Abstract
Macrophages respond to Mycobacterium tuberculosis by regulating expression of gene products that initiate a host innate response to this micro-organism. In this study, we report that exposure of murine peritoneal macrophages to heat-killed Mycobacterium tuberculosis (HK-Mtb) led to an increase in secretory leucocyte protease inhibitor (SLPI) gene expression and protein secretion in a time- and dose-dependent manner. HK-Mtb-induced SLPI mRNA expression was sensitive neither to a protein synthesis inhibitor, cycloheximide, nor to an actin polymerization blocker, cytochalasin D. Treatment of macrophages with interferon (IFN)-γ inhibited HK-Mtb-induced SLPI expression. RAW264·7 cells stably expressing SLPI produced a reduced level of tumour necrosis factor (TNF) in response to HK-Mtb as compared with mock transfectants. Aerosol infection of mice with live M. tuberculosis resulted in an induction of SLPI gene expression in infected lungs. Macrophages from Toll-like receptor 4 (TLR4)–/– or MyD88–/– mice responded to M. tuberculosis similarly to wild-type macrophages by exhibiting increased SLPI expression. In contrast, macrophages from TLR2–/– mice were incapable of inducing SLPI in response to M. tuberculosis. This induction signifies the presence of a TLR2-dependent but MyD88-independent M. tuberculosis signalling pathway, suggesting involvement of adaptor protein(s) other than MyD88 in M. tuberculosis-mediated induction of SLPI.
Keywords: rodent, macrophage, mycobacteria, signal transduction, protease inhibitor
Introduction
Toll-like receptors (TLRs) are a family of evolutionarily conserved surface molecules that contain extracellular leucine-rich repeats and an intracellular Toll/interleukin (IL)-1 receptor homology (TIR) domain.1–3 TLRs play a crucial role in the host innate recognition of and response to a variety of microbial pathogens. The best studied TLR agonist is the lipopolysaccharide (LPS) of Gram-negative bacteria. LPS can be recognized by TLR4 or TLR2, depending on its bacterial origin. The immediate downstream signalling event following LPS/TLR4 interaction is the recruitment of a family of adaptor proteins, including MyD88, TIR domain-containing adapter protein (TIRAP), TIR-containing adapter inducing interferon (IFN)-β (TRIF or Lps2) and TRIF-related adapter molecule (TRAM or TIRP). In contrast, other TLRs except TLR2 and TLR3 appear to use only MyD88 to transduce signals. Activation of TLR2 utilizes TIRAP in addition to MyD88,4,5 while activation of TLR3 by double-stranded RNA only utilizes TRIF.6,7
Mycobacterium tuberculosis, the causative agent of pulmonary tuberculosis, has been shown to activate macrophages via TLR2 and TLR4.8–11M. tuberculosis-mediated macrophage activation is important to allow the host to restrain M. tuberculosis infection12 and is accompanied by induction of a wide spectrum of bioactive cellular products.13 Microarray analysis with MyD88–/– cells, however, revealed that the majority of M. tuberculosis-induced gene expression in mouse bone marrow-derived macrophages is independent of MyD88.14 It is not known what surface receptors initiate the MyD88-independent signalling pathways triggered by M. tuberculosis.
Secretory leucocyte protease inhibitor (SLPI), an 11·7-kDa cysteine-rich secretory protein, was originally isolated from human saliva15 and subsequently found in many other body fluids, including bronchial mucus16 and plasma.17 Tracheal, bronchial and type II alveolar cells as well as macrophages and neutrophils are rich sources of SLPI in the lung. Administration of exogenous recombinant SLPI via intratracheal instillation or aerosol inhalation has been shown to control lung inflammation induced by immune complex deposition,18 neutrophil elastase19 or LPS.20 The level of endogenous SLPI during infection is under strict control. Macrophages produced an elevated level of SLPI in response to constituents of Gram-negative and Gram-positive bacteria such as LPS17 and lipoteichoic acid,21 to cytokines IL-6 and IL-1021 and to apoptotic cells.22 As the agent of pulmonary tuberculosis, M. tuberculosis infects the lungs of the host. It is not known how M. tuberculosis infection affects endogenous SLPI levels, nor how endogenous SLPI levels affect host responses to M. tuberculosis. The present study was designed to characterize macrophage responses to M. tuberculosis in terms of SLPI expression. During the course of study, we discovered that M. tuberculosis-mediated SLPI induction utilizes a novel M. tuberculosis signalling pathway. This pathway depends on TLR2 but not MyD88.
Materials and methods
Mice
Adult TLR2–/– TLR4–/– MyD88–/– mice were generated by Dr S. Akira's laboratory (Osaka University, Osaka, Japan) as previously described.23–25 These mice had been backcrossed six times to the C57BL/6 background. TRIF mutant mice were provided by Dr B. Beutler (the Scripps Institute, La Jolla, CA) and TIRAP–/– mice by Dr R. Medzhitov (Yale University School of Medicine, New Haven, CT). Age-matched wild-type littermates were bred in the Animal Research Center at the Weill Medical College of Cornell University (New York, NY). Wild-type C57BL/6 mice were purchased from the Charles River Breeding Laboratories (Wilmington, MA).
Cells
Primary mouse peritoneal macrophages were collected from the peritoneal cavity 4 days after intraperitoneal injection with 2 ml of 4% Brewer's thioglycollate broth (Difco, Detroit, MI). Bone marrow-derived macrophages were isolated and differentiated in culture as previously described.14 The RAW264·7 mouse macrophage cell line was from the American Type Culture Collection (ATCC; Manassas, VA). RAW cells stably expressing high levels of SLPI were generated as previously described.22 Cells were maintained in Dulbecco's modified Eagle's minimal essential medium (DMEM; Gibco BRL, Grand Island, NY) supplemented with 10% heat-inactivated fetal bovine serum (HyClone, Logan, UT), 2 mm l-glutamine, 200 units/ml penicillin and 200 µg/ml streptomycin (complete medium) at 37° in 5% CO2/95% air. Complete culture medium was routinely monitored for LPS contamination and found to contain <25 pg LPS/ml, as determined by the limulus amebocyte lysate assay (BioWhittaker, Walkersville, MD).
Detection of SLPI protein in culture supernatants or cell lysates
The proteins in serum-free culture supernatants from 107 cells were precipitated with ice-cold 10% trichloroacetic acid (TCA) for 30 min. The precipitates were rinsed twice in acetone and boiled for 5 min in Laemmli buffer. For cell lysates, 2 × 106 cells were collected and boiled directly in Laemmli sample buffer. Proteins were separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose membrane (Schleicher and Shuell, Inc., Keene, NH). The membrane was blocked with 5% milk and blotted with an anti-mouse SLPI IgG (1 : 5000)26 followed by a donkey anti-rabbit IgG coupled to horseradish peroxidase (HRP). The bound antibody was detected by an enhanced chemiluminescent substrate for HRP (Pierce, St Louis, MO).
Mouse infections
Mice were infected with logarithmic growth phase cultures of M. tuberculosis by aerosol using a Glas-Col Inhalation Exposure System (Glas-col Inc., Terre Haute, IN). Animals were exposed for 30 min to an aerosol produced by nebulizing 5 ml of a bacterial suspension in phosphate-buffered saline at a concentration of ∼2 × 107 bacilli/ml. This resulted in an inoculum size of 50–70 colony-forming units (CFU) per lung as determined by plating homogenized lungs onto enriched 7H11 plates 24 hr post-infection.
Infection of macrophages
Thioglycollate-elicited macrophages were cultured in 100-mm-diameter culture dishes (107 cells/dish) without antibiotics for 24 hr and then infected with live or heat-killed (HK) M. tuberculosis strain 1254 from early log-phase cultures.14
Northern blot
Total RNA (20 µg/lane) was electrophoresed on a 1% agarose gel with 20 mm 3-[N-morpholino] propanesulphonic acid, pH 7·0, 50 mm sodium acetate, 1 mm ethylenediaminetetraacetic acid (EDTA) (1 × MOPS) and 2% formaldehyde, and equal loading was confirmed by ethidium bromide staining. RNA was transferred in 20 × saline sodium citrate (SSC) onto a nylon membrane (NEN; Research Products, Boston, MA). The membrane was hybridized for 18 hr at 42° with labelled probe [106 counts per minute (c.p.m.)/ml] in 5 × SSC, 5 × Denhart, 50% formamide and 1% SDS. Membranes were then washed twice with 1 × SSC and 0·1% SDS (10 min; 25°) and with 0·25 × SSC and 0·1% SDS (10 min, 55°) before exposure to X-Omat AR film (Kodak, Rochester, NY). Control probe for β-actin cDNA was amplified using the manufacturer's templates and amplimers (Clontech, Palo Alto, CA). The probes were radiolabelled with the Priming-a-gene kit from Promega (Madison, WI).
Real-time quantitative reverse transcriptase–polymerase chain reaction (qPCR)
The 5′, 3′ nuclease activity of Taq polymerase was used to detect PCR-amplified nucleic acids. Standard curves were generated for each gene product using a plasmid dilution series containing the target sequences. Probes were synthesized by Biosearch Technologies (Novato, CA) and labelled with the reporter dye fluorescent amidites (FAM) at the 5′ end and the quencher Black Hole Quencher (BHQ) at the 3′ end. Primer and probe sequences for SLPI were: qPCR primer (forward) 5′-d(GCTGTGAGGGTATATGTGGGAAA)-3′, qPCR primer (reverse) 5′-d(CGCCAATGTCAGGGATCAG)-3′, and qPCR probe 5′-FAMd(TCTGCCTGCCCCCGATGTGAG)BHQ-3′. Primer and probe sequences for GAPDH were: qPCR primer (forward) 5′-d(GGGAAGCCCATCACCATCTT)-3′, qPCR primer (reverse) 5′-d(ACATACTCAGCACCGGCCTC)-3′, and qPCR probe 5′-FAMd(AGCGAGACCCCACTAACATCAAATGGG)BHQ-3′. RNA (100 ng) was transcribed into cDNA with gene-specific primers in 20 µl using 50 U MuLV reverse transcriptase (Perkin Elmer, Wellesley, MA). cDNA was diluted to 100 µl. PCR was performed in a volume of 15 µl on the ABI PRISM 7900HT sequence detection system (Perkin Elmer).
Enzyme-linked immunosorbent assay (ELISA)
Cultured supernatants were collected and tested for tumour necrosis factor (TNF) contents using a Duoset ELISA (R & D Systems, Minneapolis, MN) according to the manufacturer's instructions.
Reagents and supplies
M. tuberculosis strain 1254, a low-passage clinical isolate, was from American Type Culture Collection 51910. Heat-killed M. tuberculosis (HK-Mtb) was prepared by incubating M. tuberculosis 1254 at 80° for 8 hr. LPS was prepared by phenol extraction according to the procedure of Qureshi et al.27 from Escherichia coli 0111:B4 LPS (Sigma, St Louis, MO). Peptidoglycan from Staphylococcus aureus was purchased from Fluka (Milwaukee, WI). Cycloheximide was from Sigma. Purified recombinant mouse IFN-γ (protein concentration 1 mg/ml; specific activity 107 U/mg; LPS content <52 pg/ml) was from Genentech (South San Francisco, CA). Oligonucleotide primers were from Invitrogen (Carlsbad, CA). G418 was from Gibco Life Technologies (Grand Island, NY); AmpliTaq DNA polymerase, dNTPs, and PCR buffer solutions and [α-32P]-deoxycytidine 5′-triphosphate (dCTP) were from Perkin Elmer Cetus (Foster City, CA). Plasmid DNA preparation columns were from Qiagen (Chasworth, CA).
Statistical analysis
The Student's t-test was used for analysis of the real-time PCR and ELISA.
Results
Heat-killed M. tuberculosis triggers SLPI release from macrophages in vitro
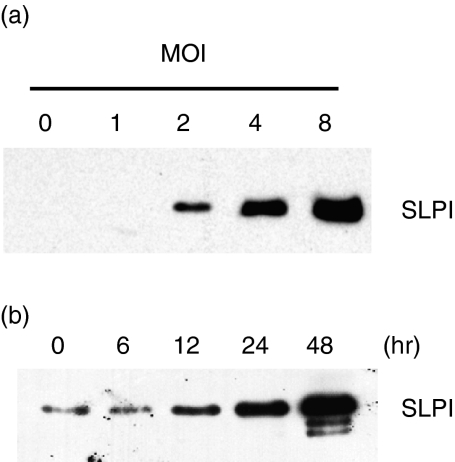
Mouse peritoneal macrophages were incubated for 90 min with HK-Mtb at a multiplicity of infection (MOI) from 1 to 8 in serum-free medium for 24 hr at 37°. Culture medium was collected and SLPI content evaluated by western blot analysis after TCA precipitation (Fig. 1a). SLPI was secreted by macrophages exposed to HK-Mtb in a dose-dependent manner. Similar results were obtained in experiments using RAW264·7 cells (data not shown). Kinetics study showed that the secretion of SLPI from primary cells increased as early as 12 hr after exposure of macrophages to HK-Mtb, and continued to increase up to 48 hr (Fig. 1b).
Figure 1.
Secretion of secretory leucocyte protease inhibitor (SLPI) by macrophages in response to heat-killed Mycobacterium tuberculosis (HK-Mtb). (a) Dose curve. Peritoneal macrophages (107) were treated with HK-Mtb [multiplicity of infection (MOI) from 1 to 8] for 24 hr. Protein samples from cultured supernatants were trichloroacetic acid (TCA)-precipitated, electrophoresed, and immunoblotted with anti-SLPI antibody. (b) Time–course. Secreted SLPIs from macrophages (107) treated with HK-Mtb (MOI = 4) for the indicated times were prepared and analysed as in (a).
Induction of SLPI expression by exposure of macrophages to HK-Mtb
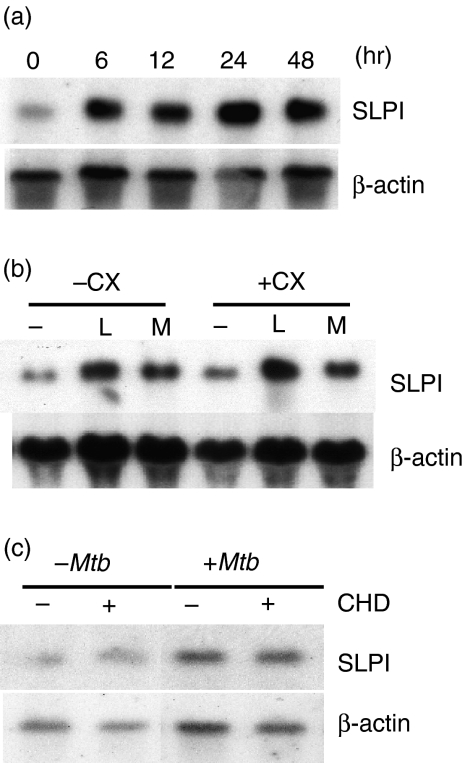
To test whether HK-Mtb-induced release of SLPI is caused by the triggering of preformed intracellular SLPI or by the induction of SLPI gene expression, we compared SLPI mRNA levels in macrophages in the presence and absence of HK-Mtb. HK-Mtb stimulated an increase in SLPI expression in a time-dependent fashion (Fig. 2a). Induction of SLPI by HK-Mtb was not detectable at 2 hr (not shown) but was readily detectable at 6 hr, and remained high throughout the study (up to 48 hr). To address the possibility that the SLPI gene might be induced indirectly by a HK-Mtb-inducible product, we examined the effect of the protein synthesis inhibitor cycloheximide. At 10 µg/ml, cycloheximide inhibited protein synthesis in primary macrophages by 95%,21 but the increased expression of SLPI by HK-Mtb, like that induced by LPS,21 was not inhibited (Fig. 2b). Next we tested whether phagocytosis of HK-Mtb was necessary for SLPI induction. Primary macrophages were pretreated with the actin polymerization blocker cytochalasin D for 30 min before the addition of HK-Mtb, and SLPI expression was analysed using northern blots. Although cytochalasin D blocked phagocytosis of HK-Mtb by macrophages (not shown), HK-Mtb-induced SLPI expression was not affected (Fig. 2c).
Figure 2.
Heat-killed Mycobacterium tuberculosis (HK-Mtb)-induced secretory leucocyte protease inhibitor (SLPI) expression and the effect of cycloheximide or cytochalasin D. (a) Peritoneal macrophages (107) were incubated alone or with HK-Mb [multiplicity of infection (MOI) = 4] for the indicated times. Total RNA was prepared, electrophoresed and hybridized with SLPI cDNA probe. The membrane was rehybridized with β-actin probe as a loading control. (b, c) Macrophages were pretreated with 10 µg/ml cycloheximide (CX) or 5 µg/ml of cytochalasin D (CHD) for 30 min, then incubated with 100 ng/ml lipopolysaccharide (L) or HK-Mtb (M, MOI = 4) for an additional 6 hr. Northern blots were prepared and analysed as in (a).
Effects of IFN-γ on SLPI expression
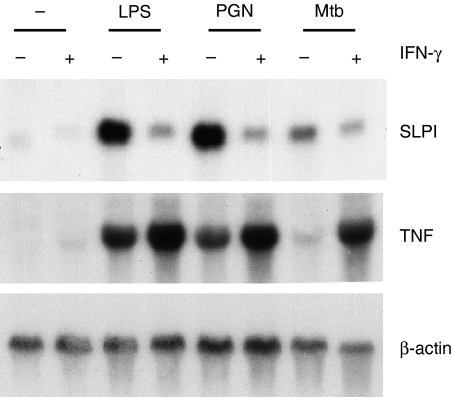
As the major activator of macrophages, IFN-γ is capable of inducing macrophages to produce a similar spectrum of immune mediators to those induced by many microbial products. The cardinal role of IFN-γ in host defence against M. tuberculosis infection is highlighted by the early onset of systemic M. tuberculosis infection and the early death of IFN-γ knockout mice.28 To test how IFN-γ affects SLPI induction by M. tuberculosis, peritoneal macrophages were incubated with LPS from E. coli, peptidoglycan from S. aureus and HK-Mtb in the presence or absence of IFN-γ for 24 hr. SLPI expression was induced by these three microbial products but not by IFN-γ alone. Co-incubation with IFN-γ suppressed SLPI induction, but enhanced TNF induction by each of these stimuli (Fig. 3). In a separate experiment, IFN-γ was preincubated with peritoneal macrophages for 24 hr before addition of HK-Mtb. Suppression of HK-Mtb-induced SLPI was observed again, regardless of whether IFN-γ was added before or together with HK-Mtb (not shown).
Figure 3.
Effect of interferon (IFN)-γ on heat-killed Mycobacterium tuberculosis (HK-Mtb)-induced secretory leucocyte protease inhibitor (SLPI) expression. Peritoneal macrophages (107) were incubated with 100 ng/ml lipopolysaccharide (LPS), or 10 µg/ml peptidoglycan (PGN), or 4 × 107 HK-Mtb in the presence or absence of 100 U/ml of IFN-γ for 24 hr. SLPI and tumour necrosis factor (TNF) mRNA levels were analysed by northern blots, controlled and expressed as in Fig. 2.
HK-Mtb-induced SLPI expression depends on TLR2, but not TLR4 or MyD88
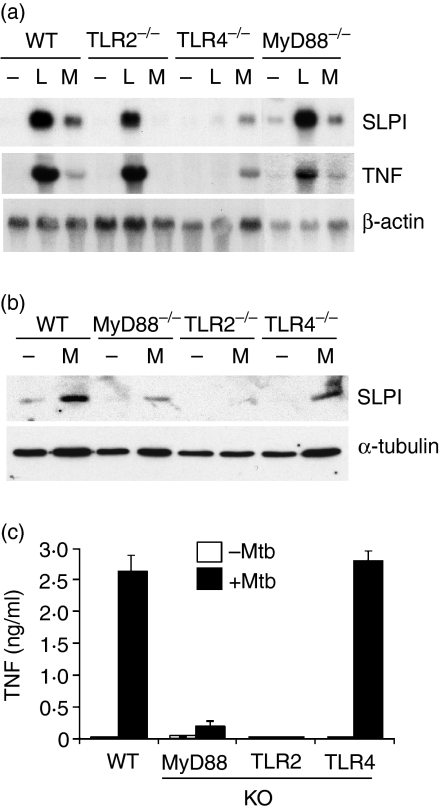
Mycobacterium tuberculosis is known to express both TLR2 and TLR4 agonists, which are usually presumed to signal via MyD88. To investigate the role of these molecules in SLPI induction by HK-Mtb, macrophages from wild-type, TLR2–/–, TLR4–/– or MyD88–/– mice were tested for SLPI induction after exposure to HK-Mtb. Figure 4(a) shows that SLPI mRNA induction by LPS and HK-Mtb was diminished in macrophages from TLR4–/– and TLR2–/– mice, respectively. Macrophages from MyD88–/– mice, however, responded to both stimuli normally. In the same experiment, the pattern of TNF mRNA induction by HK-Mtb resembled that of SLPI induction, but TNF mRNA induction by LPS was reduced in MyD88–/– cells. Induction of SLPI and TNF at the protein level was also examined. As shown in Figs 4(b) and (c), both SLPI and TNF proteins were detected from HK-Mtb-treated macrophages of wild-type and TLR4–/– mice, but not from those of TLR2–/– mice. Macrophages from MyD88–/– mice responded to HK-Mtb by producing little TNF but readily detectable levels of SLPI, albeit at a reduced level compared with wild-type cells.
Figure 4.
Induction of secretory leucocyte protease inhibitor (SLPI) expression by Mycobacterium tuberculosis depends on Toll-like receptor 2 (TLR2) but not TLR4 or MyD88. Peritoneal macrophages from wild-type (WT), TLR2–/–, TLR4–/– or MyD88–/– mice were incubated (a–c) with either heat-killed Mycobacterium tuberculosis (HK-Mtb) [M, multiplicity of infection (MOI) = 4] or lipopolysaccharide (L, 100 ng/ml) for 24 hr. (a)The expression of the secretory leucocyte protease inhibitor (SLPI) and tumour necrosis factor (TNF) mRNA was assessed by northern blot analysis as described in Fig. 2. (b) SLPI protein expression in the lysate was shown via western blot using tubulin as a loading control. (c) TNF protein in the culture supernatants was determined by enzyme-linked immunosorbent assay (ELISA). KO, knock-out mice.
To test whether live M. tuberculosis acts in a similar manner, macrophages from wild-type, TLR2–/–, TLR4–/– or MyD88–/– mice were incubated with live M. tuberculosis (MOI = 4) for 24 or 48 hr and SLPI expression was examined. As shown in Fig. 5, live M. tuberculosis induced SLPI expression in an identical fashion to HK-Mtb. Thus, LPS induced SLPI expression via a TLR4-dependent but MyD88-independent mechanism, and M. tuberculosis induced SLPI expression via a TLR2-dependent but MyD88-independent mechanism. The same induction pattern was observed using bone marrow-derived macrophages (not shown). In addition, macrophages from TIRAP-null and TRIF-mutated mice responded to live M. tuberculosis by expressing elevated levels of the SLPI gene (not shown), suggesting that none of these MyD88-like proteins has a role in mediating TLR2-dependent induction of SLPI by M. tuberculosis.
Figure 5.
Induction of secretory leucocyte protease inhibitor (SLPI) expression by live Mycobacterium tuberculosis depends on Toll-like receptor 2 (TLR2) but not TLR4 or MyD88. Peritoneal macrophages from wild-type (WT), TLR2–/–, TLR4–/– or MyD88–/– mice were incubated with live M. tuberculosis[multiplicity of infection (MOI) = 4] for the time indicated. The expression of the SLPI mRNA was assessed by northern blot analysis as described in Fig. 2.
Infection of mice with M. tuberculosis induces SLPI expression in the lung
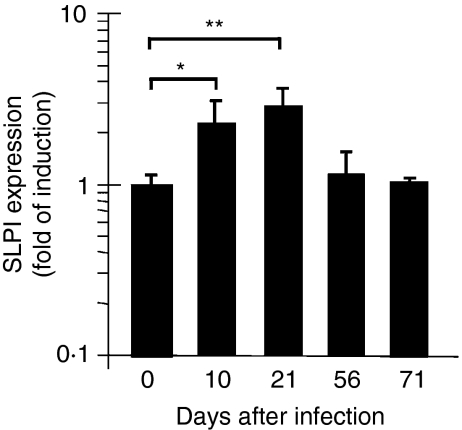
The results reported so far were from in vitro studies. To see whether SLPI can be induced by M. tuberculosis in vivo, we employed an established mouse model in which a low dose of M. tuberculosis (100 CFU/mouse) is delivered to mice via aerosol infection. This infection leads to a bacterial load of approximately 1·4 × 106 bacteria per lung at 21 days post-infection, as determined by CFU on 7H11 agar plates. SLPI expression in infected lungs was determined at day 10, 21, 56 or 71 post-infection. Induction of SLPI was detected in the early (10 and 21 days) but not late (56 and 71 days) time-points after aerosol infection (Fig. 6).
Figure 6.
Secretory leucocyte protease inhibitor (SLPI) induction in the lungs after low-dose Mycobacterium tuberculosis aerosol infection. RNA was prepared from lungs of mice that were infected with 100 colony-forming units (CFU) of M. tuberculosis for different times up to 71 days. Real-time quantitative reverse transcriptase–polymerase chain reaction (qPCR) was performed as described in the ‘Materials and methods’ section. The results are mean ± standard deviation of triplicate samples from one of two experiments. The data were analysed using Student's t-test (*P = 0·065; **P = 0·041).
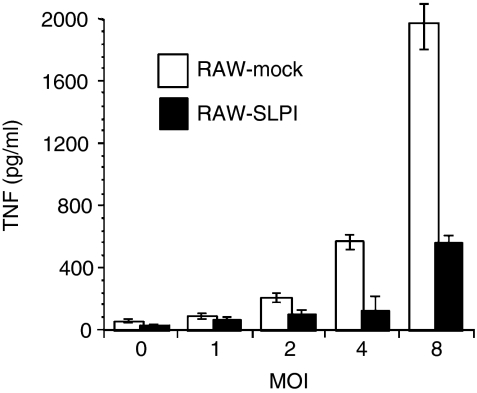
SLPI overexpression led to suppression of TNF production in response to HK-Mtb
Forced expression of SLPI in macrophage cell lines induced a LPS-hyporesponsive state.26,29 To determine whether SLPI expression has any impact on HK-Mtb-induced TNF-α production in macrophages, we compared HK-Mtb-induced TNF between two stable transfectants of RAW164·7 cells expressing either SLPI (RAW-SLPI) or vector only (RAW-mock). These cells lines have been characterized.22 SLPI-expressing cells and mock transfectants were incubated with HK-Mtb at MOIs from 1 to 8 for 16 hr. TNF levels in the media were assessed by ELISA. As shown in Fig. 7, TNF production by SLPI-expressing cells was greatly reduced in response to HK-Mtb, compared with that by mock transfectants.
Figure 7.
Secretory leucocyte protease inhibitor (SLPI) expression in macrophages leads to a suppression of tumour necrosis factor (TNF) production in response to Mycobacterium tuberculosis. RAW 264·7 cells stably transfected with SLPI (▪) or vector only (□) were treated with heat-killed Mycobacterium tuberculosis (HK-Mtb) at the indicated multiplicity of infection (MOI) for 16 hr. Conditioned media were collected for TNF determination by enzyme-linked immunosorbent assay (ELISA). Results are mean ± standard deviation of triplicates from one of four similar experiments. Student's t-test analysis shows that the differences between TNF production between two cell lines are significant at MOI 4 and 8 (P < 0·05, Student's t-test).
Discussion
The secretory leucocyte protease inhibitor (SLPI) has a multifaceted role in host inflammatory responses to infections.30 It inhibits leucocyte-derived proteases,31 exerts antimicrobial32 and antiviral activity,33 and suppresses the ongoing secretion of inflammatory mediators by macrophages.26,29,34 Because of its potent anti-inflammatory action, SLPI has been used as an effective therapy for disorders characterized by an increased inflammation, especially in the lung.35,36 We report here that both heat-killed and live M. tuberculosis induced SLPI production by mouse macrophages in vitro. M. tuberculosis-induced SLPI expression probably resulted from the direct recognition and ligation of macrophage surface receptor(s) by M. tuberculosis, as this induction was not blocked by either a protein synthesis inhibitor or an actin polymerization inhibitor. Aerosol infection of mice with live M. tuberculosis also resulted in elevated SLPI expression in infected lungs. Thus, live and heat-killed M. tuberculosis join a long list of agents that are capable of inducing SLPI in macrophages.17,21,22,37
Although SLPI was reported to possess bactericidal activity against E. coli and S. aureus in vitro, we found that recombinant SLPI alone would not kill M. tuberculosis in vitro. The time–course of the slight increase of SLPI expression after M. tuberculosis infection in vivo, observed in this study, coincided with the development of inflammation in infected lungs. Because SLPI is a potent leucocyte protease inhibitor, and macrophages expressing a high level of SLPI tend to be less responsive to many microbial stimuli, including M. tuberculosis, it is possible that enhanced SLPI expression may help to control inflammation-associated tissue damage during M. tuberculosis infection.
Surprisingly, we found that M. tuberculosis induced SLPI expression in macrophages via a novel pathway that is dependent on TLR2 but not on MyD88. This conclusion was based on the fact that macrophages from MyD88 knockout mice responded to M. tuberculosis by inducing increased expression of SLPI, while macrophages from TLR2 knockout mice could not induce SLPI in response to M. tuberculosis. SLPI protein production was diminished in MyD88–/– cells compared with that in wild-type cells, whereas no difference was observed at the mRNA level, suggesting a role for MyD88 in post-transcriptional control of SLPI. However, induction of SLPI mRNA and protein was completely abolished in TLR2–/– cells, demonstrating that TLR2 is essential for transcriptional induction of SLPI. The phenotypes of the knockout mice were confirmed by their defects in inducing TNF in response to selective TLR ligands: TLR2-null cells did not respond to Pam3, TLR4-null cells did not respond to LPS, and MyD88-null cells responded to neither stimulus (data not shown). TLR2 and TLR4 have been implicated in the recognition and signalling of M. tuberculosis in macrophages.38 Early evidence that M. tuberculosis might act via TLR2 came from the studies using forced gene expression systems.39 Brightbill et al. showed that stable expression of TLR2, but not its inactive mutant, in HEK293 cells conferred activation of a nuclear factor (NF)-κB-driven reporter gene by a 19-kDa lipoprotein from mycobacterial cell walls.39 Underhill and his colleagues40 demonstrated that mycobacterial cell wall components, including lipoarabinomannan, mycolylarabinogalactan–peptidoglycan complex, and M. tuberculosis total lipids, induced TNF in mouse RAW264·7 cells. Transient transfection of dominant negative TLR2 suppressed this response. Similar conclusions regarding the role of TLR2 were reached by Means et al. using Chinese hamster ovary cells as recipients.41,42 These authors showed that, in addition to TLR2, TLR4 also mediated signalling of a heat-sensitive mycobacterial factor in CHO cells expressing CD14.42
The role of MyD88 in the host immune response against M. tuberculosis is controversial. On one hand, MyD88 has a cardinal role in innate immune responses utilizing almost all TLRs as well as IL-1R and IL-18R.43 If TLR2 and TLR4 are responsible for M. tuberculosis-mediated cellular events in macrophages, MyD88 would be expected to be important for M. tuberculosis-mediated signals. Indeed, dominant negative MyD88 was shown to suppress the macrophage response to certain M. tuberculosis products.40 On the other hand, induction of nitric oxide by viable M. tuberculosis bacilli was found in the absence of TLR2, TLR4 and MyD88.8 These authors concluded that, while the TLR-dependent pathway depended on MyD88, the non-TLR-dependent pathway did not. In a transcriptome-wide analysis of M. tuberculosis-inducible genes in bone marrow-derived macrophages, the majority of M. tuberculosis-induced genes could be induced in the absence of MyD88.14 In a follow-up study, most of these MyD88-independent genes were found also to be induced in macrophages from TLR2, TLR4 double-deficient mice (Shi et al., unpublished observation). However, SLPI was regulated in a TLR2-dependent, MyD88-independent manner, as also demonstrated in this work.
Our finding suggests the presence of a MyD88-like adaptor protein(s) in TLR2-mediated signalling that functions independently of MyD88. Three MyD88-like proteins have been identified and implicated in LPS responses via TLR4. These include TIRAP/Mal,4,44 TRIF/Lps26,45 and TRAM/TIRP.46,47 TIRAP/Mal was found to be shared by TLR2 and TLR4. TIRAP/Mal-deficient cells responded to LPS in an identical fashion to MyD88-deficient cells, and thus did not function independently from MyD88. The adaptors TRIF/Lps2 and TRAM/TIRP do function in the absence of MyD88, but they are shared by TLR3 and TLR4 in response to double-stranded RNA and LPS, but not by TLR2 in response to peptidoglycan.6,45 We found that macrophages from TIRAP knockout or TRIF-mutated mice were still capable of inducing SLPI after M. tuberculosis exposure, suggesting that these molecules are unlikely to be the adaptors that mediate TLR2-dependent SLPI induction by M. tuberculosis. Tests in TRAM knockout mice should help clarify whether TRAM or an unidentified adaptor is necessary for M. tuberculosis-induced SLPI expression as well as for TLR2-mediated signalling in general.
Acknowledgments
This work was supported by Public Health Grants AI30165, GM61710 (AD) and HL68525 (SE) and a Cancer Research Institute Predoctoral Fellowship Training Grant (SS). We thank S. Akira for providing TLR2–/– TLR4–/– MyD88–/– mice, R. Medzhitov for TIRAP–/– mice, B. Beutler for TRIF mutated mice, and C. Nathan for critical reading of the manuscript. The Department of Microbiology and Immunology acknowledges the support of the William Randolph Hearst Foundation.
References
- 1.Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300:1524–5. doi: 10.1126/science.1085536. [DOI] [PubMed] [Google Scholar]
- 2.O'Neill LA, Fitzgerald KA, Bowie AG. The Toll-IL-1 receptor adaptor family grows to five members. Trends Immunol. 2003;24:286–90. doi: 10.1016/s1471-4906(03)00115-7. [DOI] [PubMed] [Google Scholar]
- 3.Modlin RL. Activation of toll-like receptors by microbial lipoproteins: role in host defense. J Allergy Clin Immunol. 2001;108:S104–6. doi: 10.1067/mai.2001.118299. [DOI] [PubMed] [Google Scholar]
- 4.Horng T, Barton GM, Medzhitov R. TIRAP: an adapter molecule in the Toll signaling pathway. Nat Immunol. 2001;2:835–41. doi: 10.1038/ni0901-835. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto M, Sato S, Hemmi H, et al. Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature. 2002;420:324–9. doi: 10.1038/nature01182. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto M, Sato S, Hemmi H, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–3. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 7.Sato S, Sugiyama M, Yamamoto M, Watanabe Y, Kawai T, Takeda K, Akira S. Toll/IL-1 receptor domain-containing adaptor inducing IFN-beta (TRIF) associates with TNF receptor-associated factor 6 and TANK-binding kinase 1, and activates two distinct transcription factors, NF-kappa B and IFN-regulatory factor-3, in the Toll-like receptor signaling. J Immunol. 2003;171:4304–10. doi: 10.4049/jimmunol.171.8.4304. [DOI] [PubMed] [Google Scholar]
- 8.Means TK, Jones BW, Schromm AB, et al. Differential effects of a Toll-like receptor antagonist on Mycobacterium tuberculosis-induced macrophage responses. J Immunol. 2001;166:4074–82. doi: 10.4049/jimmunol.166.6.4074. [DOI] [PubMed] [Google Scholar]
- 9.Lim SK. Freund adjuvant induces TLR2 but not TLR4 expression in the liver of mice. Int Immunopharmacol. 2003;3:115–8. doi: 10.1016/s1567-5769(02)00256-4. [DOI] [PubMed] [Google Scholar]
- 10.Tobian AA, Potter NS, Ramachandra L, Pai RK, Convery M, Boom WH, Harding CV. Alternate class I MHC antigen processing is inhibited by Toll-like receptor signaling pathogen-associated molecular patterns. Mycobacterium tuberculosis 19-kDa lipoprotein, CpG DNA, and lipopolysaccharide. J Immunol. 2003;171:1413–22. doi: 10.4049/jimmunol.171.3.1413. [DOI] [PubMed] [Google Scholar]
- 11.Noss EH, Pai RK, Sellati TJ, Radolf JD, Belisle J, Golenbock DT, Boom WH, Harding CV. Toll-like receptor 2-dependent inhibition of macrophage class II MHC expression and antigen processing by 19-kDa lipoprotein of Mycobacterium tuberculosis. J Immunol. 2001;167:910–8. doi: 10.4049/jimmunol.167.2.910. [DOI] [PubMed] [Google Scholar]
- 12.Fenton MJ. Macrophages and tuberculosis. Curr Opin Hematol. 1998;5:72–8. doi: 10.1097/00062752-199801000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Ehrt S, Schnappinger D, Bekiranov S, et al. Reprogramming of the macrophage transcriptome in response to interferon-gamma and Mycobacterium tuberculosis: signaling roles of nitric oxide synthase-2 and phagocyte oxidase. J Exp Med. 2001;194:1123–40. doi: 10.1084/jem.194.8.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi S, Nathan C, Schnappinger D, et al. MyD88 primes macrophages for full-scale activation by interferon-gamma yet mediates few responses to Mycobacterium tuberculosis. J Exp Med. 2003;198:987–97. doi: 10.1084/jem.20030603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson RC, Ohlsson K. Isolation, properties, and complete amino acid sequence of human secretory leukocyte protease inhibitor, a potent inhibitor of leukocyte elastase. Proc Natl Acad Sci USA. 1986;83:6692–6. doi: 10.1073/pnas.83.18.6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hutchison DC. The role of proteases and antiproteases in bronchial secretions. Eur J Respir Dis. 1987;153(Suppl.):78–85. [PubMed] [Google Scholar]
- 17.Grobmyer SR, Barie PS, Nathan CF, et al. Secretory leukocyte protease inhibitor, an inhibitor of neutrophil activation, is elevated in serum in human sepsis and experimental endotoxemia. Crit Care Med. 2000;28:1276–82. doi: 10.1097/00003246-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Mulligan MS, Desrochers PE, Chinnaiyan AM, Gibbs DF, Varani J, Johnson KJ, Weiss SJ. In vivo suppression of immune complex-induced alveolitis by secretory leukoproteinase inhibitor and tissue inhibitor of metalloproteinases 2. Proc Natl Acad Sci USA. 1993;90:11523–7. doi: 10.1073/pnas.90.24.11523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lucey EC, Stone PJ, Ciccolella DE, Breuer R, Christensen TG, Thompson RC, Snider GL. Recombinant human secretory leukocyte-protease inhibitor: in vitro properties, and amelioration of human neutrophil elastase-induced emphysema and secretory cell metaplasia in the hamster. J Lab Clin Med. 1990;115:224–32. [PubMed] [Google Scholar]
- 20.Rudolphus A, Stolk J, Dijkman JH, Kramps JA. Inhibition of lipopolysaccharide-induced pulmonary emphysema by intratracheally instilled recombinant secretory leukocyte proteinase inhibitor. Am Rev Respir Dis. 1993;147:442–7. doi: 10.1164/ajrccm/147.2.442. [DOI] [PubMed] [Google Scholar]
- 21.Jin F, Nathan CF, Radzioch D, Ding A. Lipopolysaccharide-related stimuli induce expression of the secretory leukocyte protease inhibitor, a macrophage-derived lipopolysaccharide inhibitor. Infect Immun. 1998;66:2447–52. doi: 10.1128/iai.66.6.2447-2452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Odaka C, Mizuochi T, Yang J, Ding A. Murine macrophages produce secretory leukocyte protease inhibitor during clearance of apoptotic cells: implications for resolution of the inflammatory response. J Immunol. 2003;171:1507–14. doi: 10.4049/jimmunol.171.3.1507. [DOI] [PubMed] [Google Scholar]
- 23.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–51. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 24.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–50. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 25.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–52. [PubMed] [Google Scholar]
- 26.Jin FY, Nathan C, Radzioch D, Ding A. Secretory leukocyte protease inhibitor: a macrophage product induced by and antagonistic to bacterial lipopolysaccharide. Cell. 1997;88:417–26. doi: 10.1016/s0092-8674(00)81880-2. [DOI] [PubMed] [Google Scholar]
- 27.Qureshi N, Takayama K, Sievert TR, Manthey CL, Vogel SN, Hronowski XL, Cotter RJ. Novel method for the purification and characterization of lipopolysaccharide from Escherichia coli D31m3. Prog Clin Biol Res. 1995;392:151–60. [PubMed] [Google Scholar]
- 28.Mogues T, Goodrich ME, Ryan L, LaCourse R, North RJ. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J Exp Med. 2001;193:271–80. doi: 10.1084/jem.193.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu J, Nathan C, Ding A. Suppression of macrophage responses to bacterial lipopolysaccharide by a non-secretory form of secretory leukocyte protease inhibitor. Biochim Biophys Acta. 1999;1451:219–23. doi: 10.1016/s0167-4889(99)00111-1. [DOI] [PubMed] [Google Scholar]
- 30.Ding A, Zhu J, Jin F, Grobmyer SR, Nathan C. Anti-inflammatory function of secretory leukocyte protease inhibitor. J Endotoxin Res. 1999;5:167–9. [Google Scholar]
- 31.Eisenberg SP, Hale KK, Heimdal P, Thompson RC. Location of the protease-inhibitory region of secretory leukocyte protease inhibitor. J Biol Chem. 1990;265:7976–81. [PubMed] [Google Scholar]
- 32.Hiemstra PS, Maassen RJ, Stolk J, Heinzel-Wieland R, Steffens GJ, Dijkman JH. Antibacterial activity of antileukoprotease. Infect Immun. 1996;64:4520–4. doi: 10.1128/iai.64.11.4520-4524.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McNeely TB, Dealy M, Dripps DJ, Orenstein JM, Eisenberg SP, Wahl SM. Secretory leukocyte protease inhibitor: a human saliva protein exhibiting anti-human immunodeficiency virus 1 activity in vitro. J Clin Invest. 1995;96:456–64. doi: 10.1172/JCI118056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song X, Zeng L, Jin W, et al. Secretory leukocyte protease inhibitor suppresses the inflammation and joint damage of bacterial cell wall-induced arthritis. J Exp Med. 1999;190:535–42. doi: 10.1084/jem.190.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Birrer P, McElvaney NG, Gillissen A, Hoyt RF, Bloedow DC, Hubbard RC, Crystal RG. Intravenous recombinant secretory leukoprotease inhibitor augments antineutrophil elastase defense. J Appl Physiol. 1992;73:317–23. doi: 10.1152/jappl.1992.73.1.317. [DOI] [PubMed] [Google Scholar]
- 36.McElvaney NG, Nakamura H, Birrer P, et al. Modulation of airway inflammation in cystic fibrosis. In vivo suppression of interleukin-8 levels on the respiratory epithelial surface by of recombinant secretory leukoprotease inhibitor. J Clin Invest. 1992;90:1296–301. doi: 10.1172/JCI115994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mukherjee S, Belbin TJ, Spray DC, et al. Microarray analysis of changes in gene expression in a murine model of chronic chagasic cardiomyopathy. Parasitol Res. 2003;91:187–96. doi: 10.1007/s00436-003-0937-z. [DOI] [PubMed] [Google Scholar]
- 38.Heldwein KA, Fenton MJ. The role of Toll-like receptors in immunity against mycobacterial infection. Microbes Infect. 2002;4:937–44. doi: 10.1016/s1286-4579(02)01611-8. [DOI] [PubMed] [Google Scholar]
- 39.Brightbill HD, Libraty DH, Krutzik SR, et al. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732–6. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 40.Underhill DM, Ozinsky A, Smith KD, Aderem A. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc Natl Acad Sci USA. 1999;96:14459–63. doi: 10.1073/pnas.96.25.14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Means TK, Lien E, Yoshimura A, Wang S, Golenbock DT, Fenton MJ. The CD14 ligands lipoarabinomannan and lipopolysaccharide differ in their requirement for Toll-like receptors. J Immunol. 1999;163:6748–55. [PubMed] [Google Scholar]
- 42.Means TK, Wang S, Lien E, Yoshimura A, Golenbock DT, Fenton MJ. Human toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J Immunol. 1999;163:3920–7. [PubMed] [Google Scholar]
- 43.Akira S. Toll-like receptors: lessons from knockout mice. Biochem Soc Trans. 2000;28:551–6. doi: 10.1042/bst0280551. [DOI] [PubMed] [Google Scholar]
- 44.Fitzgerald KA, Palsson-McDermott EM, Bowie AG, et al. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature. 2001;413:78–83. doi: 10.1038/35092578. [DOI] [PubMed] [Google Scholar]
- 45.Beutler B, Du Hoebe KX, Janssen E, Georgel P, Tabeta K. Lps2 and signal transduction in sepsis: at the intersection of host responses to bacteria and viruses. Scand J Infect Dis. 2003;35:563–7. doi: 10.1080/00365540310016295. [DOI] [PubMed] [Google Scholar]
- 46.Bin LH, Xu LG, Shu HB. TIRP, a novel Toll/interleukin-1 receptor (TIR) domain-containing adapter protein involved in TIR signaling. J Biol Chem. 2003;278:24526–32. doi: 10.1074/jbc.M303451200. [DOI] [PubMed] [Google Scholar]
- 47.Fitzgerald KA, Rowe DC, Barnes BJ, et al. LPS-TLR4 signaling to IRF-3/7 and NF-kappaB involves the toll adapters TRAM and TRIF. J Exp Med. 2003;198:1043–55. doi: 10.1084/jem.20031023. [DOI] [PMC free article] [PubMed] [Google Scholar]