Abstract
Anti-α4 and anti-αL integrin chain monoclonal antibodies have shown a clear-cut beneficial effect in different animal models of autoimmune and inflammatory disorders as well as in human diseases, including multiple sclerosis, inflammatory bowel disease, and psoriasis. It has been widely assumed that this therapeutic effect is mainly consequence of the blockade of leucocyte adhesion to endothelium, inhibiting thus their extravasation and the inflammatory phenomenon. However, it is evident that both α4β1 (very late antigen-4) and αLβ2 (leucocyte function-associated antigen-1) integrins have additional important roles in other immune phenomena, including the formation of the immune synapse and the differentiation of T helper 1 lymphocytes. Therefore, it is very feasible that the long-term administration of blocking agents directed against these integrins to patients with inflammatory/autoimmune conditions may have undesirable or unexpected effects.
Keywords: antibody responses, immunoglobulins, genetics, xenotransplantation
Very late antigen-4 and leucocyte function-associated antigen-1 integrins
Integrins are a large family of adhesion receptors that are expressed by many different cell types. These receptors are involved in both extracellular matrix/cell and cell–cell interactions. In addition to their adhesive function, these receptors modulate key intracellular phenomena, including cell activation, proliferation, and apoptosis.1,2 All integrins are composed by an α subunit non-covalently linked to a β chain. These adhesion molecules have been classified in different subfamilies, including the β1 integrins (very late antigen integrins), and the β2 or leucocyte integrins.
Very late activation antigen-4 (VLA-4) belongs to the integrin family of adhesion receptors and is composed by an α4 (CD49d) and a β1 (CD29) chain.3,4 This integrin mainly interacts with fibronectin, and vascular cell adhesion molecule-1 (VCAM-1, CD106), mediating adhesion to the extracellular matrix and activated endothelium, respectively.5,6 VLA-4 also interacts with the junctional adhesion molecule-2 (JAM-2) which is expressed by endothelial cells, an interaction that is facilitated by JAM-3.7 In addition, it has been proposed, although not confirmed, that VLA-4 interacts with itself, potentially mediating interactions among leucocytes.8 Furthermore, it has been reported that VLA-4 is able to interact with the mucosal addressin MAdCAM-1,9 and with the metalloprotease MDC-L (ADAM 28), which is expressed on the surface of lymphoid cells.10 Finally, it has been described that VLA-4, as αv integrins, interacts with intercellular adhesion molecule-4 (ICAM-4), a counter-receptor for leucocyte functio-associated antigen-1 (LFA-1) expressed by erythropoietic cells.11 Unlike the α5β1 integrin that binds to the classical RGD amino acid sequence of fibronectin, VLA-4 interacts with the LDV tripeptide sequence on the connecting segment-1 (CS-1) of this protein. Two additional sites of VLA-4/fibronectin interaction have been described at the 3Fn8 and 3Fn10 type III fibronectin modules.12 On the other hand, a cryptic antiadhesion region in fibronectin for VLA-4 has been detected at the FNIII14 repeat of this protein.13
In contrast with other VLA integrins, the α4β1 heterodimer is expressed by most resting lymphocytes, and its expression is not significantly up-regulated following cell activation. In addition, VLA-4 is detected in eosinophils and, at moderate levels, in monocytes. Furthermore, it has been reported that human monocyte-derived antigen presenting dendritic cells (DCs) up-regulate their expression of VLA-4 during maturation.14 Although peripheral blood neutrophils from healthy individuals do not express this integrin, it has been described that after their extravasation VLA-4 is detected on the cell membrane of these cells, participating in their migration through the extracellular matrix.15 On the other hand, VCAM-1 is expressed by activated endothelium and it has been reported that macrophages, follicular dendritic cells and bone marrow-derived DCs are also able to synthesize in vivo this adhesion receptor.16–19 However, the latter point remains controversial.20,21
The α4 integrin chain is shared by other adhesion receptors. The α4β7 integrin also interacts with VCAM-1 and is expressed by a subset of lymphoid cells that show a preferential homing to Peyer's patches. In addition, this integrin interacts with MadCAM-1.22,23 Given that VLA-4 and α4β7 have a common chain, it is expectable that anti-α4 integrin chain blocking agents affect the function of both integrins.
LFA-1 is composed of the αL (CD11a) and β2 (CD18) integrin chains and is expressed by all leucocytes. The β2 integrin chain is shared by other three integrins, Mac-1 (αMβ2), gp150.95 (αXβ2), and the αDβ2 heterodimer.1,2,22 LFA-1 increases its expression upon cell activation, and interacts with ICAM-1, and ICAM-2, which are expressed by different cell types, including endothelium.2,22 Upon activation, endothelial cells show a marked up-regulation of ICAM-1, but not ICAM-2. LFA-1 also interacts with ICAM-3 that is detected exclusively in bone marrow-derived cells, including DCs and Langerhans cells.24 Additional intercellular adhesion molecules (ICAM-4, ICAM-5) that interact with LFA-1 have been described, but these ligands are expressed in cells other than leucocytes and endothelium. Finally, it has been described that JAM-1 (or JAM-A) is an additional ligand for LFA-1.25
An important functional feature of VLA-4 and LFA-1 (and other integrins) is its ability to change the adhesiveness for their ligands. This phenomenon is mainly consequence of the redistribution of these adhesion receptors on cell membrane and of conformational changes that enhances their affinity/avidity for the ligand (integrin activation).1,2,22,26 Therefore leucocytes are able to change rapidly their adhesiveness mediated by integrins to both other cells and the extracellular matrix.
Functional roles of α4 and αL under physiological and pathological conditions
VLA-4 is involved in the development and differentiation of several tissues and cell types. This integrin is expressed by muscle cells during embryonic development and has an important role in myotube formation and the differentiation of these cells.27,28 In addition, VLA-4 is detected in thymic epithelial cells, likely mediating cellular interactions and participating in thymocyte development.29 Furthermore, it has been reported that this integrin is expressed by bone marrow CD34+ haematopoietic stem cells, having an important role in their adhesion to stromal cells and in the inhibition of its migration towards peripheral tissues.30 It has also been demonstrated that the α4 integrin chain is required for normal haematopoiesis, and B- and T-cell precursor development in bone marrow.31–33 Finally, it has been reported that osteoclastogenesis is dependent on the expression of VCAM-1 by stromal cells.34 All these data account for the essential role of VLA-4 in embryo development.35
Different evidences indicate that VLA-4 also has an important role in the generation of the immune response through its involvement in the formation of the immune synapse. In this regard, α4β1 integrin is relocated to the peripheral supramolecular activation complex (pSMAC) during immune synapse formation between antigen-presenting cells (APC) and T lymphocytes36(Fig. 1). In addition, the in vitro engagement of VLA-4 with specific monoclonal antibodies (mAb) during the T lymphocyte–APC interaction favours the activation of T cells37 and the differentiation of T helper 1 (Th1) lymphocytes, interfering thus with the generation of Th2 cells.36 The in vivo administration of anti-VLA-4 mAb to rats also induces a shift towards a Th1 type immune response.36,38 It has also been reported that in experimental cardiac allograft rejection, Th1 cytokine synthesis is depressed in the absence of fibronectin, a major ligand of VLA-4·39 Furthermore, VLA-4 expression is up-regulated upon DC cell maturation.14 These interesting observations strongly suggest that during the formation of the immune synapse, VLA-4, expressed by both the T lymphocyte and DC, interacts with one or more ligands, thus participating in the generation of the immune response. In this regard, and as stated above, it has been reported that follicular dendritic cells and bone marrow-derived DCs could express VCAM-1, another major ligand of VLA-4.16–19 An additional interesting possibility is that the expression of VLA-4 by T lymphocytes and DCs allows a homophilic interaction of this integrin8 during the immune synapse. Finally, it is possible that there are additional ligands of VLA-4 expressed by the APC.
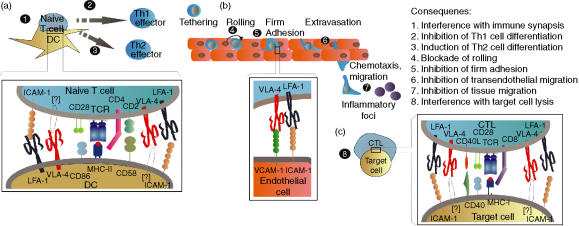
Figure 1.
Possible consequences of the in vivo blockade of LFA-1 and VLA-4. The long-term administration of anti-αL or anti-α4 integrin chain mAb may affect the generation of the immune response and differentiation of T cells (a), the travelling of leucocytes towards inflammatory foci (b) and the effector phase of the cell-mediated immune response (c). When an antagonist agent directed against VLA-4 is administered, an interference effect could be exerted on the immune synapse formed by CD4+ T cells and bone marrow-derived antigen presenting dendritic cells (1), inhibiting the differentiation of Th1 lymphocytes, and thus favouring the activation of Th2 cells (2). However, some anti-VLA-4 agents may act as agonistic molecules, simulating the physiological ligand, and promoting the differentiation of Th1 lymphocytes (3). Although the putative counter-receptor of VLA-4 on these APC might correspond to VCAM-1 or VLA-4, this possibility requires further studies. The best characterized effect of anti-VLA-4 agents is exerted on the extravasation of leucocytes to inflammatory foci, specifically during their tethering/rolling (4), and firm adhesion (5) on and to activated endothelium, and their transendothelial migration (6), as a consequence of the blockade of VLA-4/VCAM-1, VLA-4/MadCAM-1, and VLA-4/JAM2 interactions. Furthermore, VLA-4 blockade could interfere with the migration of leucocytes through the extracellular matrix by the inhibition of its interaction with fibronectin (7). Finally, the possible involvement of VLA-4 in the interaction of CD8+ cytotoxic T cells with some target cells (8), through its interaction with VCAM-1 or other putative counter-receptors, is an interesting point to be explored. LFA-1 blockade would have similar consequences on the immune synapse, but with an additional clear-cut effect on T cell–APC adhesion (1). Likewise, LFA-1 blockade would also interfere with the differentiation of Th0 cells to Th1 effector lymphocytes (3), favouring the generation of activated Th2 cells (2). On the process of leucocyte extravasation, the blockade of the interaction of LFA-1 with ICAM-1/-2, and JAM-1/JAM-A would inhibit the firm adherence of leucocytes to endothelium (5) as well as their transendothelial migration (6). Lastly, it would be also expected that LFA-1 blockade has an inhibitory effect on the adhesion of CD8+ T cytotoxic cells to target cells, interfering thus with their lytic function (8).
VLA-4 has a clear-cut role in the extravasation of leucocytes (lymphocytes, monocytes, eosinophils, neutrophils) towards inflammatory foci.40,41 Under the current paradigmatic sequential model of leucocyte extravasation22 this integrin participates in the early steps of lymphocyte–endothelial cell interaction (Fig. 1). In this regard, it has been demonstrated that VLA-4/VCAM-1 interaction mediates the tethering/rolling of T cells on activated endothelium, and their firm adhesion.22 In addition, it seems that VLA-4 also participates in the transendothelial migration of lymphocytes.7,42 According to a recent report, the firm adhesion of lymphocytes to endothelium mediated by VLA-4 requires its physical association with the hyaluronan receptor CD44.43 Finally, VLA-4 participates in the migration of leucocytes towards the inflammatory foci through the extracellular matrix by its interaction with fibronectin.
An additional putative functional role of VLA-4 is the possible involvement in the cell interaction between cytotoxic T cells and target cells (Fig. 1).44,45 As in the case of the immune synapse, VLA-4 molecules expressed by CD8+ cytotoxic T cells may interact with VCAM-1, when target cells express it, contributing to cell adhesion, a phenomenon necessary for the lytic event. As discussed above, other potential cellular ligands of VLA-4 in this type of cellular interactions may be ADAM 28, VLA-4 and a not yet characterized molecule. In addition to its adhesive function, the possible contribution of the intracellular signals generated through VLA-422 in cytotoxic cells to the activation of the lytic event, remains an interesting point to be explored. On the other hand, it has been described that VLA-4 exerts an antiapoptotic effect in lymphocytes46 a phenomenon that has been well characterized for β1 integrins in epithelial cells.47
LFA-1 has a well-defined role during the immune synapse. This integrin is redistributed towards the peripheral SMAC during immune synapse formation, and has an important role in the T lymphocyte-DC adhesion,48–50 (Fig. 1). In addition, LFA-1 contributes to the generation of costimulatory signals necessary for T-cell activation. A similar role could be exerted by LFA-1 in the activation of DCs, because these cells also express this integrin and T cells the corresponding receptors.51,52
As in the case of VLA-4, it has been described that LFA-1 engagement favours the differentiation of naïve T cells to Th1 effector lymphocytes.53In vivo experiments further support this functional role of LFA-1.38 A similar effect seems to be exerted by ICAM-1.54 Likewise, LFA-1 engagement exerts an antiapoptotic activity similar to that described for VLA-4.46 This integrin also has an important role in the extravasation of leucocytes, mainly by mediating the firm adherence of these cells to the activated endothelium.2,22 In this regard, it is worth mentioning that during leucocyte rolling, intracellular signals generated through l-selectin and chemokine receptors, induce the activation of LFA-1, increasing its affinity for their counter-receptors. In addition, LFA-1, through its interaction with ICAM-1 and JAM-1/JAM-A, is involved in the transendothelial migration of leucocytes.55 Finally, this integrin participates in both the adhesion of cytotoxic lymphocytes (T and natural killer cells) to target cells and the triggering of the lytic event.56,57
α4 and αL integrin chains as targets for therapy of inflammatory diseases
Different specific anti-α4 integrin chain mAb have been employed to explore the therapeutic effect of VLA-4 blockade in experimental inflammatory and autoimmune conditions. In this regard, it has been reported that, for example, experimental allergic encephalomyelitis, adjuvant induced arthritis, diabetes mellitus of non-obese diabetic mice, experimental graft-versus-host disease, allograft rejection and immediate hypersensitivity reactions are prevented and/or show a good response to the administration of anti-VLA-4 mAb.4,58–65 Similar results have been obtained with different synthetic blockers of VLA-4·66 The promising results obtained in these animal studies have prompted different groups and drug developers to move into clinical trials. In this regard, a humanized anti-VLA-4 mAb has showed a clear-cut therapeutic effect in relapsing multiple sclerosis,67 Crohn's disease68 and rheumatoid arthritis.69,70 In fact, in 2004 the Food and Drug Administration of USA (FDA) approved the use of this anti-α4 integrin chain mAb (Natalizumab, Tysabri®) for the relapsing form of multiple sclerosis.71 Other potential uses of this type of therapeutic mAb include inflammatory and/or autoimmune conditions with a high impact in general population, including asthma, type I diabetes mellitus and the arterial intimal hyperplasia associated with angioplasty.72–74
Anti-LFA-1 mAbs also have a remarkable effect in different animal models of inflammatory diseases.75–79 Accordingly, as in the case of VLA-4, it has been found that anti-LFA-1 mAb have a significant therapeutic potential in humans.80–84 In this regard, a humanized anti-αL mAb (Efalizumab, Raptiva®) has been approved by FDA (2003), and the European Medicines Evaluation Agency (EMEA, 2004) for the therapy of moderate to severe forms of psoriasis.85 The potential therapeutic uses of anti-LFA-1 mAb include the prevention of bone marrow graft failure, and the treatment of kidney allograft rejection, asthma, and graft-versus-host disease, among others.80–83,86
All these data indicate that anti-VLA-4 and anti-LFA-1 mAb as well as other murine, humanized or fully human antibodies are emerging as additional therapeutic options in a wide array of human disorders.87 Future directions in this field mainly includes the use of recombinant chimeric antibody fragments, and synthetic blockers.87,88 In addition, the characterization of novel mechanisms of action of currently available drugs is another interesting possibility for the blockade of action of VLA-4 and LFA-1.
Therapeutic anti-α4 and anti-αL agents, two-edged swords?
It has been assumed that the main in vivo effect of anti-α4 integrin chain biological agents is the inhibition of leucocyte extravasation. This assumption has been the basis for the use of Natalizumab in multiple sclerosis or Crohn's disease. In this regard, the expression of α4β1 and α4β7 integrins by the infiltrating leucocytes in the central nervous system and gut of patients with these conditions has been widely reported.89,90 However, it is evident that VLA-4 blockade must have additional effects in vivo (Fig. 1). In addition, it is evident that although anti-α4 integrin chain mAb block the interaction of VLA-4 with their counter-receptors these biological agents could also simultaneously act as ligands, generating intracellular signals in target cells (agonistic antibodies). In this regard, we have found that during the formation of the immune synapse, VLA-4 engagement with specific mAb induces their relocation to the cSMAC and favours the differentiation of Th1 cells.36 In addition, it is worth mentioning that multiple sclerosis is an autoimmune/inflammatory condition mediated by a Th1-type immune response, and that the deviation towards a Th2 response may be beneficial in these patients.91,92 Therefore, although VLA-4 blockade, with inhibition of leucocyte extravasation, exerts a beneficial effect in most multiple sclerosis patients, the effect of VLA-4 engagement might have undesirable or unpredictable consequences, including the exacerbation of the disease. This possibility has been reported in experimental allergic encephalomyelitis93 and similar results have been obtained with synthetic blockers of VLA-4.94 On the other hand, antagonistic anti-VLA-4 mAb (that do not generate intracellular signals in target cells) may block the putative physiological role of VLA-4 during the immune synapses, interfering thus with the generation of the immune response, and the differentiation of Th1 lymphocytes. Therefore, this type of anti-VLA-4 mAb would exert a suppressive effect on the cellular immune response, whereas agonistic antibodies would favour it. Additional potential effects of VLA-4 blockade/engagement include the interference with the function of thymic epithelial cells29 and, possibly, with the cytotoxic activity of CD8+ T cells (Fig. 1). Finally, the engagement of VLA-4 with mAb in lymphocytes would alter their programmed cell death46 a phenomenon with potential consequences on immune tolerance (antagonistic antibodies) or immune competence (agonistic antibodies).95 Whether or not this wide array of potential effects is causally related with the two cases (one confirmed, and one suspected) of progressive multifocal leukoencephalopathy in patients with multiple sclerosis receiving Tysabri® and interferon β-1a, remains an important issue to be clarified*. Therefore, we can conclude that with the current knowledge on the different physiological roles of VLA-4, it is expected that its blockade has additional effects besides the inhibition of leucocyte extravasation. These effects may be responsible of unexpected or undesirable phenomena in patients under anti-VLA-4 therapy. In this regard, it is well know that the therapeutic blockade of tumour necrosis factor-α in patients with rheumatoid arthritis is associated with both an increased risk for tuberculosis and autoimmune phenomena.96,97
LFA-1 is also involved in several physiological phenomena, and thus it is expected that its engagement/blockade with specific mAb has different consequences (Fig. 1). In this regard, it has been well demonstrated the important role of LFA-1 in both the costimulation of T cells and the adhesion of T lymphocytes and APC during the formation of the immune synapse.48,49,98 In addition, the role of LFA-1 in the effector phase of the cellular immune response has been properly characterized.56,57 Thus, it is expected that antagonistic anti-αL mAb exert an important anti-inflammatory and immunosuppressive effect. However, as in the case of VLA-4, LFA-1 engagement also alters the differentiation of Th1 lymphocytes and exacerbates Th1-mediated conditions.99,100 In this regard, different data indicate that psoriasis, the current indication for the humanized anti-αL mAb Efalizumab (Raptiva®), is an immune-mediated disease mainly caused by a Th1-type immune response.101–103 In this disease, it has also been assumed that the main mechanism of action of Efalizumab is the blockade of lymphocyte extravasation85 but, as in the case of anti-VLA-4 mAb and multiple sclerosis, it is evident that LFA-1 blockade must have additional consequences, including the perturbation in the differentiation of Th1/Th2 lymphocytes.53,99,100,104 Therefore, an agonistic anti-αL mAb would block the extravasation of T cells to the skin in patients with psoriasis, but would also promote the differentiation of Th1 cells. As expected, the latter phenomenon could have an undesirable effect in these patients. Finally, the putative role of α4 integrins in the development of inducible T regulatory (Treg) cells may also contribute to the complex in vivo effect of integrin blockade. In this regard, it has been described that α4β1+ Treg lymphocytes induce the development of interleukin-10-producing regulatory cells (Tr1-like lymphocytes), whereas α4β7 + Treg cells favor the differentiation of Th3-like cells, which synthesize transforming growth factor-β.105 This effect adds an element of caution that should be took into account for the therapeutic use of anti-integrin agents.
Conclusions
There is no doubt that therapeutic mAb directed against adhesion/costimulation molecules are a major step for the therapy of inflammatory and autoimmune diseases. However, these biological agents, as anti-α4 and anti-αL integrin chain mAb, are directed against receptors with many different biological roles, including the generation of the immune response, the differentiation of Th1/Th2 lymphocytes, the apoptosis of immune cells, and the extravasation of leucocytes to inflammatory foci, among others. In addition, it is evident that some mAb may act as agonistic molecules, generating thus intracellular signals upon antigen engagement. Therefore, the long-term administration of this type of therapeutic agents may have unexpected and even undesirable consequences. It would be very important to take into account all the information derived from both basic studies and preclinical works to properly guide the future clinical trials with this type of biological agents.
Acknowledgments
This work was supported by grants BMC02-00536, GEN 2003-649CO6 and Ayuda a la Investigación Básica Juan March 2002 to F. Sánchez-Madrid. M.M. is supported by GEN 2003–649CO6.
Footnotes
Due to these serious adverse events in February 2005 Biogen Idec/Elan Pharmaceuticals announced the voluntary suspension in the marketing of Tysabri®.
References
- 1.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 2.Hogg N, Laschinger M, Giles K, McDowall A. T-cell integrins: more than just sticking points. J Cell Sci. 2003;116:4695–705. doi: 10.1242/jcs.00876. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez-Madrid F, De Landazuri MO, Morago G, Cebrian M, Acevedo A, Bernabeu C. VLA-3: a novel polypeptide association within the VLA molecular complex: cell distribution and biochemical characterization. Eur J Immunol. 1986;16:1343–9. doi: 10.1002/eji.1830161106. [DOI] [PubMed] [Google Scholar]
- 4.Hemler ME, Huang C, Schwarz L. The VLA protein family. Characterization of five distinct cell surface heterodimers each with a common 130,000 molecular weight beta subunit. J Biol Chem. 1987;262:3300–9. [PubMed] [Google Scholar]
- 5.Elices MJ, Osborn L, Takada Y, Crouse C, Luhowskyj S, Hemler ME, Lobb RR. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990;60:577–84. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- 6.Wayner EA, Garcia-Pardo A, Humphries MJ, McDonald JA, Carter WG. Identification and characterization of the T lymphocyte adhesion receptor for an alternative cell attachment domain (CS-1) in plasma fibronectin. J Cell Biol. 1989;109:1321–30. doi: 10.1083/jcb.109.3.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunningham SA, Rodriguez JM, Arrate MP, Tran TM, Brock TA. JAM2 interacts with alpha4beta1. Facilitation by JAM3. J Biol Chem. 2002;277:27589–92. doi: 10.1074/jbc.C200331200. [DOI] [PubMed] [Google Scholar]
- 8.Altevogt P, Hubbe M, Ruppert M, et al. The alpha 4 integrin chain is a ligand for alpha 4 beta 7 and alpha 4 beta 1. J Exp Med. 1995;182:345–55. doi: 10.1084/jem.182.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newham P, Craig SE, Clark K, Mould AP, Humphries MJ. Analysis of ligand-induced and ligand-attenuated epitopes on the leukocyte integrin alpha4beta1: VCAM-1, mucosal addressin cell adhesion molecule-1, and fibronectin induce distinct conformational changes. J Immunol. 1998;160:4508–17. [PubMed] [Google Scholar]
- 10.Bridges LC, Tani PH, Hanson KR, Roberts CM, Judkins MB, Bowditch RD. The lymphocyte metalloprotease MDC-L (ADAM 28) is a ligand for the integrin alpha4beta1. J Biol Chem. 2002;277:3784–92. doi: 10.1074/jbc.M109538200. [DOI] [PubMed] [Google Scholar]
- 11.Spring FA, Parsons SF, Ortlepp S, Olsson ML, Sessions R, Brady RL, Anstee DJ. Intercellular adhesion molecule-4 binds alpha (4) beta (1) and alpha (V) -family integrins through novel integrin-binding mechanisms. Blood. 2001;98:458–66. doi: 10.1182/blood.v98.2.458. [DOI] [PubMed] [Google Scholar]
- 12.Mould AP, Komoriya A, Yamada KM, Humphries MJ. The CS5 peptide is a second site in the IIICS region of fibronectin recognized by the integrin alpha 4 beta 1. Inhibition of alpha 4 beta 1 function by RGD peptide homologues. J Biol Chem. 1991;266:3579–85. [PubMed] [Google Scholar]
- 13.Kato R, Ishikawa T, Kamiya S, et al. A new type of antimetastatic peptide derived from fibronectin. Clin Cancer Res. 2002;8:2455–62. [PubMed] [Google Scholar]
- 14.Puig-Kroger A, Sanz-Rodriguez F, Longo N, Sanchez-Mateos P, Botella L, Teixido J, Bernabeu C, Corbi AL. Maturation-dependent expression and function of the CD49d integrin on monocyte-derived human dendritic cells. J Immunol. 2000;165:4338–45. doi: 10.4049/jimmunol.165.8.4338. [DOI] [PubMed] [Google Scholar]
- 15.Werr J, Xie X, Hedqvist P, Ruoslahti E, Lindbom L. Beta1 integrins are critically involved in neutrophil locomotion in extravascular tissue in vivo. J Exp Med. 1998;187:2091–6. doi: 10.1084/jem.187.12.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Oosten M, van de Bilt E, de Vries HE, van Berkel TJ, Kuiper J. Vascular adhesion molecule-1 and intercellular adhesion molecule-1 expression on rat liver cells after lipopolysaccharide administration in vivo. Hepatology. 1995;22:1538–46. doi: 10.1002/hep.1840220529. [DOI] [PubMed] [Google Scholar]
- 17.Marazuela M, Postigo AA, Acevedo A, Diaz-Gonzalez F, Sanchez-Madrid F, de Landazuri MO. Adhesion molecules from the LFA-1/ICAM-1,3 and VLA-4/VCAM-1 pathways on T lymphocytes and vascular endothelium in Graves' and Hashimoto's thyroid glands. Eur J Immunol. 1994;24:2483–90. doi: 10.1002/eji.1830241034. [DOI] [PubMed] [Google Scholar]
- 18.Walton LJ, Macey MG, Thornhill MH, Farthing PM. Intra-epithelial subpopulations of T lymphocytes and Langerhans cells in oral lichen planus. J Oral Pathol Med. 1998;27:116–23. doi: 10.1111/j.1600-0714.1998.tb01926.x. [DOI] [PubMed] [Google Scholar]
- 19.Koopman G, Parmentier HK, Schuurman HJ, Newman W, Meijer CJ, Pals ST. Adhesion of human B cells to follicular dendritic cells involves both the lymphocyte function-associated antigen 1/intercellular adhesion molecule 1 and very late antigen 4/vascular cell adhesion molecule 1 pathways. J Exp Med. 1991;173:1297–304. doi: 10.1084/jem.173.6.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheeren RA, Koopman G, Van der Baan S, Meijer CJ, Pals ST. Adhesion receptors involved in clustering of blood dendritic cells and T lymphocytes. Eur J Immunol. 1991;21:1101–5. doi: 10.1002/eji.1830210503. [DOI] [PubMed] [Google Scholar]
- 21.Kriegsmann J, Keyszer GM, Geiler T, Brauer R, Gay RE, Gay S. Expression of vascular cell adhesion molecule-1 mRNA and protein in rheumatoid synovium demonstrated by in situ hybridization and immunohistochemistry. Lab Invest. 1995;72:209–14. [PubMed] [Google Scholar]
- 22.Gonzalez-Amaro R, Sanchez-Madrid F. Cell adhesion molecules: selectins and integrins. Crit Rev Immunol. 1999;19:389–429. [PubMed] [Google Scholar]
- 23.Berlin C, Bargatze RF, Campbell JJ, et al. alpha 4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell. 1995;80:413–22. doi: 10.1016/0092-8674(95)90491-3. [DOI] [PubMed] [Google Scholar]
- 24.Acevedo A, del Pozo MA, Arroyo AG, Sanchez-Mateos P, Gonzalez-Amaro R, Sanchez-Madrid F. Distribution of ICAM-3-bearing cells in normal human tissues. Expression of a novel counter-receptor for LFA-1 in epidermal Langerhans cells. Am J Pathol. 1993;143:774–83. [PMC free article] [PubMed] [Google Scholar]
- 25.Fraemohs L, Koenen RR, Ostermann G, Heinemann B, Weber C. The functional interaction of the beta 2 integrin lymphocyte function-associated antigen-1 with junctional adhesion molecule-A is mediated by the I domain. J Immunol. 2004;173:6259–64. doi: 10.4049/jimmunol.173.10.6259. [DOI] [PubMed] [Google Scholar]
- 26.Hogg N, Henderson R, Leitinger B, McDowall A, Porter J, Stanley P. Mechanisms contributing to the activity of integrins on leukocytes. Immunol Rev. 2002;186:164–71. doi: 10.1034/j.1600-065x.2002.18614.x. [DOI] [PubMed] [Google Scholar]
- 27.Rosen GD, Sanes JR, LaChance R, Cunningham JM, Roman J, Dean DC. Roles for the integrin VLA-4 and its counter receptor VCAM-1 in myogenesis. Cell. 1992;69:1107–19. doi: 10.1016/0092-8674(92)90633-n. [DOI] [PubMed] [Google Scholar]
- 28.Takada Y, Strominger JL, Hemler ME. The very late antigen family of heterodimers is part of a superfamily of molecules involved in adhesion and embryogenesis. Proc Natl Acad Sci USA. 1987;84:3239–43. doi: 10.1073/pnas.84.10.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nieto M, Gomez M, Sanchez-Mateos P, et al. Expression of functionally active alpha 4 beta 1 integrin by thymic epithelial cells. Clin Exp Immunol. 1996;106:170–8. doi: 10.1046/j.1365-2249.1996.d01-819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prosper F, Stroncek D, McCarthy JB, Verfaillie CM. Mobilization and homing of peripheral blood progenitors is related to reversible downregulation of alpha4 beta1 integrin expression and function. J Clin Invest. 1998;101:2456–67. doi: 10.1172/JCI188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyake K, Weissman IL, Greenberger JS, Kincade PW. Evidence for a role of the integrin VLA-4 in lympho-hemopoiesis. J Exp Med. 1991;173:599–607. doi: 10.1084/jem.173.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arroyo AG, Yang JT, Rayburn H, Hynes RO. Alpha4 integrins regulate the proliferation/differentiation balance of multilineage hematopoietic progenitors in vivo. Immunity. 1999;11:555–66. doi: 10.1016/s1074-7613(00)80131-4. [DOI] [PubMed] [Google Scholar]
- 33.Arroyo AG, Yang JT, Rayburn H, Hynes RO. Differential requirements for alpha4 integrins during fetal and adult hematopoiesis. Cell. 1996;85:997–1008. doi: 10.1016/s0092-8674(00)81301-x. [DOI] [PubMed] [Google Scholar]
- 34.Feuerbach D, Feyen JH. Expression of the cell-adhesion molecule VCAM-1 by stromal cells is necessary for osteoclastogenesis. FEBS Lett. 1997;402:21–4. doi: 10.1016/s0014-5793(96)01495-0. [DOI] [PubMed] [Google Scholar]
- 35.Spence S, Vetter C, Hagmann WK, et al. Effects of VLA-4 antagonists in rat whole embryo culture. Teratology. 2002;65:26–37. doi: 10.1002/tera.1095. [DOI] [PubMed] [Google Scholar]
- 36.Mittelbrunn M, Molina A, Escribese MM, et al. VLA-4 integrin concentrates at the peripheral supramolecular activation complex of the immune synapse and drives T helper 1 responses. Proc Natl Acad Sci U S A. 2004;101:11058–63. doi: 10.1073/pnas.0307927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nojima Y, Humphries MJ, Mould AP, Komoriya A, Yamada KM, Schlossman SF, Morimoto C. VLA-4 mediates CD3-dependent CD4+ T cell activation via the CS1 alternatively spliced domain of fibronectin. J Exp Med. 1990;172:1185–92. doi: 10.1084/jem.172.4.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suzuki J, Isobe M, Izawa A, Takahashi W, Yamazaki S, Okubo Y, Amano J, Sekiguchi M. Differential Th1 and Th2 cell regulation of murine cardiac allograft acceptance by blocking cell adhesion of ICAM-1/LFA-1 and VCAM-1/VLA-4. Transpl Immunol. 1999;7:65–72. doi: 10.1016/s0966-3274(99)80021-5. [DOI] [PubMed] [Google Scholar]
- 39.Coito AJ, Onodera K, Kato H, Busuttil RW, Kupiec-Weglinsk JW. Fibronectin–mononuclear cell interactions regulate type 1 helper T cell cytokine network in tolerant transplant recipients. Am J Pathol. 2000;157:1207–18. doi: 10.1016/S0002-9440(10)64636-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lobb RR, Hemler ME. The pathophysiologic role of alpha 4 integrins in vivo. J Clin Invest. 1994;95:94(5):1722–8. doi: 10.1172/JCI117519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ibbotson GC, Doig C, Kaur J, Gill V, Ostrovsky L, Fairhead T, Kubes P. Functional alpha4-integrin: a newly identified pathway of neutrophil recruitment in critically ill septic patients. Nat Med. 2001;7:465–70. doi: 10.1038/86539. [DOI] [PubMed] [Google Scholar]
- 42.Hourihan H, Allen TD, Ager A. Lymphocyte migration across high endothelium is associated with increases in alpha 4 beta 1 integrin (VLA-4) affinity. J Cell Sci. 1993;104:1049–59. doi: 10.1242/jcs.104.4.1049. [DOI] [PubMed] [Google Scholar]
- 43.Nandi A, Estess P, Siegelman M. Bimolecular complex between rolling and firm adhesion receptors required for cell arrest; CD44 association with VLA-4 in T cell extravasation. Immunity. 2004;20:455–65. doi: 10.1016/s1074-7613(04)00077-9. [DOI] [PubMed] [Google Scholar]
- 44.Schlegel PG, Vaysburd M, Chen Y, Butcher EC, Chao NJ. Inhibition of T cell costimulation by VCAM-1 prevents murine graft-versus-host disease across minor histocompatibility barriers. J Immunol. 1995;155:3856–65. [PubMed] [Google Scholar]
- 45.Rodrigues M, Nussenzweig RS, Romero P, Zavala F. The in vivo cytotoxic activity of CD8+ T cell clones correlates with their levels of expression of adhesion molecules. J Exp Med. 1992;175:895–905. doi: 10.1084/jem.175.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koopman G, Keehnen RM, Lindhout E, Newman W, Shimizu Y, van Seventer GA, de Groot C, Pals ST. Adhesion through the LFA-1 (CD11a/CD18) -ICAM-1 (CD54) and the VLA-4 (CD49d)-VCAM-1 (CD106) pathways prevents apoptosis of germinal center B cells. J Immunol. 1994;152:3760–7. [PubMed] [Google Scholar]
- 47.Boudreau N, Sympson CJ, Werb Z, Bissell MJ. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995;267:891–3. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Montoya M, Sancho D, Vicente-Manzanares M, Sanchez-Madrid F. Cell adhesion and polarity during immune interactions. Immunol Rev. 2002;186:68–82. doi: 10.1034/j.1600-065x.2002.18607.x. [DOI] [PubMed] [Google Scholar]
- 49.Sims TN, Dustin ML. The immunological synapse: integrins take the stage. Immunol Rev. 2002;186::100–17. doi: 10.1034/j.1600-065x.2002.18610.x. [DOI] [PubMed] [Google Scholar]
- 50.Monks C, Freiberg B, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–6. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 51.Hart DN, McKenzie JL. Isolation and characterization of human tonsil dendritic cells. J Exp Med. 1988;168:157–70. doi: 10.1084/jem.168.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ardavin C, Shortman K. Cell surface marker analysis of mouse thymic dendritic cells. Eur J Immunol. 1992;22:859–62. doi: 10.1002/eji.1830220334. [DOI] [PubMed] [Google Scholar]
- 53.Smits HH, de Jong EC, Schuitemaker JH, Geijtenbeek TB, van Kooyk Y, Kapsenberg ML, Wierenga EA. Intercellular adhesion molecule-1/LFA-1 ligation favors human Th1 development. J Immunol. 2002;168:1710–6. doi: 10.4049/jimmunol.168.4.1710. [DOI] [PubMed] [Google Scholar]
- 54.Chirathaworn C, Kohlmeier JE, Tibbetts SA, Rumsey LM, Chan MA, Benedict SH. Stimulation through intercellular adhesion molecule-1 provides a second signal for T cell activation. J Immunol. 2002;168:5530–7. doi: 10.4049/jimmunol.168.11.5530. [DOI] [PubMed] [Google Scholar]
- 55.Oppenheimer-Marks N, Davis LS, Bogue DT, Ramberg J, Lipsky PE. Differential utilization of ICAM-1 and VCAM-1 during the adhesion and transendothelial migration of human T lymphocytes. J Immunol. 1991;147:2913–21. [PubMed] [Google Scholar]
- 56.Rutigliano JA, Johnson TR, Hollinger TN, Fischer JE, Aung S, Graham BS. Treatment with anti-LFA-1 delays the CD8+ cytotoxic-T-lymphocyte response and viral clearance in mice with primary respiratory syncytial virus infection. J Virol. 2004;78:3014–23. doi: 10.1128/JVI.78.6.3014-3023.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barber DF, Faure M, Long EO. LFA-1 contributes an early signal for NK cell cytotoxicity. J Immunol. 2004;173:3653–9. doi: 10.4049/jimmunol.173.6.3653. [DOI] [PubMed] [Google Scholar]
- 58.Baron JL, Madri JA, Ruddle NH, Hashim G, Janeway CA. Surface expression of alpha 4 integrin by CD4 T cells is required for their entry into brain parenchyma. J Exp Med. 1993;177:57–68. doi: 10.1084/jem.177.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Isobe M, Suzuki J, Yagita H, Okumura K, Yamazaki S, Nagai R, Yazaki Y, Sekiguchi M. Immunosuppression to cardiac allografts and soluble antigens by anti-vascular cellular adhesion molecule-1 and anti-very late antigen-4 monoclonal antibodies. J Immunol. 1994;153:5810–8. [PubMed] [Google Scholar]
- 60.Itoh S, Matsuzaki Y, Kimura T, et al. Suppression of hepatic lesions in a murine graft-versus-host reaction by antibodies against adhesion molecules. J Hepatol. 2000;32:587–95. doi: 10.1016/s0168-8278(00)80220-2. [DOI] [PubMed] [Google Scholar]
- 61.Issekutz AC, Ayer L, Miyasaka M, Issekutz TB. Treatment of established adjuvant arthritis in rats with monoclonal antibody to CD18 and very late activation antigen-4 integrins suppresses neutrophil and T-lymphocyte migration to the joints and improves clinical disease. Immunology. 1996;88:569–76. doi: 10.1046/j.1365-2567.1996.d01-695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yednock TA, Cannon C, Fritz LC, Sanchez-Madrid F, Steinman L, Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992;356:63–6. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- 63.Tsukamoto K, Yokono K, Amano K, et al. Administration of monoclonal antibodies against vascular cell adhesion molecule-1/very late antigen-4 abrogates predisposing autoimmune diabetes in NOD mice. Cell Immunol. 1995;165:193–201. doi: 10.1006/cimm.1995.1205. [DOI] [PubMed] [Google Scholar]
- 64.Abraham WM, Ahmed A, Sielczak MW, Narita M, Arrhenius T, Elices MJ. Blockade of late-phase airway responses and airway hyperresponsiveness in allergic sheep with a small-molecule peptide inhibitor of VLA-4. Am J Respir Crit Care Med. 1997;156:696–703. doi: 10.1164/ajrccm.156.3.9609039. [DOI] [PubMed] [Google Scholar]
- 65.Sagara H, Matsuda H, Wada N, Yagita H, Fukuda T, Okumura K, Makino S, Ra C. A monoclonal antibody against very late activation antigen-4 inhibits eosinophil accumulation and late asthmatic response in a guinea pig model of asthma. Int Arch Allergy Immunol. 1997;112:287–94. doi: 10.1159/000237468. [DOI] [PubMed] [Google Scholar]
- 66.Jackson DY, Quan C, Artis DR, et al. Potent alpha 4 beta 1 peptide antagonists as potential anti-inflammatory agents. J Med Chem. 1997;40:3359–68. doi: 10.1021/jm970175s. [DOI] [PubMed] [Google Scholar]
- 67.Chaudhuri A, Behan PO. Natalizumab for relapsing multiple sclerosis. N Engl J Med. 2003;348:1598–9. doi: 10.1056/NEJM200304173481614. [DOI] [PubMed] [Google Scholar]
- 68.Lew EA, Stoffel EM. Natalizumab for active Crohn's disease. N Engl J Med. 2003;348:1599. doi: 10.1056/NEJM200304173481615. [DOI] [PubMed] [Google Scholar]
- 69.von Andrian UH, Engelhardt B. Alpha4 integrins as therapeutic targets in autoimmune disease. N Engl J Med. 2003;348:68–72. doi: 10.1056/NEJMe020157. [DOI] [PubMed] [Google Scholar]
- 70.Noseworthy JH, Kirkpatrick P. Natalizumab. Nat Rev Drug Discov. 2005;4:101–2. doi: 10.1038/nrd1637. [DOI] [PubMed] [Google Scholar]
- 71.Stacy KM. Therapeutic mAbs: saving lives and making billions. The Scientist. 2005;19:14. [Google Scholar]
- 72.Ma X, O'Brien ER. Antagonism of the alpha4 integrin subunit attenuates the acute inflammatory response to stent implantation yet is insufficient to prevent late intimal formation. J Leukoc Biol. 2004;75:1016–21. doi: 10.1189/jlb.1203618. [DOI] [PubMed] [Google Scholar]
- 73.Yang XD, Karin N, Tisch R, Steinman L, McDevitt HO. Inhibition of insulitis and prevention of diabetes in nonobese diabetic mice by blocking 1-selectin and very late antigen 4 adhesion receptors. Proc Natl Acad Sci USA. 1993;90:10494–8. doi: 10.1073/pnas.90.22.10494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kudlacz E, Whitney C, Andresen C, et al. Pulmonary eosinophilia in a murine model of allergic inflammation is attenuated by small molecule alpha4beta1 antagonists. J Pharmacol Exp Ther. 2002;301:747–52. doi: 10.1124/jpet.301.2.747. [DOI] [PubMed] [Google Scholar]
- 75.Vedder NB, Winn RK, Rice CL, Chi EY, Arfors KE, Harlan JM. A monoclonal antibody to the adherence-promoting leukocyte glycoprotein, CD18, reduces organ injury and improves survival from hemorrhagic shock and resuscitation in rabbits. J Clin Invest. 1988;81:939–44. doi: 10.1172/JCI113407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blazar BR, Carroll SF, Vallera DA. Prevention of murine graft-versus-host disease and bone marrow alloengraftment across the major histocompatibility barrier after donor graft preincubation with anti-LFA1 immunotoxin. Blood. 1991;78:3093–102. [PubMed] [Google Scholar]
- 77.Kakimoto K, Nakamura T, Ishii K, Takashi T, Iigou H, Yagita H, Okumura K, Onoue K. The effect of anti-adhesion molecule antibody on the development of collagen-induced arthritis. Cell Immunol. 1992;142:326–37. doi: 10.1016/0008-8749(92)90294-y. [DOI] [PubMed] [Google Scholar]
- 78.Nishikawa K, Guo YJ, Miyasaka M, Tamatani T, Collins AB, Sy MS, McCluskey RT, Andres G. Antibodies to intercellular adhesion molecule 1/lymphocyte function-associated antigen 1 prevent crescent formation in rat autoimmune glomerulonephritis. J Exp Med. 1993;177:667–77. doi: 10.1084/jem.177.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hasegawa Y, Yokono K, Taki T, et al. Prevention of autoimmune insulin-dependent diabetes in non-obese diabetic mice by anti-LFA-1 and anti-ICAM-1 mAb. Int Immunol. 1994;6:831–8. doi: 10.1093/intimm/6.6.831. [DOI] [PubMed] [Google Scholar]
- 80.Fischer A, Friedrich W, Fasth A, et al. Reduction of graft failure by a monoclonal antibody (anti-LFA-1 CD11a) after HLA nonidentical bone marrow transplantation in children with immunodeficiencies, osteopetrosis, and Fanconi's anemia: a European Group for Immunodeficiency/European Group for Bone Marrow Transplantation report. Blood. 1991;77:249–56. [PubMed] [Google Scholar]
- 81.Le Mauff B, Hourmant M, Rougier JP, et al. Effect of anti-LFA1 (CD11a) monoclonal antibodies in acute rejection in human kidney transplantation. Transplantation. 1991;52:291–6. doi: 10.1097/00007890-199108000-00020. [DOI] [PubMed] [Google Scholar]
- 82.Stoppa AM, Maraninchi D, Blaise D, et al. Anti-LFA1 monoclonal antibody (25.3) for treatment of steroid-resistant grade III–IV acute graft-versus-host disease. Transpl Int. 1991;4:3–7. doi: 10.1007/BF00335508. [DOI] [PubMed] [Google Scholar]
- 83.Gauvreau GM, Becker AB, Boulet LP, et al. The effects of an anti-CD11a mAb, efalizumab, on allergen-induced airway responses and airway inflammation in subjects with atopic asthma. J Allergy Clin Immunol. 2003;112:331–8. doi: 10.1067/mai.2003.1689. [DOI] [PubMed] [Google Scholar]
- 84.Lebwohl M, Tyring SK, Hamilton TK, et al. A novel targeted T-cell modulator, efalizumab, for plaque psoriasis. N Engl J Med. 2003;349:2004–13. doi: 10.1056/NEJMoa030002. [DOI] [PubMed] [Google Scholar]
- 85.Marecki S, Kirkpatrick P. Efalizumab. Nat Rev Drug Discov. 2004;3:473–4. doi: 10.1038/nrd1420. [DOI] [PubMed] [Google Scholar]
- 86.Yusuf-Makagiansar H, Anderson ME, Yakovleva TV, Murray JS, Siahaan TJ. Inhibition of LFA-1/ICAM-1 and VLA-4/VCAM-1 as a therapeutic approach to inflammation and autoimmune diseases. Med Res Rev. 2002;22:146–67. doi: 10.1002/med.10001. [DOI] [PubMed] [Google Scholar]
- 87.Aruffo A, Hollenbaugh D. Therapeutic intervention with inhibitors of co-stimulatory pathways in autoimmune disease. Curr Opin Immunol. 2001;13:683–6. doi: 10.1016/s0952-7915(01)00279-5. [DOI] [PubMed] [Google Scholar]
- 88.Greener M. MAbs turn. The Scientist. 2005;30::14. [Google Scholar]
- 89.Alter A, Duddy M, Hebert S, et al. Determinants of human B cell migration across brain endothelial cells. J Immunol. 2003;170:170(9):4497, 505. doi: 10.4049/jimmunol.170.9.4497. [DOI] [PubMed] [Google Scholar]
- 90.Ghosh S. Therapeutic value of alpha-4 integrin blockade in inflammatory bowel disease: the role of natalizumab. Expert Opin Biol Ther. 2003;3:995–1000. doi: 10.1517/14712598.3.6.995. [DOI] [PubMed] [Google Scholar]
- 91.Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 92.Renno T, Krakowski M, Piccirillo C, Lin JY, Owens T. TNF-alpha expression by resident microglia and infiltrating leukocytes in the central nervous system of mice with experimental allergic encephalomyelitis. Regulation by Th1 cytokines. J Immunol. 1995;154:944–53. [PubMed] [Google Scholar]
- 93.Theien BE, Vanderlugt CL, Eagar TN, Nickerson-Nutter C, Nazareno R, Kuchroo VK, Miller SD. Discordant effects of anti-VLA-4 treatment before and after onset of relapsing experimental autoimmune encephalomyelitis. J Clin Invest. 2001;107:995–1006. doi: 10.1172/JCI11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Theien BE, Vanderlugt CL, Nickerson-Nutter C, Cornebise M, Scott DM, Perper SJ, Whalley ET, Miller SD. Differential effects of treatment with a small-molecule VLA-4 antagonist before and after onset of relapsing EAE. Blood. 2003;102:4464–71. doi: 10.1182/blood-2003-03-0974. [DOI] [PubMed] [Google Scholar]
- 95.Ferguson TA, Stuart PM, Herndon JM, Griffith TS. Apoptosis, tolerance, and regulatory T cells – old wine, new wineskins. Immunol Rev. 2003;193::111–23. doi: 10.1034/j.1600-065x.2003.00042.x. [DOI] [PubMed] [Google Scholar]
- 96.Gomez-Reino JJ, Carmona L, Valverde VR, Mola EM, Montero MD, Group B. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active-surveillance report. Arthritis Rheum. 2003;48:2085–91. doi: 10.1002/art.11137. [DOI] [PubMed] [Google Scholar]
- 97.Cush JJ. Unusual toxicities with TNF inhibition. heart failure and drug-induced lupus. Clin Exp Rheumatol. 2004;22:S141–7. [PubMed] [Google Scholar]
- 98.Kandula S, Abraham C. LFA-1 on CD4+ T cells is required for optimal antigen-dependent activation in vivo. J Immunol. 2004;173:4443–51. doi: 10.4049/jimmunol.173.7.4443. [DOI] [PubMed] [Google Scholar]
- 99.Welsh CT, Rose JW, Hill KE, Townsend JJ. Augmentation of adoptively transferred experimental allergic encephalomyelitis by administration of a monoclonal antibody specific for LFA-1 alpha. J Neuroimmunol. 1993;43:161–7. doi: 10.1016/0165-5728(93)90087-f. [DOI] [PubMed] [Google Scholar]
- 100.Isobe M, Suzuki J, Yamazaki S, et al. Regulation by differential development of Th1 and Th2 cells in peripheral tolerance to cardiac allograft induced by blocking ICAM-1/LFA-1 adhesion. Circulation. 1997;96:2247–53. doi: 10.1161/01.cir.96.7.2247. [DOI] [PubMed] [Google Scholar]
- 101.Gottlieb AB, Krueger JG, Wittkowski K, Dedrick R, Walicke PA, Garovoy M. Psoriasis as a model for T-cell-mediated disease: immunobiologic and clinical effects of treatment with multiple doses of efalizumab, an anti-CD11a antibody. Arch Dermatol. 2002;138:591–600. doi: 10.1001/archderm.138.5.591. [DOI] [PubMed] [Google Scholar]
- 102.Gottlieb A, Krueger JG, Bright R, et al. Effects of administration of a single dose of a humanized monoclonal antibody to CD11a on the immunobiology and clinical activity of psoriasis. J Am Acad Dermatol. 2000;42:428–35. doi: 10.1016/s0190-9622(00)90214-7. [DOI] [PubMed] [Google Scholar]
- 103.Papp K, Bissonnette R, Krueger JG, et al. The treatment of moderate to severe psoriasis with a new anti-CD11a monoclonal antibody. J Am Acad Dermatol. 2001;45:665–74. doi: 10.1067/mjd.2001.117850. [DOI] [PubMed] [Google Scholar]
- 104.Takeshita K, Yamasaki T, Akira S, Gantner F, Bacon KB. Essential role of MHC II-independent CD4+ T cells, IL-4 and STAT6 in contact hypersensitivity induced by fluorescein isothiocyanate in the mouse. Int Immunol. 2004;16:685–95. doi: 10.1093/intimm/dxh073. [DOI] [PubMed] [Google Scholar]
- 105.Stassen M, Fondel S, Bopp T, et al. Human CD25+ regulatory T cells: two subsets defined by the integrins alpha 4 beta 7 or alpha 4 beta 1 confer distinct suppressive properties upon CD4+ T helper cells. Eur J Immunol. 2004;34:1303–11. doi: 10.1002/eji.200324656. [DOI] [PubMed] [Google Scholar]