Abstract
It has been previously shown that the subset of human natural killer (NK) cells which express CD8 in a homodimeric α/α form are more cytotoxic than their CD8– counterparts but the mechanisms behind this differential cytolytic activity remained unknown. Target cell lysis by CD8– NK cells is associated with high levels of effector cell apoptosis, which is in contrast to the significantly lower levels found in the CD8α+ cells after lysis of the same targets. We report that cross-linking of the CD8α chains on NK cells induces rapid rises in intracellular Ca2+ and increased expression of CD69 at the cell surface by initiating the influx of extracellular Ca2+ ions. We demonstrate that secretion of cytolytic enzymes initiates NK-cell apoptosis from which CD8α+ NK cells are protected by an influx of exogenous calcium following ligation of CD8 on the NK-cell surface. This ligation is through interaction with fellow NK cells in the cell conjugate and can occur when the target cells lack major histocompatibility complex (MHC) Class I expression. Protection from apoptosis is blocked by preincubation of the NK cells with anti-MHC Class I antibody. Thus, in contrast to the CD8– subset, CD8α+ NK cells are capable of sequential lysis of multiple target cells.
Keywords: apoptosis, cytotoxicity, natural killer cells, tumour immunotherapy
Introduction
Human CD8 is a glycoprotein containing an N-terminal immunoglobulin-like extracellular domain, extended and glycosylated hinge and stalk regions, a transmembrane portion and a short cytoplasmic tail that interacts with p56lck, an src- like tyrosine kinase.1
CD8 on thymocytes and thymus-derived T cells usually consists of disulphide-linked α- and β-chains each with a molecular weight of 34 000. The extracellular regions of the α- and β-chains of CD8 are homologous and consist of a single N-terminal immunoglobulin-like domain and a 50 (α-chain) or 30 (β-chain) amino acid ‘hinge’ region. Infrequently CD8 can be expressed as a homodimer of α-chains (α/α). On T cells, both hetero- and homodimer forms function as low-affinity adhesion co-receptors for the trimolecular ligation of T-cell receptor–antigen–major histocompatibility complex (MHC) Class I.2
A small subset of human natural killer (NK) cells (CD3– CD56+) expresses CD8. Only the α/α homodimer is expressed and the density of expression is approximately a log-fold lower than that of CD8α/α or CD8α/β on T cells as determined by flow cytometry.3 The expression of CD8α/α on CD3– NK cells is presumably of fundamental significance because it has recently been reported to identify the lytic subset in avian NK cells.4 The CD56+ CD3– CD8+ subset of human NK cells has been shown to have higher cytolytic function than the CD8– subset both at rest5 and after culture,6 and was reported by our group in 1997 to mediate autologous cytotoxicity of myeloid leukaemic cells from patients in clinical remission after autologous stem-cell transplantation.7 This was subsequently extended to acute myeloid leukaemia patients in complete remission after chemotherapy alone.8
NK cells mediate the lysis of tumour cells and virus-infected cells via natural cytotoxicity and antibody-dependent cellular cytotoxicity (ADCC) and are controlled by positive and negative cytolytic signals.9,10 Negative (inhibitory) signals are transduced by C-lectin-domain-containing receptors CD94/NKG2A and by some killer immunoglobulin-like receptors (KIRs)10.
ADCC is mediated via CD16, while the triggering receptors responsible for natural cytotoxicity and the innate ability of NK cells to lyse virally infected and tumour cells remain largely elusive. Using receptor-specific monoclonal antibodies (mAb) to redirect lysis of FcγR+ cells a number of cytotoxic triggering molecules have been identified, including CD2, CD38, CD69, NKRP-1, CD40, B7-2, NK-TR, NKp46, NKp30 and NKp44.
Materials and methods
Monoclonal antibodies and reagents
The following murine mAbs were used in cell culture experiments: anti-CD8α immunoglobulin G1 (IgG1) RPA-T8 (pure), anti-CD16 IgG1 3G8 (pure) clone, CD3ε IgG1 (pure) and anti-CD56 IgG1 MY31, all purchased from Becton Dickenson, Cowley, UK. Anti-CD8α IgG2 OKT8 supernatant (American Type Culture Collection, LGC Promochem, Teddington, UK) was also used.
Goat F(ab′)2 anti-mouse IgG (H + L) was purchased from Caltag Laboratories, Invitrogen, Paisley, UK.
Recombinant human interleukin-2 (IL-2) was purchased from R & D Systems, Minneapolis, USA. All cell cultures were maintained in complete media (CM) consisting of RPMI-1640 supplemented with 10% fetal calf serum (FCS), 100 i.u. penicillin and 100 i.u.streptomycin (all supplied by Gibco, Paisley, UK).
Immunophenotyping
To analyse cell surface antigen expression, 105 cells in 100 μl Hanks' balanced salt solution (HBSS) were incubated with commercially supplied, fluorochrome-conjugated mAbs (Table 1) at the manufacturers' recommended concentration for 15 min at room temperature. After washing, the cells were analysed by flow cytometry (FACS Calibur with cellquest software, Becton Dickinson, Oxford, UK). Forward and side light scatter characteristics were used to gate the viable lymphocyte population before acquisition of at least 10 000 cells from each sample.
Table 1.
Suppliers of the commercially supplied, fluorochrome-conjugated monoclonal antibodies used in this study
| Target antigen | Fluorochrome | Manufacturer |
|---|---|---|
| CD2 | FITC | Becton Dickinson |
| CD11a | PE | Becton Dickinson |
| CD11b | FITC | Becton Dickinson |
| CD54 | FITC | Becton Dickinson |
| CD58 | FITC | Becton Dickinson |
| CD69 | PE | Becton Dickinson |
| NKp30 | PE | Beckman Coulter |
| NKp44 | PE | Beckman Coulter |
| NKp46 | PE | Beckman Coulter |
| NKG2D | PE | Beckman Coulter |
| Perforin | PE | Becton Dickinson |
| Granzyme A | PE | Becton Dickinson |
| MHC Class I | FITC | Serotec |
FITC, fluorescein isothiocyanate; PE, phycoerythrin.
Addresses: Becton Dickinson, Oxford, UK; Beckman Coulter, High Wycombe, UK, Serotec, Oxford, UK.
Isolation of human NK cells and target cells
Fresh heparinized peripheral blood samples were obtained from normal healthy donors and from four patients in complete remission (CR) after chemotherapy for acute myeloid leukaemia (AML). Each of the four patients had previously donated bone marrow samples at the time of their disease presentation and the leukaemic blasts were cryopreserved in multiple aliquots. Two cases were AML M4; the third was AML M2 and the fourth was AML M5. All patients' samples were obtained after informed consent.
Mononuclear cells (PBMCs) were isolated from venous blood by discontinuous density gradient separation (Lymphoprep, Nycomed, UK). CD56+ CD3– cells were purified from PBMCs by direct immunomagnetic separation using a CD56 Multisort kit (Miltenyi Biotec, Cologne, Germany) and subsequent depletion with CD3 fluorescein isothiocyanate (FITC) and anti-FITC beads. All selected cells were confirmed as >98% CD56+ and <3% CD3+. CD8+ NK cells were then isolated using the OKT8 antibody because this was shown not to induce NK activation, as shown by increased intracellular Ca2+, phosphorylation of p56lck or up-regulation of CD69 (data not shown).
Intracellular measurement of perforin and granzyme A
CD56+ CD3– NK cells were immunophenotyped with anti-CD8 allophycocyanin (SK1, Becton Dickenson) and washed extensively. Intracellular staining with anti-Perforin FITC and anti-Granzyme A phycoerythrin (PE) was carried out using Cytofix/Cytoperm kits, all from Becton Dickenson, and analysed by flow cytometry.
Cytoplasmic Ca2+-measurement
Cytoplasmic Ca2+ was measured using Fluo-3/AM (Molecular Probes, Invitrogen, Paisley, UK) as described previously.11
In these experiments freshly isolated NK cells were washed with HBSS containing 1% FCS and 106 cells were loaded with 1 ml of 5 μmol Fluo3/AM with 0·05% Pluronic F-127 (Molecular Probes) for 30 min at room temperature. Cells were washed three times and kept on ice in either HEPES-buffered saline (137 mmol/l NaCl, 5 mmol/l KCl, 1 mmol Na2HPO4, 5 mmol/l glucose, 1 mmol/l CaCl2, 0·5 mmol/l MgCl2, 1 mg/ml bovine serum albumin and 10 mmol/l HEPES, pH 7·4) or HEPES-buffered saline (calcium-free). Cells were warmed to 37° 5 min prior to analysis and 10 μg/ml anti-CD8 (RPA-T8), or anti-CD56 was added. In some experiments goat anti-mouse IgG was added prior to the anti-CD8 to try to enhance CD8 signalling by cross-linking. Fluo-3/AM loading was checked by adding 2 μg/ml of ionomycin (Sigma, Poole, Dorset, UK) as a positive control.
Cytotoxicity assay
Primary AML cells isolated from bone marrow aspirates at disease presentation from consenting patients were cryopreserved until use. All samples were assessed morphologically as containing >90% blasts and consisted of one AML M2, two M4 and one M5. K562 cells (American Type Culture Collection), P815 cells (gift from Dr M. Glennie, Southampton, UK), 721.721 cells and 721.221 transfected with Cw*0304 (gift from Dr Linda Barber, Anthony Nolan Research Institute, London) were maintained in continuous suspension culture. Prior to their use as target cells each of these cell types was washed in HBSS, resuspended in labelling buffer (Diluent C) and labelled with PHK-26 dye (Sigma) as previously described.12 Briefly target cells in continuous exponential growth phase were recovered from culture and washed in HBSS and resuspended in 1·0 ml of PHK-26 labelling diluent at a concentration of 4 × 106/ml. A 4-μl aliquot of PKH-26 was added to 1·0 ml of labelling diluent and then this mixture was added to the cell suspension for 2 min at room temperature. The labelling reaction was stopped by the addition of 1·0 ml neat FCS for 1 min. Finally the labelled cells were washed twice in CM and resuspended in CM at 106/ml; 106 effector cells were incubated for 30 min at room temperature with and without mAb (10 μg/ml). 10 000 PKH-26 labelled target cells in 100 μl RPMI-1640 (10% FCS) were added to 300 μl of effector cells and pelleted at 200 g for 1 min.
Cytotoxicity was measured in triplicate samples using a 4-hr cytotoxicity assay at 37°. After the incubation period the cells were resuspended in a solution of To-Pro-3 iodide (Molecular Probes) in PBS (1 μm) and analysed by flow cytometry. At least 10 000 target cells were acquired with 1024-channel resolution after electronic gating on red fluorescence and the mean proportion of To-Pro iodide-positive cells from the triplicate samples was determined. Background target cell death was determined from cells incubated in the absence of effector cells. Cell-mediated cytotoxicity was reported as percentage killing over background cell death averaged from the three samples: mean (% cell lysis in test − % spontaneous lysis). Less than 5% spontaneous lysis of target cells was observed in these experiments.
Results
Expression of CD8α molecule on peripheral blood NK cells
Peripheral blood samples from 20 healthy adult donors were tested for frequency of CD8+ cells within the CD3– CD56+ cell population. Frequencies ranged from 13 to 81% of NK cells (mean 46·1%, SD 18·9). In contrast, the intensity of expression was constant between donors (data not shown).
CD8+ NK cells are more cytotoxic than CD8– NK cells
To confirm our previous findings and those of others4–7 CD56+ CD8+ and CD56+ CD8– NK cells were isolated from normal donors and from patients in clinical remission after chemotherapy for AML and tested against K562 cells and autologous leukaemia cells that were cryopreserved at patient presentation. In all cases the CD8+ subset was significantly more lytic than CD8– NK cells from the same donor (P < 0·01) (Fig. 1). We hypothesized that this increase in lytic activity could be the result of increased adhesion molecule expression, decreased KIR expression, increased perforin and or granzyme content, increased expression of NK natural cytotoxicity receptors (NCRs), a direct effect of signalling through CD8, a differential response to activating signals or combinations of one or more of these.
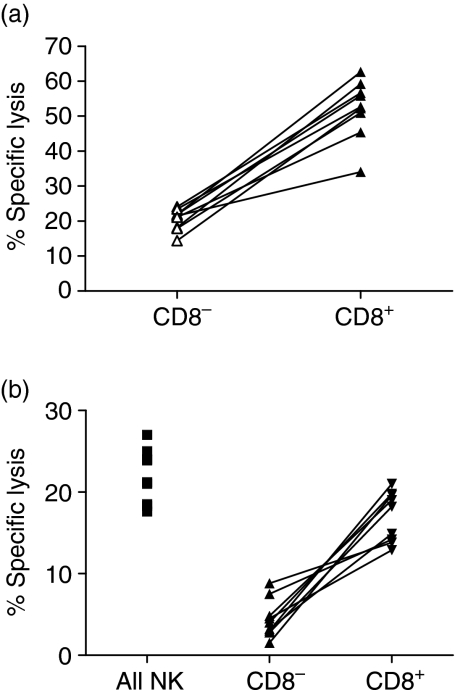
Figure 1.
CD8+ NK cells from normal donors and AML patients in remission are more cytotoxic than CD8– NK cells against K562 cells and autologous AML blasts, respectively. Freshly isolated CD8+ or CD8– NK cells from normal donors (n = 5) or patients with AML in complete remission after chemotherapy (n = 4) were incubated in triplicate with PKH-26-labelled K562 cells (a) or autologous AML blasts (b), respectively, in 4-hr assays at 37°. Cell death was determined by flow cytometric measurement of influx of To-Pro iodide into PKH26+ target cells. Results are presented as paired results for 5 : 1 effector : target ratios. Paired Student's t-test showed consistent significant increases (P < 0·05; Student's paired t-test) in cytotoxicity of CD8+ NK subset compared to the CD8– subset from the matched donor in all cases.
Expression of NCRs and adhesion molecules
Peripheral blood NK cells from normal donors were analysed by flow cytometry for the expression of NKp30, NKp44, NKp46 and NKG2D; the relative proportions of cells expressing these NCRs were comparable between the CD8+ and CD8– fractions, as were the densities of expression (data not shown). The relative proportions of cells within each subset expressing the adhesion molecules CD2, CD11a, CD11b, CD11c, CD16, CD54, CD56, CD58 and CD102 were comparable between the two subsets (data not shown) and there were no significant differences in the intensity of expression of any of these molecules, although there was a tendency towards higher expression of CD2 on CD8+ NK cells (P = 0·08; data not shown).
CD8+ and CD8– NK cells show identical levels of intracellular granzyme A and perforin
No differences could be detected in the levels of intracellular perforin or granzyme A between the two NK-cell subsets (data not shown).
CD8+ and CD8– NK cells show identical KIR expression and CD8 expression does not block KIR-mediated NK inhibition by HLA
NK cells from 10 normal healthy donors were assessed for the frequency and intensity of expression of CD158a, CD158b and NKB1 to determine whether the increased lytic activity of the CD8+ subset could be explained by a deficiency in these KIR molecules. Of the CD8+ NK subset the mean proportions of CD158a+, CD158b+ and NKB1+ cells were 18·48% (SD 11·32), 39·8% (SD 14·67) and 22·92% (SD 12·93), respectively. The proportions for the same subsets of CD8– NK cells were 13·87% (SD 8·67 P = NS), 36·26% (SD 21·96 P = NS) and 23·65% (SD 15·63 P = NS). The relative fluorescence intensities showed no significant differences either (data not shown).
To determine whether the increased lytic activity against autologous AML cells was the result of interference of the NKIR/MHC Class I interaction by the CD8α molecule, CD56+ CD3–CD8+ NK cells were incubated in cytotoxic assays (effector to target ratio 5 : 1) with 721.221 cells and with 721.221 cells transfected with Cw*0304, a ligand for CD158b (data not shown). All three normal donors used had high frequencies of CD158b+ NK cells and low frequencies of CD158a+ cells. CD8+ NK cells from all donors lysed the non-transfected 721.221 target cells whilst the Cw*0304 transfected cells were protected from lysis despite the presence of CD8α on the NK cells (data not shown).
CD8+ and CD8– NK cells show differential levels of cytotoxicity-induced apoptosis
Having found no discernible differences between the expression of molecules controlling NK-cell activation and cytolysis we investigated the effects of target cell ligation on NK-cell viability. CD8+ and CD8– NK cells were isolated from normal healthy donors (n = 5) and incubated with equal numbers of PKH-26-labelled K562 target cells for up to 3 hr at 37°. The cell suspensions were labelled with anti-annexin V–FITC and analysed by flow cytometry. Viable and dead K562 were excluded from analysis on the basis of forward scatter (FSC) and PKH-26 signals. In all cases, the proportion of annexin V + NK cells was significantly greater in the CD8– fraction than in the CD8+(Fig. 2a,c,e). This was confirmed using the Caspase 3 substrate PhiPhiLux which was used to load resting NK cells prior to co-incubation with unlabelled K562 cells. The cell suspensions were labelled with anti-CD8 APC and analysed by flow cytometry. Figure 2(b) shows the high frequency of Caspase 3+ cells within the CD8– NK fraction as compared to that in the CD8+ cells (Fig. 2d) in the same assay.
Figure 2.
Co-incubation of NK cells with target cells initiates apoptosis in the CD8– subset which is not apparent in the CD8+ cells. Purified NK cells from normal donors (n = 5) were incubated with equal numbers of PKH-26-labelled K562 cells for 1 and 3 hr. Cells were labelled with anti-CD8 APC and target cells were excluded from the analysis by virtue of PKH26 expression. CD8– NK cells (a) showed a marked increase in the proportion of annexin V+/FSClow (apoptotic) cells within 1 hr of co-incubation which was absent in the CD8+ subset even at 3 hr (c). The apoptosis of the CD8– NK cells at 1 hr was confirmed by the detection of activated Caspase 3 (b) as determined by PhiPhiLux which was not apparent in the CD8+ subset (d). The figures in each plot represent the proportion of apoptotic cells. The paired changes in annexin V expression in CD8– and CD8+ cells for the five donors at 1 and 3 hr is shown in (e).
Signalling through the CD8α chain by cross-linking with anti-CD8 (RPA-T8) induces activation in freshly isolated NK cells but not cytotoxicity
Ligation of CD8 with mAbs induces an increase in intracellular Ca2+ and phosphorylation of p56lck in human T cells.13 We therefore asked whether CD8 cross-linking could trigger Ca2+ mobilization in CD56+ CD8+ NK cells. CD8+ NK cells were isolated from peripheral blood lymphocytes by sequential immunomagnetic sorting as described above. Sorted cells were treated with RPA-T8 in either the presence or absence of extracellular Ca2+. In the presence of extracellular Ca2+, ligation of the cells with RPA-T8 induced a rapid but transient rise in intracellular free calcium (Fig. 3a). When conducted in Ca2+-free media, CD8 ligation failed to induce a detectable increase in intracellular Ca2+, even when the cells were preincubated with goat anti-mouse immunoglobulin to enhance the CD8 signalling by cross-linking (Fig. 3b). In contrast to CD8 ligation, incubation with anti-CD56 antibody failed to increase intracellular Ca2+ (Fig. 3c), whilst treatment with calcium ionophore caused rapid and sustained Ca2+ mobilization (Fig. 3d).
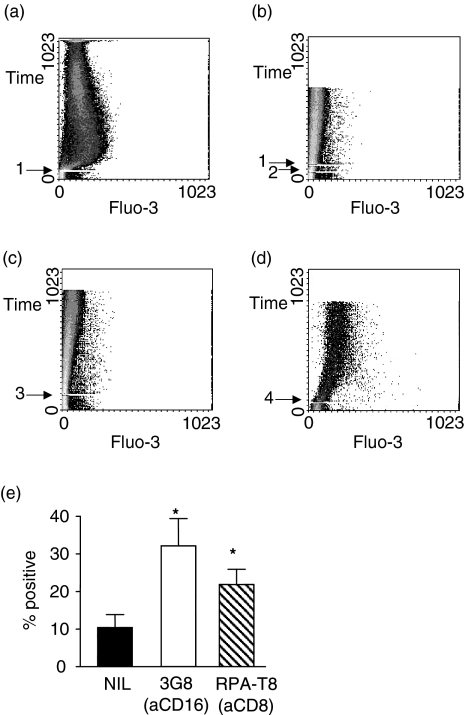
Figure 3.
Induction of intracellular Ca2+ in purified CD8+ ve NK cells by anti-CD8 MAb. Purified CD8+ ve (OKT8) NK cells were loaded with the fluorescent calcium indicator Fluo-3/AM and stimulated with RPA-T81 in the presence of extracellular calcium (A) or sequentially with goat-antimouse Ig2 and RPA-T81 in the absence of extra-cellular calcium (B). Stimulation with anti-CD563 was used as a negative control (C). Calcium ionophore4 was used as a positive control (D). Ligation of CD8 with RPA-T8 induces surface expression of CD69 within 4 h (E). Asterisks indicate results which are significantly greater (P < 0·05) than the untreated cells.
Moreover, the ligation of CD8 on these cells in the presence of extracellular Ca2+ induced rapid and sustained expression of CD69 on the cell surface (Fig. 3e), a phenomenon which is dependent upon influx of calcium and which does not occur following mobilization of Ca2+ from intracellular stores.14
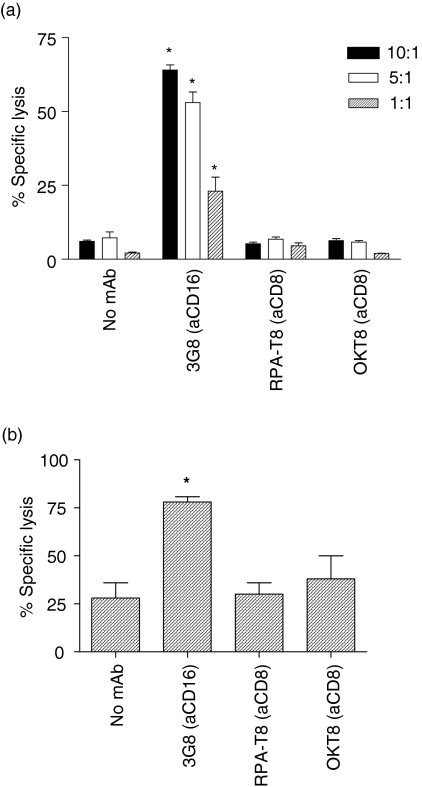
To determine whether CD8 cross-linking could trigger NK cytotoxicity of an NK-resistant cell line we employed the reversed ADCC assay of the P815 murine cell line using either resting or IL-2-stimulated NK cells. Briefly, murine Fc-receptor-bearing P815 cells were coated with the relevant mAb or left in their native state and then co-incubated with human NK cells for 4 hr; cytotoxicity was then determined as described above. As shown in Fig. 4 neither resting NK cells (Fig. 4a) nor IL-2-activated NK cells (Fig. 4b) showed increased lytic activity in the presence of anti-CD8 mAbs in contrast to the significant increase in specific lysis induced by anti-CD16.
Figure 4.
CD8 is not a cytotoxic triggering molecule on freshly purified or IL-2-stimulated NK cells. Neither freshly isolated NK cells at various effector : target ratios (a) nor IL-2-activated NK cells at an effector : target ratio of 5 : 1 (b) were triggered by either anti-CD8 (RPA-T8) or anti-CD8 (OKT8) to mediate reverse ADCC of P815 target cells (a kind gift from Prof. M. Glennie, University of Southampton). Anti-CD16 (3G8), was used as a positive control. Asterisks indicate results which are significantly greater (P < 0·05) than the background NK lysis in the absence of mAb.
The anti-apoptotic effect of CD8 ligation is not dependent upon the NK : target cell synapse but can be provided by co-localized NK cells
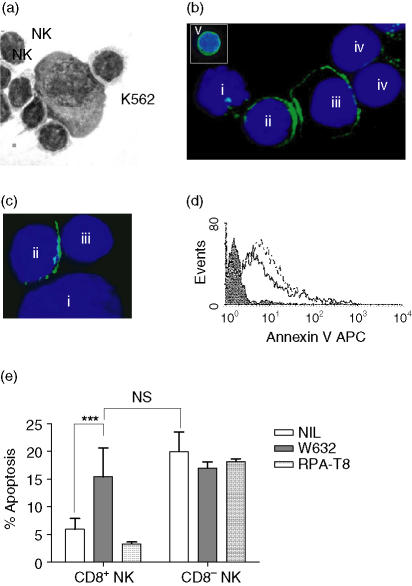
It was paradoxical that the CD8+ NK cells appeared protected from cytotoxicity-induced cell death (CICD) through CD8 ligation even when the target cells lacked HLA-Class I. CD8+ NK cells from a normal donor were incubated at a 5 : 1 effector : target ratio with K562 cells for 30 min. NK cells formed tight conjugates which were resistant to vigorous pipetting and which could be visualized by light microscopy (Fig. 5a). NK cells formed immune synapses with both the K562 target cell and with co-localized NK cells. Confocal microscopy confirmed that CD8 molecules capped at the synapse formed between the NK cells in the conjugate in contrast to the natural homogeneous expression of CD8 on non-conjugated NK (cell ‘v’ in Fig. 5b). Ligation of CD8 on CD8+ NK after target cell binding can be provided by both CD8+ and CD8– NK cells (Fig. 5c) although we were unable to find NK : NK conjugates in the absence of NK : target cell binding. Furthermore, in 3-hr cytotoxicity assays the addition of the anti-HLA Class I mAb W632 to the cytotoxicity assay rendered the CD8+ NK cells susceptible to CICD (Fig. 5d).
Figure 5.
NK cells conjugate simultaneously to target cells and to other NK cells and provide CD8 ligation. Freshly isolated NK cells (CD8+ and CD8–) were co-incubated with K562 cells for 30–240 min. At 60 min NK–NK and NK–target cell conjugates formed (a). (b) shows a 90-minute conjugate of a single K562 target cell (i) and a CD8+ NK cell (ii) with capping of the CD8 molecule (Leu2 FITC) at the cell–cell synapse between two CD8+ NK cells (ii and iii). Furthermore, the second CD8+ NK cell has formed synapses with two CD8– NK cells (iv). The insert (v) shows the homogeneous expression of CD8 on a non-conjugated NK cell. Smaller conjugates were apparent involving a CD8+ NK cell (ii) and a target cell (i) with CD8 ligation by a single CD8– NK cell (iii) (c). Apoptosis was measured at 240 min by flow cytometric assessment of annexin V expression on CD8+ and CD8– NK cells in the presence or absence of W632 (d). CD8+ NK showed low expression of annexin V (shaded histogram) in the absence of W632 in contrast to the high expression on the CD8– cells (dashed histogram) and coincident reduction in forward angle light scatter (not shown) indicative of apoptosis. In the presence of the MHC Class I blocking mAb W632 the CD8+ NK cells showed equivalent levels of apoptosis to the CD8– cells (solid line). In 1-hr K562 conjugation assays with five normal donors (e), blockade of the CD8–MHC Class I interaction with W632 (solid bars) increased the activation-induced apoptosis within the CD8+ NK subset significantly (P < 0·001; paired t-test; n = 5) to levels equivalent to those of CD8– NK cells in the same culture and this could be prevented by direct ligation of CD8 on the NK-cell surface with RPA-T8 mAb (hatched bars). Neither mAb had any effect on the CD8– NK cells in the culture (e).
Whilst blockade of the CD8 : MHC interaction with W632 mAb rendered the CD8+ NK susceptible to activation-induced apoptosis at 1 hr, it was possible to overcome this by concomitant direct ligation of CD8 through addition of RPA-T8 mAb to the cultures (Fig. 5e).
Discussion
The role of CD8 as an ‘accessory molecule’ on T cells is well established. However, the CD8 molecule is expressed on approximately 40% of NK cells and in this study our results confirm previous findings that the CD8+ NK subset is more cytolytic than its CD8– counterpart.4–8 The principal mechanism of NK-mediated lysis is via the secretion of perforin and granzymes but the increased cytolytic activity of the CD8+ cells was not the result of differences in the levels of either of these. It is an established fact that the initiation of NK-cell activity is a balance between inhibitory and activatory signals.9,10 We initially hypothesized that CD8+ NK cells might express lower levels of inhibitory KIRs or increased levels of NCRs but could find no evidence of this using cell surface phenotyping techniques. Since the extracellular domains of inhibitory and activating KIRs are indiscernible with respect to antibody binding it remains possible that the CD8+ NK cells express higher proportions of activating KIR with short intracytoplasmic domains but we were unable to investigate this.
It was possible that the binding of cell membrane CD8 to class I HLA on target cells might interfere with the KIR–MHC interaction, so reducing inhibitory function, but this was also not supported by the experimental evidence.
Although CD8 ligation did not initiate cytolytic activity it did lead to a consistent increase in intracellular calcium. This increase was not the result of mobilization of calcium from intracellular stores but rather it was the result of the increased influx of calcium from the extracellular milieu. This was shown both by the fact that the increase only occurred when the experiment was conducted in calcium-containing media and by the fact that CD8 ligation was associated with increased expression of CD69. It has been shown previously that CD69 expression requires an influx of calcium from extracellular sources14 and that CD8 ligation leads to ingress of extracellular calcium.15
This study is the first to demonstrate the induction of apoptosis in NK cells after tumour cell lysis although others have previously reported target-induced anergy in NK cells16 which may have been because of the onset of apoptosis. We confirmed the onset of apoptosis using two established flow cytometric assays which allowed us to measure apoptosis in effector cell subsets whilst excluding any concomitant target cell apoptosis. Others have recorded apoptosis by annexin V expression in K562 target cells after co-incubation with NK cells but did not assess the NK cells specifically.17 Furthermore, it should be noted that the flow cytometric assay used did not exclude NK cells by membrane dye labelling and at least some of the apoptotic cells reported may have been free NK cells or NK cells within NK-K562 conjugates.
We hypothesized that the post-conjugation/lysis apoptosis was secretion-induced, as has been previously shown for a variety of cell types,18 and found evidence for this when we reduced the level of post-conjugation apoptosis by adding brefeldin to the coculture.
Secretion of granzymes and perforin is calcium-dependent and requires mobilization of bound calcium from intracellular stores such as the endoplasmic reticulum. The reduction in intracellular calcium stores below a predetermined threshold is a known trigger of apoptosis.19
From these experiments we propose the following model of NK-cell function. NK–target cell conjugation is associated with concomitant NK–NK conjugation as we have demonstrated here and elsewhere.8 The initiation of NK lytic activity through NCR and KIR ligation at the target cell immune synapse, results in calcium-dependent secretion of intracellular lytic granules and the reduction of stored calcium below the threshold for apoptosis. NK cells expressing CD8 are rescued from secretion-induced apoptosis by ligation of CD8 by MHC class I molecules expressed on NK cells in the NK–NK synapse and the subsequent CD8-induced influx of calcium from extracellular sources. It is possible that the CD8–MHC ligation also occurs at the NK–target cell synapse when the target cells express sufficient density of MHC Class I but we have shown here that this is not a requirement by using MHC Class I-deficient target cells. CD8α/α is known also to bind non-classical MHC molecules such as HLA-G,12 murine TL20 and the CD1 family of proteins. These are non-polymorphic glycoproteins related in structure and evolutionary origin to the MHC-encoded molecules but with more restricted tissue distribution. It is possible that one or more of these molecules may be present on tumour cells and affect the same result.
The ligation of CD8 also increases the expression of CD69 on the CD8+ NK cells. This molecule is an activatory ligand for cytotoxicity13,21 and we hypothesize that its up-regulation further enhances target cell lysis by binding to its ligand on the target cell. The ligand for CD69 is currently unknown.
In contrast to our findings here, a recent study from a single group reported MHC class I-induced apoptosis of human CD8+ NK cells.15,22 The CD8α/α molecules were ligated with soluble human MHC class I molecules and the resultant NK apoptosis was mediated by the secretion of soluble FasL binding to Fas on the NK surface. These studies were restricted to NK clones, which expressed surface Fas, whereas we did not detect Fas expression on the freshly isolated NK cells used in our experiments (data not shown). We were unable to induce apoptosis through CD8 ligation with either mAb studied nor by co-incubation with MHC class I transfected 721.221 cells. Furthermore, the increased lysis of MHC class I-positive autologous AML cells by CD8+ NK cells argues that the presence of membrane-bound MHC class I antigens in the presence of stimulatory signals does not lead to apoptosis of the CD8+ NK cells.
In summary, our findings indicate that the CD8 molecule provides NK cells with a survival mechanism after target cell lysis, potentially allowing conjugation and lysis of multiple target cells. CD8+ NK cells may be regarded as the ‘waSPS’ of innate cellular immunity whist CD8– NK are the ‘bees’!
Acknowledgments
We are grateful to Dr M. Glennie (University of Southampton, UK) for the provision of the P815 cells and to Dr L. Barber and Prof. A. Madrigal (Anthony Nolan Research Institute) for supplying the 721.221 cell transfectants. We also thank Prof. Madrigal for his helpful comments and advice. This work was supported by the Leukaemia Research Fund and the Association for International Cancer Research. J.K.D. was supported by an MRC Clinical Training Fellowship. We wish to acknowledge colleagues within the Department of Haematology for provision of clinical material.
References
- 1.Veillette A, Bookman MA, Horak EM, Bolen JB. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988;55:301–8. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- 2.Miceli MC, Parnes JR. Role of CD4 and CD8 in T cell activation and differentiation. Adv Immunol. 1993;53:59–122. doi: 10.1016/s0065-2776(08)60498-8. [DOI] [PubMed] [Google Scholar]
- 3.Parnes JR. Molecular biology and function of CD4 and CD8. Adv Immunol. 1989;44:265–311. doi: 10.1016/s0065-2776(08)60644-6. [DOI] [PubMed] [Google Scholar]
- 4.Kushima K, Fujita M, Shigeta A, Horiuchi H, Matsuda H, Furusawa S. Flow cytometric analysis of chicken NK activity and its use on the effect of restraint stress. J Vet Med Sci. 2003;65:995–1000. doi: 10.1292/jvms.65.995. [DOI] [PubMed] [Google Scholar]
- 5.Srour EF, Leemhuis T, Jenski L, Redmond R, Jansen J. Cytolytic activity of human natural killer cells subpopulations by four-colour immunofluorescence flow cytometric sorting. Cytometry. 1990;11:442–6. doi: 10.1002/cyto.990110316. [DOI] [PubMed] [Google Scholar]
- 6.Fuchshuber PR, Lotzova E. Differential oncolytic effect of NK-enriched subsets in long-term interleukin-2 cultures. Lymphokine Cytokine Res. 1992;11:271–6. [PubMed] [Google Scholar]
- 7.Lowdell MW, Ray N, Craston R, Corbett T, Deane M, Prentice HG. The in vitro detection of anti-leukaemia-specific cytotoxicity after autologous bone marrow transplantation for acute leukaemia. Bone Marrow Transplant. 1997;19:891–7. doi: 10.1038/sj.bmt.1700756. [DOI] [PubMed] [Google Scholar]
- 8.Lowdell MW, Craston R, Samuel D, Wood ME, O'Neill E, Saha V, Prentice HG. Evidence that continued remission in patients treated for acute leukaemia is dependent upon autologous natural killer cells. Br J Haematol. 2002;117:821–7. doi: 10.1046/j.1365-2141.2002.03495.x. [DOI] [PubMed] [Google Scholar]
- 9.Bakker AB, Wu J, Phillips JH, Lanier LL. NK cell activation: distinct stimulatory pathways counterbalancing inhibitory signals. Human Immunol. 2000;61:18–27. doi: 10.1016/s0198-8859(99)00160-3. [DOI] [PubMed] [Google Scholar]
- 10.Moretta A, Biassoni R, Bottino C, Mingari MC, Moretta L. Natural cytotoxicity receptors that trigger human NK-cell-mediated cytolysis. Immunol Today. 2000;21:228–34. doi: 10.1016/s0167-5699(00)01596-6. [DOI] [PubMed] [Google Scholar]
- 11.Vandenberghe PA, Ceuppens JL. Flow cytometric measurement of cytoplasmic free calcium in human peripheral blood T lymphocytes with Fluo-3, a new fluorescent calcium indicator. J Immunol Meth. 1990;127:197–205. doi: 10.1016/0022-1759(90)90069-8. [DOI] [PubMed] [Google Scholar]
- 12.Sanders SK, Giblin PA, Kavathas P. Cell-cell adhesion mediated by CD8 and human histocompatibility leukocyte antigen G, a nonclassical major histocompatibility complex class 1 molecule on cytotrophoblasts. J Exp Med. 1991;174:737–40. doi: 10.1084/jem.174.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Testi R, D'Ambrosio D, De Maria R, Santoni A. The CD69 receptor: a multipurpose cell surface trigger for hematopoietic cells. Immunol Today. 1994;15:479–83. doi: 10.1016/0167-5699(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 14.Mascarenhas SR, Echevarria-Lima J, dos Santos NF, Rumjanek VM. CD69 expression induced by thapsigagin, phorbol ester and oubain on thymocytes is dependent on external Ca2+ entry. Life Sci. 2003;73:1037–51. doi: 10.1016/s0024-3205(03)00377-1. [DOI] [PubMed] [Google Scholar]
- 15.Puppo F, Contini P, Ghio M, Indiveri F. Soluble HLA-class I molecules/CD8 ligation trigger apoptosis of CD8+ cells by Fas/Fas–ligand interaction. Sci World J. 2002;2:421–3. doi: 10.1100/tsw.2002.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jewett A, Bonavida B. Target-induced anergy of natural killer cytotoxic function is restricted to the NK-target conjugate subset. Cellular Immunol. 1995;160:91–7. doi: 10.1016/0008-8749(95)80013-9. [DOI] [PubMed] [Google Scholar]
- 17.Umemoto M, Azuma E, Hirayama M, et al. Two cytotoxic pathways of natural killer cells in human cord blood: implications in cord blood transplantation. Br J Haematol. 1997;98:1037–40. doi: 10.1046/j.1365-2141.1997.3183135.x. [DOI] [PubMed] [Google Scholar]
- 18.Maag RS, Hicks SW, Machamer CE. Death from within: apoptosis and the secretory pathway. Curr Opin Cell Biol. 2003;15:456–61. doi: 10.1016/s0955-0674(03)00075-9. [DOI] [PubMed] [Google Scholar]
- 19.McConkey DJ, Orrenius S. The role of calcium in the regulation of apoptosis. Biochem Biophys Res Comm. 1997;239:357–66. doi: 10.1006/bbrc.1997.7409. [DOI] [PubMed] [Google Scholar]
- 20.Teitell M, Mescher MF, Olson CA, Littman DR, Kronenberg M. The thymus leukemia antigen binds human and mouse CD8. J Exp Med. 1991;174:1131–8. doi: 10.1084/jem.174.5.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moretta A, Poggi A, Pende D, Tripodi G, et al. CD69-mediated pathway of lymphocyte activation: anti-CD69 monoclonal antibodies trigger the cytolytic activity of different lymphoid effector cells with the exception of cytolytic T lymphocytes expressing T cell receptor alpha/beta. J Exp Med. 1991;174:1393–8. doi: 10.1084/jem.174.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spaggiari GM, Contini P, Carosio R, et al. Soluble HLA class I molecules induce natural killer cell apoptosis through the engagement of CD8: evidence for a negative regulation exerted by members of the inhibitory receptor superfamily. Blood. 2002;99:1706–14. doi: 10.1182/blood.v99.5.1706. [DOI] [PubMed] [Google Scholar]