 12/stromal cell-derived factor-1/pre-B cell growth stimulating factor and its receptor CXCR4 in fetal and adult T cell development in vivo. J Immunol. 2003;170:4649–55. doi: 10.4049/jimmunol.170.9.4649. [DOI] [PubMed] [Google Scholar]
- 26.Uehara S, Grinberg A, Farber JM, Love PE. A role for CCR9 in T lymphocyte development and migration. J Immunol. 2002;168:2811–9. doi: 10.4049/jimmunol.168.6.2811. [DOI] [PubMed] [Google Scholar]
- 27.Misslitz A, Pabst O, Hintzen G, Ohl L, Kremmer E, Petrie HT, Forster R. Thymic T cell development and progenitor localization depend on CCR7. J Exp Med. 2004;200:481–91. doi: 10.1084/jem.20040383. 10.1084/jem.20040383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwan J, Killeen N. CCR7 directs the migration of thymocytes into the thymic medulla. J Immunol. 2004;172:3999–4007. doi: 10.4049/jimmunol.172.7.3999. [DOI] [PubMed] [Google Scholar]
- 29.Ueno T, Saito F, Gray DH, et al. CCR7 signals are essential for cortex–medulla migration of developing thymocytes. J Exp Med. 2004;200:493–505. doi: 10.1084/jem.20040643. 10.1084/jem.20040643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Witt CM, Robey EA. The ins and outs of CCR7 in the thymus. J Exp Med. 2004;200:405–9. doi: 10.1084/jem.20041110. 10.1084/jem.20041110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma Q, Jones D, Springer TA. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity. 1999;10:463–71. doi: 10.1016/s1074-7613(00)80046-1. 10.1016/S1074-7613(00)80046-1. [DOI] [PubMed] [Google Scholar]
- 32.Plotkin J, Prockop SE, Lepique A, Petrie HT. Critical role for CXCR4 signaling in progenitor localization and T cell differentiation in the postnatal thymus. J Immunol. 2003;171:4521–7. doi: 10.4049/jimmunol.171.9.4521. [DOI] [PubMed] [Google Scholar]
- 33.Hernandez-Lopez C, Varas A, Sacedon R, Jimenez E, Munoz JJ, Zapata AG, Vicente A. Stromal cell-derived factor 1/CXCR4 signaling is critical for early human T-cell development. Blood. 2002;99:546–54. doi: 10.1182/blood.v99.2.546. [DOI] [PubMed] [Google Scholar]
- 34.Ueno T, Hara K, Willis MS, et al. Role for CCR7 ligands in the emigration of newly generated T lymphocytes from the neonatal thymus. Immunity. 2002;16:205–18. doi: 10.1016/s1074-7613(02)00267-4. 10.1016/S1074-7613(02)00267-4. [DOI] [PubMed] [Google Scholar]
- 35.Matloubian M, Lo CG, Cinamon G, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–60. doi: 10.1038/nature02284. 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 36.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–9. doi: 10.1038/31269. 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 37.Tachibana K, Hirota S, Iizasa H, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–4. doi: 10.1038/31261. 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 38.Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci USA. 1998;95:9448–53. doi: 10.1073/pnas.95.16.9448. 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Egawa T, Kawabata K, Kawamoto H, et al. The earliest stages of B cell development require a chemokine stromal cell-derived factor/pre-B cell growth-stimulating factor. Immunity. 2001;15:323–34. doi: 10.1016/s1074-7613(01)00185-6. 10.1016/S1074-7613(01)00185-6. [DOI] [PubMed] [Google Scholar]
- 40.Glodek AM, Honczarenko M, Le Y, Campbell JJ, Silberstein LE. Sustained activation of cell adhesion is a differentially regulated process in B lymphopoiesis. J Exp Med. 2003;197:461–73. doi: 10.1084/jem.20021477. 10.1084/jem.20021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bowman EP, Campbell JJ, Soler D, Dong Z, Manlongat N, Picarella D, Hardy RR, Butcher EC. Developmental switches in chemokine response profiles during B cell differentiation and maturation. J Exp Med. 2000;191:1303–18. doi: 10.1084/jem.191.8.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pillai S, Cariappa A, Moran ST. Positive selection and lineage commitment during peripheral B-lymphocyte development. Immunol Rev. 2004;197:206–18. doi: 10.1111/j.0105-2896.2003.097.x. 10.1111/j.0105-2896.2003.097.x. [DOI] [PubMed] [Google Scholar]
- 43.Okada T, Ngo VN, Ekland EH, Forster R, Lipp M, Littman DR, Cyster JG. Chemokine requirements for B cell entry to lymph nodes and Peyer's patches. J Exp Med. 2002;196:65–75. doi: 10.1084/jem.20020201. 10.1084/jem.20020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scimone ML, Felbinger TW, Mazo IB, Stein JV, Von Andrian UH, Weninger W. CXCL12 mediates CCR7-independent homing of central memory cells, but not naive T cells, in peripheral lymph nodes. J Exp Med. 2004;199:1113–20. doi: 10.1084/jem.20031645. 10.1084/jem.20031645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ebisuno Y, Tanaka T, Kanemitsu N, Kanda H, Yamaguchi K, Kaisho T, Akira S, Miyasaka M. Cutting edge. the B cell chemokine CXC chemokine ligand 13/B lymphocyte chemoattractant is expressed in the high endothelial venules of lymph nodes and Peyer's patches and affects B cell trafficking across high endothelial venules. J Immunol. 2003;171:1642–6. doi: 10.4049/jimmunol.171.4.1642. [DOI] [PubMed] [Google Scholar]
- 46.Warnock RA, Campbell JJ, Dorf ME, Matsuzawa A, McEvoy LM, Butcher EC. The role of chemokines in the microenvironmental control of T versus B cell arrest in Peyer's patch high endothelial venules. J Exp Med. 2000;191:77–88. doi: 10.1084/jem.191.1.77. 10.1084/jem.191.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cinamon G, Shinder V, Alon R. Shear forces promote lymphocyte migration across vascular endothelium bearing apical chemokines. Nat Immunol. 2001;2:515–22. doi: 10.1038/88710. [DOI] [PubMed] [Google Scholar]
- 48.Nie Y, Waite J, Brewer F, Sunshine MJ, Littman DR, Zou YR. The role of CXCR4 in maintaining peripheral B cell compartments and humoral immunity. J Exp Med. 2004;200:1145–56. doi: 10.1084/jem.20041185. 10.1084/jem.20041185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Förster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 50.Förster R, Mattis AE, Kremmer E, Wolf E, Brem G, Lipp M. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996;87:1037–47. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- 51.Kabashima K, Murata T, Tanaka H, et al. Thromboxane A2 modulates interaction of dendritic cells and T cells and regulates acquired immunity. Nat Immunol. 2003;4:694–701. doi: 10.1038/ni943. 10.1038/ni943. [DOI] [PubMed] [Google Scholar]
- 52.Gunn MD, Ngo VN, Ansel KM, Ekland EH, Cyster JG, Williams LT. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt's lymphoma receptor-1. Nature. 1998;391:799–803. doi: 10.1038/35876. [DOI] [PubMed] [Google Scholar]
- 53.Cinamon G, Matloubian M, Lesneski MJ, Xu Y, Low C, Lu T, Proia RL, Cyster JG. Sphingosine 1-phosphate receptor 1 promotes B cell localization in the splenic marginal zone. Nat Immunol. 2004;5:713–20. doi: 10.1038/ni1083. [DOI] [PubMed] [Google Scholar]
- 54.Lo CG, Xu Y, Proia RL, Cyster JG. Cyclical modulation of sphingosine-1-phosphate receptor 1 surface expression during lymphocyte recirculation and relationship to lymphoid organ transit. J Exp Med. 2005;201:291–301. doi: 10.1084/jem.20041509. 10.1084/jem.20041509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14:617–29. doi: 10.1016/s1074-7613(01)00129-7. 10.1016/S1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- 56.Ansel KM, Harris RB, Cyster JG. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity. 2002;16:67–76. doi: 10.1016/s1074-7613(01)00257-6. 10.1016/S1074-7613(01)00257-6. [DOI] [PubMed] [Google Scholar]
- 57.Campbell DJ, Kim CH, Butcher EC. Separable effector T cell populations specialized for B cell help or tissue inflammation. Nat Immunol. 2001;2:876–81. doi: 10.1038/ni0901-876. [DOI] [PubMed] [Google Scholar]
- 58.Reif K, Ekland EH, Ohl L, Nakano H, Lipp M, Förster R, Cyster JG. Balanced responsiveness to chemoattractants from adjacent zones determines B-cell position. Nature. 2002;416:94–9. doi: 10.1038/416094a. 10.1038/416094a. [DOI] [PubMed] [Google Scholar]
- 59.Liao F, Shirakawa AK, Foley JF, Rabin RL, Farber JM. Human B cells become highly responsive to macrophage-inflammatory protein-3 alpha/CC chemokine ligand-20 after cellular activation without changes in CCR6 expression or ligand binding. J Immunol. 2002;168:4871–80. doi: 10.4049/jimmunol.168.10.4871. [DOI] [PubMed] [Google Scholar]
- 60.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–38. doi: 10.1016/j.immuni.2004.08.011. 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 61.Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, Von Andrian UH. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 62.Mora JR, von Andrian UH. Retinoic acid: an educational ‘vitamin elixir’ for gut-seeking T cells. Immunity. 2004;21:458–60. doi: 10.1016/j.immuni.2004.10.002. 10.1016/j.immuni.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 63.Mora JR, Cheng G, Picarella D, Briskin M, Buchanan N, von Andrian UH. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J Exp Med. 2005;201:303–16. doi: 10.1084/jem.20041645. 10.1084/jem.20041645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi: 10.1038/44385. 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 65.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–5. doi: 10.1038/35065111. 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 66.Weninger W, Crowley MA, Manjunath N, von Andrian UH. Migratory properties of naive, effector, and memory CD8(+) T cells. J Exp Med. 2001;194:953–66. doi: 10.1084/jem.194.7.953. 10.1084/jem.194.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim CH, Rott L, Kunkel EJ, Genovese MC, Andrew DP, Wu L, Butcher EC. Rules of chemokine receptor association with T cell polarization in vivo. J Clin Invest. 2001;108:1331–9. doi: 10.1172/JCI13543. 10.1172/JCI200113543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Unsoeld H, Krautwald S, Voehringer D, Kunzendorf U, Pircher H. Cutting edge: CCR7+ and CCR7- memory T cells do not differ in immediate effector cell function. J Immunol. 2002;169:638–41. doi: 10.4049/jimmunol.169.2.638. [DOI] [PubMed] [Google Scholar]
- 69.Debes GF, Hopken UE, Hamann A. In vivo differentiated cytokine-producing CD4(+) T cells express functional CCR7. J Immunol. 2002;168:5441–7. doi: 10.4049/jimmunol.168.11.5441. [DOI] [PubMed] [Google Scholar]
- 70.Debes GF, Bonhagen K, Wolff T, Kretschmer U, Krautwald S, Kamradt T, Hamann A. CC chemokine receptor 7 expression by effector/memory CD4+ T cells depends on antigen specificity and tissue localization during influenza A virus infection. J Virol. 2004;78:7528–35. doi: 10.1128/JVI.78.14.7528-7535.2004. 10.1128/JVI.78.14.7528-7535.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roman E, Miller E, Harmsen A, Wiley J, Von Andrian UH, Huston G, Swain SL. CD4 effector T cell subsets in the response to influenza. heterogeneity, migration, and function. J Exp Med. 2002;196:957–68. doi: 10.1084/jem.20021052. 10.1084/jem.20021052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weninger W, Carlsen HS, Goodarzi M, Moazed F, Crowley MA, Baekkevold ES, Cavanagh LL, von Andrian UH. Naive T cell recruitment to nonlymphoid tissues: a role for endothelium-expressed CC chemokine ligand 21 in autoimmune disease and lymphoid neogenesis. J Immunol. 2003;170:4638–48. doi: 10.4049/jimmunol.170.9.4638. [DOI] [PubMed] [Google Scholar]
- 73.Alt C, Laschinger M, Engelhardt B. Functional expression of the lymphoid chemokines CCL19 (ELC) and CCL 21 (SLC) at the blood–brain barrier suggests their involvement in G-protein-dependent lymphocyte recruitment into the central nervous system during experimental autoimmune encephalomyelitis. Eur J Immunol. 2002;32:2133–44. doi: 10.1002/1521-4141(200208)32:8<2133::AID-IMMU2133>3.0.CO;2-W. 10.1002/1521-4141(200208)32:8<2133::AID-IMMU2133>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 74.Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, Förster R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192:1545–52. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192:1553–62. doi: 10.1084/jem.192.11.1553. 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ward SG, Bacon K, Westwick J. Chemokines and T lymphocytes: more than an attraction. Immunity. 1998;9:1–11. doi: 10.1016/s1074-7613(00)80583-x. 10.1016/S1074-7613(00)80583-X. [DOI] [PubMed] [Google Scholar]
- 77.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–62. doi: 10.1146/annurev.immunol.21.120601.141122. 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 78.Huehn J, Siegmund K, Lehmann JC, et al. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J Exp Med. 2004;199:303–13. doi: 10.1084/jem.20031562. 10.1084/jem.20031562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bystry RS, Aluvihare V, Welch KA, Kallikourdis M, Betz AG. B cells and professional APCs recruit regulatory T cells via CCL4. Nat Immunol. 2001;2:1126–32. doi: 10.1038/ni735. [DOI] [PubMed] [Google Scholar]
- 80.Huffnagle GB, McNeil LK, McDonald RA, Murphy JW, Toews GB, Maeda N, Kuziel WA. Cutting edge: role of C-C chemokine receptor 5 in organ-specific and innate immunity to Cryptococcus neoformans. J Immunol. 1999;163:4642–6. [PubMed] [Google Scholar]
- 81.Allen CD, Ansel KM, Low C, Lesley R, Tamamura H, Fujii N, Cyster JG. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat Immunol. 2004;5:943–52. doi: 10.1038/ni1100. 10.1038/ni1100. [DOI] [PubMed] [Google Scholar]
- 82.Mori S, Nakano H, Aritomi K, Wang CR, Gunn MD, Kakiuchi T. Mice lacking expression of the chemokines CCL21-ser and CCL19 (plt mice) demonstrate delayed but enhanced T cell immune responses. J Exp Med. 2001;193:207–18. doi: 10.1084/jem.193.2.207. 10.1084/jem.193.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Junt T, Nakano H, Dumrese T, Kakiuchi T, Odermatt B, Zinkernagel RM, Hengartner H, Ludewig B. Antiviral immune responses in the absence of organized lymphoid T cell zones in plt/plt mice. J Immunol. 2002;168:6032–40. doi: 10.4049/jimmunol.168.12.6032. [DOI] [PubMed] [Google Scholar]
- 84.Junt T, Scandella E, Förster R, Krebs P, Krautwald S, Lipp M, Hengartner H, Ludewig B. Impact of CCR7 on priming and distribution of antiviral effector and memory CTL. J Immunol. 2004;173:6684–93. doi: 10.4049/jimmunol.173.11.6684. [DOI] [PubMed] [Google Scholar]
- 85.Cook DN, Prosser DM, Forster R, et al. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity. 2000;12:495–503. doi: 10.1016/s1074-7613(00)80201-0. [DOI] [PubMed] [Google Scholar]
- 86.Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinase grammar for immune cells. Annu Rev Immunol. 2004;22:891–928. doi: 10.1146/annurev.immunol.22.012703.104543. 10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]