Abstract
We previously reported that ligation of CD3 induces antiapoptotic signals in T helper 2 (Th2) cells, and in contrast causes Th1 cells to undergo apoptosis. Here we show that Cbl-b is accountable for the unequal response, revealing a previously unknown cell-specific regulatory function for the molecule. Absence of Cbl-b resulted in resistance to activation-induced apoptosis in murine Th1 cells following CD3 ligation, akin to what is observed in Th2 cells containing Cbl-b. Concurrent with the apoptosis profile, CD3 ligation in the absence of Cbl-b induced raft mobilization and cytoskeletal rearrangement in Th1 cells. Despite their ability to signal from CD3, Th2 cells did not aggregate their rafts, providing an explanation for cell-specific activity of Cbl-b.
Keywords: Th1/Th2 cells, apoptosis, Cbl-b, rafts, costimulation, CD28
Introduction
The E3 ubiquitin ligase, Casitas B cell lymphoma-b (Cbl-b)1 has recently emerged as an important negative regulator of T-cell activation.2 In response to T-cell receptor (TCR) stimulation alone, peripheral T cells from Cbl-b–/– mice exhibit deregulated activation. The cells are hyperproliferative and secrete enhanced levels of Interleukin-2. Consistent with increased T-cell activation, Cbl-b–/– mice develop spontaneous autoimmunity.3,4 Mutation of cbl-b uncouples raft aggregation, antigen receptor clustering and phosphorylation of the guanosine diphosphate/guanosine triphosphate exchange factor Vav1 from CD28 costimulation dependency5 resulting in enhanced T-cell activation. The major pathway affected by Cbl-b is the CD28 initiated Vav-1-WASP signalling pathway which is necessary for full T-cell activation.5
Despite a large number of studies on CD28 costimulation, none have specifically addressed the costimulation requirements of mature differentiated effector T helper 1 (Th1) and Th2 populations. Furthermore, how Cbl-b regulates signals generated from costimulatory molecules on the two subsets has also not been studied. Our laboratory has been studying activation-induced cell death in Th1 and Th2 cells and has reported that CD95-mediated apoptosis in response to CD3/TCR complex ligation without engagement of costimulatory molecules occurs only in Th1, and not in Th2 clones.6 We showed that mechanistically the difference in sensitivity to apoptosis of the two subsets was caused by a selective lack of up-regulation of phosphoinositol-3′-kinase (PI3′-K) activity in Th1 clones following CD3 ligation.7 In continued investigations, we also reported that PI3′-K abrogated apoptosis by mediating changes in the cytoskeleton, which in turn inhibited the lateral diffusion of CD95 in the membrane.8 Notwithstanding this accumulated understanding of activation-induced apoptosis in Th1 and Th2 cells, the mechanism behind why analogous receptors triggered different outcomes in the two subsets continued to be unclear. In this study, we therefore examined whether Cbl-b functioned in a cell-specific manner, exclusively in Th1 cells.
Materials and methods
Mice
Female wild-type C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Breeding pairs of Cbl-b-deficient mice5 were provided by Amgen Inc. (Thousand Oaks, CA). Breeding pairs of Ovalbumin (OVA) peptide-specific DO11.10 TCR transgenic mice on the BALB/c background9 were purchased from Jackson Laboratory. The knockout and the transgenic mice were bred and maintained under pathogen-free conditions in the Rodent Barrier Facility at Temple University School of Medicine (Philadelphia, PA).
Antibodies and recombinant cytokines
The monoclonal antibodies (mAb) used in this study were as follows: anti-interleukin (IL)-4 (11B11 clone) from the National Cancer Institute (Bethesda, MD); anti-CD3 (2C11 clone), anti-CD28 (PV-1 clone), anti-interferon-γ (IFN-γ; R4-6A2 clone) from American Type Culture Collection (ATCC, Bethesda, MD); anti-IL-12 (C17.15 clone) from Wistar Institute (Philadelphia, PA). Anti-p85α antibody, anti-Cbl-b antibody, and anti-β-tubulin antibody were purchased from Santa Cruz Biotech (Santa Cruz, CA). The recombinant cytokines IL-12 and IL-4 were purchased from BD Biosciences Pharmingen (San Diego, CA).
Biasing of naïve splenocytes to Th1 and Th2 lines
CD4+ T lymphocytes were positively selected from the spleens of wild type (Cbl-b+/+) and Cbl-b-deficient mice (Cbl-b–/–) using BD Image anti-mouse CD4 particles (BD Biosciences Pharmingen), and were cultured in Dulbecco's modified Eagle's Medium supplemented with anti-IL-4 mAb (10 µg/ml), recombinant IL-12 (5 ng/ml) and stimulated in the presence of plate-bound anti-CD3 mAb and 1 µg/ml of soluble anti-CD28 mAb to generate Cbl-b+/+ and Cbl-b–/– Th1 cells. For generation of Th2 cells, stimulation was carried out in the presence of anti-IFN-γ mAb (10 µg/ml), anti-IL-12 mAb (10 µg/ml) and recombinant IL-4 (5 ng/ml). On day 3, all cultures received IL-2 to induce cell expansion, and on day seven the cells were harvested for use in the apoptosis, raft aggregation or actin polymerization experiments described below. An aliquot of the day-7 cells was restimulated with CD3 antibodies and supernatants were assayed for the presence of IFN-γ and IL-4 by enzyme-linked immunosorbent assay (ELISA) to confirm that appropriate biasing of the lines had occurred.
Apoptosis
Cell apoptosis was measured using a previously described sandwich ELISA method.10 This method is based on detection of nucleosomal fragments released from nuclei of apoptotic cells. Percentage apoptosis is calculated as follows:
Visualization of raft aggregation
Red fluorescent fluorospheres (560 nm excitation, Molecular Probes, Eugene, OR) were coated with CD3 mAb, and with or without CD28 mAb or immunoglobulin G (IgG) isotype control. Th1 and Th2 lines (5 × 104 each) generated from Cbl-b+/+ and Cbl-b–/– mice were stimulated with the mAb-coated fluorospheres for 20 min at 37° in chamber slides. Cells were washed twice with phosphate-buffered saline (PBS) and fixed with 1% formaldehyde. Rafts were stained with 8 µg/ml fluoroscein isothiocyanate (FITC)-conjugated cholera toxin B subunit for 45 min. Cell:bead interactions were visualized and imaged using an Olympus confocal microscope with Fluoview software (Olympus, Melville, NY). A hundred cell:bead conjugates were counted for each condition. Of the hundred cell:bead interactions, raft polarization was scored dependent upon the polarization of the FITC-positive rafts at the site of cell:bead interaction.
Visualization of actin polymerization
5 × 104 each of Th1 and Th2 lines generated from Cbl-b+/+ and Cbl-b–/– mice were reacted with 106 beads coated with mabs at 37° for 20 min in a 96 well plate. The plate was then centrifuged to pellet cell-bead conjugates, re-suspended in 20 µl PBS, and transferred to poly l-Lysine (Sigma, Poole, UK) coated slides (Fisher, Hanover Park, IL), fixed, washed and reacted with 50 µg/ml tetramethylrhodamine isothiocyanate-conjugated phalloidin (Sigma) diluted in PBS containing 1% saponin and 1% bovine serum albumin (BSA) for 45 min at room temperature. The slides were washed and mounted in Slow Fade, an antifade solution (Molecular Probes). Confocal microscopy was performed with Olympus Fluoview 300 microscope to visualize cell:bead interactions and images captured via Fluoview software (Olympus). A minimum of 50 cell:bead interactions were counted for each condition. A positive actin polymerization was scored when a cap, or increased intensity of the phalloidin staining, was visualized at one pole of the cell.
Statistical analysis
For statistical analysis of samples, Student t-test was performed when comparing two conditions, while anova was used for comparison of multiple conditions (Prizm v. 4.0, Graph Pad Software, San Diego, CA). P-values of <0·05 were considered significant.
Results
Cbl-b regulates the divergence in apoptosis pathway that ensues in Th1 and Th2 cells following CD3 ligation
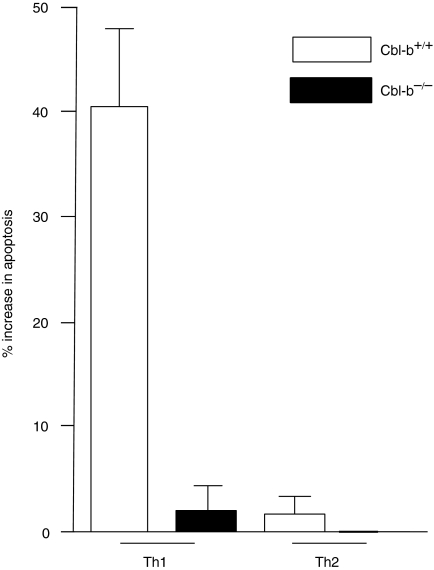
One variable that could affect CD3 signalling from Th1 and Th2 cells to result in differential resistance to apoptosis is the expression level of CD3 and CD28 on the two cell lines. To rule out this possibility, we first examined by flow cytometry expression levels of CD3 and CD28 on Th1 and Th2 cell lines, and found that both lines expressed equivalent amounts of the two molecules (data not shown). Knowing Cbl-b's negative regulatory effects on peripheral T cells5 we then examined if Cbl-b was responsible for the differential ability of Th1 and Th2 cells to resist activation-induced apoptosis. For these studies Th1 and Th2 lines were generated from both Cbl-b sufficient wild type (Cbl-b+/+) and Cbl-b-deficient mice (Cbl-b–/–). The lines exhibited the characteristic cytokine profile following stimulation, with Th1 cells producing IFN-γ and Th2 cells producing IL-4 (data not shown). Examination of the apoptosis profile of Th1 cells revealed that Cbl-b+/+ Th1 cells, as would be expected, underwent apoptosis following CD3 stimulation, and in contrast, Cbl-b–/– Th1 cells were protected from apoptosis following CD3 stimulation alone (P < 0·001, Fig. 1). Additionally, if CD28 costimulation was provided to Cbl-b+/+ Th1 cells during CD3 stimulation, apoptosis was also reduced significantly [4 ± 1% (P < 0·01) data not shown]. Therefore, in the absence of Cbl-b, CD3 stimulation alone could signal for Th1 protection from apoptosis. Notably, both Cbl-b–/– and Cbl-b+/+ Th2 cells showed no significant apoptosis (Fig. 1). Thus, Cbl-b functions differently in the Th subsets; as a negative regulator in the Th1 CD3 signalling cascade, but having a nominal effect in the Th2 signalling pathway.
Figure 1.
Th1 cells resist activation-induced apoptosis in the absence of Cbl-b. Cbl-b+/+ and Cbl-b–/– Th1 and Th2 cells were stimulated for 5 hr with plate bound anti-CD3 antibody and extent of apoptosis was measured. Data are mean ± SD from three separate experiments.
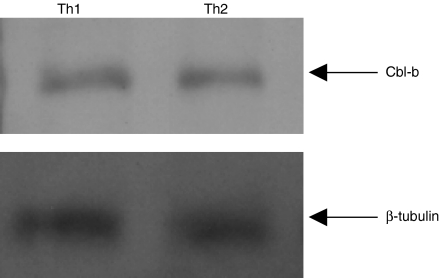
Results presented in Fig. 2 demonstrate that the expression levels of Cbl-b are similar in the two subsets, indicating that the mere absence of Cbl-b protein in Th2 cells is not responsible for its resistance to activation-induced apoptosis.
Figure 2.
Equivalent Cbl-b protein expression levels in Th1 and Th2 cells. Th1 and Th2 (2 × 106) cells were lysed in ice-cold buffer containing 25 mm Tris, pH 7·5, 150 mm NaCl, 5 mm ethylenediaminetetra-acetic acid, 1 mm phenylmethylsulphonyl fluoride, 1 mm Na3VO4, 1 µg/ml aprotinin, 1 µg/ml leupeptin and 1% NP-40. The proteins were separated on a 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis and immunoblotted with anti-Cbl-b antibody and developed using ECL Western blotting detection system (Amersham, UK). The blot was stripped and reprobed with anti-β-tubulin antibody. The data are representative of two individual experiments.
Th1 and Th2 cells signal from different compartments allowing Cbl-b to function in a cell-specific manner
Rafts are detergent insoluble glycolipid enriched raft microdomains (DIGS) of cholesterol and sphingolipid responsible for the aggregation of signalling molecules at the immunological synapse.11 Sequential to CD3 and CD28 costimulation, the GM1 positive rafts coalesce at the site of receptor engagement creating a stable platform for T-cell signalling.12 Specifically, following T-cell activation PI3′-K that can protect Th1 cells from apoptosis7 is enriched in the DIG fractions.13 We reasoned that the disparity in Cbl-b's influence on Th1 and Th2 cells was a reflection of the signals necessary for raft partitioning, and as a result, CD3 triggering of Th2 cells would induce raft aggregation. In contrast, the Th1 population would require removal of Cbl-b to achieve raft aggregation, or possibly addition of CD28 that is known to negate Cbl-b's functions.
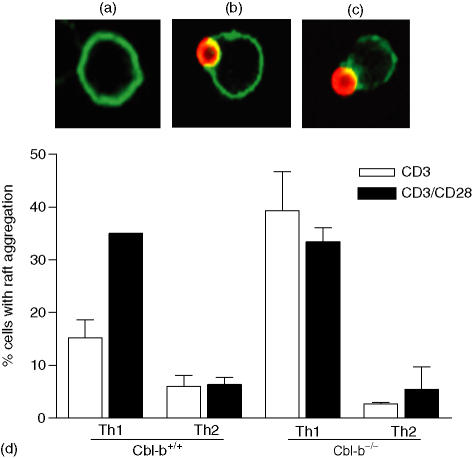
To investigate whether there was a difference in signal requirement for raft clustering, Cbl-b+/+ and Cbl-b–/– Th1 and Th2 cells were stimulated with anti-CD3 antibody-coated fluorescent beads that mimicked the T cell and antigen-presenting cell interaction. To test the role of CD28, cells were also stimulated with beads coated with both anti-CD3 and anti-CD28 antibodies. CD3 stimulation alone induced a nominal number of Th1 cells to aggregate their rafts, however, significantly more Th1 cells partitioned their rafts to the site of receptor engagement either when Cbl-b was absent (P < 0·01, Fig. 3) or when CD28 costimulation was provided (P < 0·05, Fig. 3). Thus in Th1 cells, in the absence of Cbl-b, CD3 stimulation alone was sufficient for the coalescing of membrane microdomains, without additional costimulation from CD28. Similar analysis with Th2 lines gave unexpected results, with the cells showing no evidence of membrane partitioning following stimulation. In the presence or absence of Cbl-b, and with or without CD28 costimulation, Th2 cells did not have raft polarization at the site of receptor engagement (Fig. 3).
Figure 3.
Absence of Cbl-b induces Raft aggregation in Th1 cells. Th1 and Th2 lines derived from Cbl-b+/+ and Cbl-b–/– mice, were stimulated with either CD3 or CD3/CD28 mAb coated red fluorescent fluorospheres for 20 min at 37°. Cells were stained with FITC-tagged cholera toxin B subunit to detect ganglioside rich membrane microdomains (rafts) as shown in (a). For each condition 100 cell:bead interactions were visualized by confocal microscopy (b), and raft aggregation in the 100 cells interacting with beads was determined as an aggregation of over 50% of the GM1 staining to the site of receptor engagement (c). The percentage of raft aggregation was calculated as: raft aggregation = (raft positive cells/total cell bead interactions) × 100 (d). Data are mean ± SD of three separate experiments.
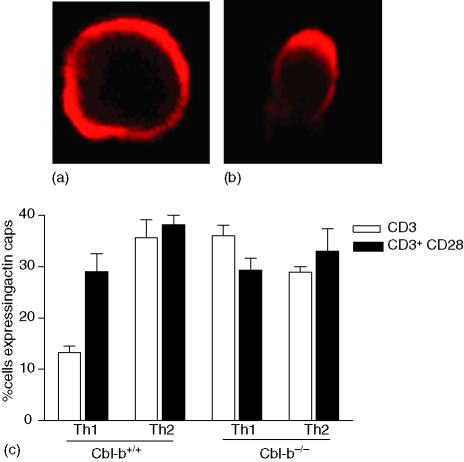
Next we wanted to confirm that in Th2 cells, despite lack of raft polarization, receptor ligation was initiating a signalling cascade. This was determined by examining cytoskeletal reorganization in the cells following stimulation. All four lines were stimulated with anti-CD3 antibody coated or anti-CD3 and anti-CD28 antibody coated beads, and stained with TRITC conjugated phalloidin to detect actin filaments (Fig. 4a, b). Cell:bead interactions were visualized by confocal microscopy, and the percentage of cells with actin caps was determined as an indication of actin reorganization and CD3/TCR clustering. Actin caps were compared between Th1 and Th2 lines derived from wild type and knockout mice. There was significant difference (P < 0·001, Fig. 4c) in actin capping between Cbl-b+/+ Th1 and Cbl-b+/+ Th2 cells when stimulated with CD3 alone, indicating that in Th2 cells signalling was initiated from the CD3/TCR, despite lack of raft aggregation. As observed with raft aggregation, when Th1 cells were stimulated with both CD3 and CD28 antibodies, they were able to reorganize their actin (P < 0·01, Fig. 4) and displayed actin caps equivalent to that seen in Th2 cells stimulated with CD3 alone (Fig. 4). Comparison of actin caps in Cbl-b+/+ and Cbl-b–/– Th1 cells revealed that CD3 stimulation alone was sufficient to induce enhanced actin caps in the absence of Cbl-b (P < 0·01), and CD28 costimulation did not further enhance the capping.
Figure 4.
Cbl-b deficiency induces actin reorganization in Th1 cells. Th1 and Th2 cells derived from Cbl- b +/+ and Cbl-b–/– mice were stimulated with CD3 or CD3/CD28 antibody coated polysytrene beads. Actin polymerization was visualized by phalloidin staining using confocal microscopy. Cell:bead interactions were counted. Cells displaying even staining all around were considered negative for actin capping (a) and actin polymerization was defined as capping or aggregation of phalloidin staining at one pole of the cell (b). The percentage of cells with actin caps was calculated as (actin capping/total cell:bead interactions) × 100 (c) Data are mean ± SD from three separate experiments.
Together, these data suggest that Th2 cells signal from a compartment that is different from that present in Th1 cells. This probably results in enhanced and sustained signalling from the CD3/TCR of Th2 cells, which empowers the cells to override Cbl-b's suppressive effects without help from CD28 costimulation.
Discussion
Deregulation of T-cell activation has been previously implicated for Cbl-b.14 The findings presented here delineate a new cell-specific regulatory function for Cbl-b. Together with our previously published work that Th2 cells but not Th1 cells activate PI3′-K in response to CD3 stimulation alone7 the data presented here suggest that the strength of signalling generated from CD3/TCR of Th2 cells may be stronger. Therefore two cells do not require CD28 costimulation to achieve full activation necessary to resist apoptosis. Studies in Cbl-b and ubiquitin transfected Jurkat T cells have shown that CD28 costimulation leads to the ubiquitination and degradation of Cbl-b.15 The amount of ubiquitinated Cbl-b targeted for degradation was directly related to the degree and duration of CD28 engagement.15 Similarly, Th1 cells may require CD28 costimulation to block Cbl-b's negative regulation of T-cell activation by targeting the ligase for degradation. In contrast, in Th2 cells ligation of the TCR complex alone is probably sufficient to ubiquitinate Cbl-b thereby inhibiting its negative regulation and allowing for the threshold of activation to be achieved.
Prior to this report, the contribution of raft aggregation to generation of antiapoptotic signals in Th1 and Th2 cell subsets had not been addressed. Our results demonstrate that raft coalescence is not critical for Th2 cells to generate antiapoptotic signals. In contrast, Th1 cells need to aggregate their rafts in a CD28-dependent manner to resist apoptosis. There are other studies that support TCR signalling can occur independent of raft integrity. For example, proteins associated with the TCR complex are found to aggregate with rafts at the site of MHC/peptide contact in Th1 cells but not Th2 cells16 and signalling in Jurkat cells in response to CD3 antibody occurs outside of raft microdomains.17 A recent study using fluorescence resonance energy transfer (FRET) analysis of Jurkat T cells showed that signalling molecules had a random distribution during localized activation of the T-cell receptor, and were not necessarily clustered in the rafts.18 A mechanism that may explain Th2 cell's ability to signal in the absence of raft aggregation is TCR avidity. Activation of naïve T cells enhances their TCR avidity for peptide–MHC complexes by 20- to 50-fold caused by increases in cross-linking of TCR.19 In a similar manner, as cells differentiate into effector Th1 and Th2 cells the TCR avidity of the two subsets may diverge, presenting a so far unappreciated means of regulating signalling from the two subsets. It is possible that TCR avidity of Th1 cells is low and is therefore dependent on CD28 costimulation to aggregate rafts and achieve the same quantum of signalling that is generated from the higher avidity TCR of Th2 cells independent of CD28 and raft coalescing. Depletion of cholesterol in Th1 and Th2 cells caused a significant reduction in calcium mobilization only in the Th1 cells following ligation with a high affinity TCR agonist.20 Together, these data suggest that there is an intrinsic difference between the TCRs of Th1 and Th2 cells leading to differences in the requirement of raft compartmentalization as a prerequisite for complete signalling. A future area of investigation would be to examine TCR avidity of Th1 and Th2 cells and to determine its sensitivity to cholesterol content in the membrane.
A role for Cbl-b in the maintenance of tolerance and in the development of autoimmunity has been implicated. Based on data presented here, it will be interesting to explore if Cbl-b also functions in the temporal regulation of Th1 effector activity generated in response to an infectious challenge. Cytokines produced by pathogen-specific Th1 cells are highly inflammatory and when unregulated can result in excessive tissue destruction. Therefore, the immune system may have created an internal mechanism for regulating the Th1 immune response by requiring costimulation for effector Th1 proliferation, and resistance from apoptosis. When danger from the pathogen is removed, B7 molecules are down regulated, however, the TCRs are still engaged from the plethora of peptides that are generated during infection. Cbl-b may function to prevent activation signals from being generated from the engaged TCR and instead target the Th1 cells for apoptosis. In such a scenario, Th2 cells may continue to be active for a longer time to further down-regulate inflammation. Cbl-b–/– mice offer us a unique model to further examine in vivo the role of Cbl-b and costimulation on pathogen-specific Th1 effector activity.
Acknowledgments
We thank Amgen for providing the Cbl-b-deficient mice and NCI for 11B11 antibodies. Allison Hanlon was supported by the MD/PhD program at Temple University School of Medicine.
References
- 1.Keane MM, Rivero-Lezcano OM, Mitchell JA, Robbins KC, Lipkowitz S. Cloning and characterization of cbl-b: a SH3 binding protein with homology to the c-cbl proto-oncogene. Oncogene. 1995;10:2367–77. [PubMed] [Google Scholar]
- 2.Liu YC, Gu H. Cbl and Cbl-b in T-cell regulation. Trends Immunol. 2002;23:140–3. doi: 10.1016/s1471-4906(01)02157-3. [DOI] [PubMed] [Google Scholar]
- 3.Bachmaier K, Krawczyk C, Kozieradzki I, et al. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature. 2000;403:211–6. doi: 10.1038/35003228. [DOI] [PubMed] [Google Scholar]
- 4.Chiang YJ, Kole HK, Brown K, et al. Cbl-b regulates the CD28 dependence of T-cell activation. Nature. 2000;403:216–20. doi: 10.1038/35003235. [DOI] [PubMed] [Google Scholar]
- 5.Krawczyk C, Bachmaier K, Sasaki T, et al. Cbl-b is a negative regulator of receptor clustering and Raft aggregation in T cells. Immunity. 2000;13:463–73. doi: 10.1016/s1074-7613(00)00046-7. [DOI] [PubMed] [Google Scholar]
- 6.Varadhachary A, Perdow S, Hu C, Ramanarayanan M, Salgame P. Differential ability of T cell subsets to undergo activation-induced cell death. Proc Natl Acad Sci USA. 1997;94:5778–83. doi: 10.1073/pnas.94.11.5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varadhachary A, Peter ME, Perdow SN, Krammer PH, Salgame P. Selective upregulation of Phosphatidylinositol 3′-kinase activity in Th2 cells inhibits caspase-8 cleavage at the death-inducing complex: a mechanism for Th2 resistance from Fas-mediated apoptosis. J Immunol. 1999;163:4772–9. [PubMed] [Google Scholar]
- 8.Varadhachary AS, Edidin M, Hanlon AM, Peter ME, Krammer PH, Salgame P. Phosphatidylinositol 3′-kinase blocks CD95 aggregation and caspase-8 cleavage at the death-inducing signaling complex by modulating lateral diffusion of CD95. J Immunol. 2001;166:6564–9. doi: 10.4049/jimmunol.166.11.6564. [DOI] [PubMed] [Google Scholar]
- 9.Hsieh C-S, Macatonia SE, O'Garra A, Murphy KM. T cell genetic background determined default T helper phenotype development in vitro. J Exp Med. 1995;181:713–21. doi: 10.1084/jem.181.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salgame P, Varadhachary A, Primiano LL, Fincke JE, Muller S, Monestier M. An ELISA for detection of apoptosis. Nucl Acids Res. 1997;25:680–1. doi: 10.1093/nar/25.3.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–72. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 12.Viola A, Schroeder S, Sakakibara Y, Lanzavecchia A. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science. 1999;283:680–2. doi: 10.1126/science.283.5402.680. [DOI] [PubMed] [Google Scholar]
- 13.Xavier R, Brennan T, Qingqin L, McCormack C, Seed B. Membrane compartmentalization is required for efficient T cell activation. Immunity. 1998;8:723–32. doi: 10.1016/s1074-7613(00)80577-4. [DOI] [PubMed] [Google Scholar]
- 14.Krawczyk C, Penninger JM. Molecular controls of antigen receptor clustering and autoimmunity. Trends Cell Biol. 2001;11:212–20. doi: 10.1016/s0962-8924(01)01981-x. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Bardos T, Li D, et al. Cutting edge. Regulation of T cell activation threshold by CD28 costimulation through targeting Cbl-b for ubiquitination. J Immunol. 2002;169:2236–40. doi: 10.4049/jimmunol.169.5.2236. [DOI] [PubMed] [Google Scholar]
- 16.Balamuth F, Leitenberg D, Unternaehrer J, Mellman I, Bottomly K. Distinct patterns of membrane microdomain partitioning in Th1 and Th2 cells. Immunity. 2001;15:729–38. doi: 10.1016/s1074-7613(01)00223-0. [DOI] [PubMed] [Google Scholar]
- 17.Pizzo P, Giurisato E, Tassi M, Benedetti A, Pozzan T, Viola A. Lipid rafts and T cell receptor signaling: a critical re-evaluation. Eur J Immunol. 2002;32:3082–91. doi: 10.1002/1521-4141(200211)32:11<3082::AID-IMMU3082>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 18.Glebov OO, Nichols BJ. Lipid raft proteins have a random distribution during localized activation of the T-cell receptor. Nat Cell Biol. 2004;6:238–43. doi: 10.1038/ncb1103. [DOI] [PubMed] [Google Scholar]
- 19.Fahmy TM, Bieler JG, Edidin M, Schneck JP. Increased TCR avidity after T cell activation: a mechanism for sensing low-density antigen. Immunity. 2001;14:135–43. [PubMed] [Google Scholar]
- 20.Leitenberg D, Balamuth F, Bottomly K. Changes in the T cell receptor macromolecular signaling complex and membrane microdomains during T cell development and activation. Semin Immunol. 2001;13:129–38. doi: 10.1006/smim.2000.0304. [DOI] [PubMed] [Google Scholar]