Abstract
The NOD-derived islet-reactive CD4+ T cell clone, BDC-2·5, is able to transfer diabetes to neonatal non-obese diabetic (NOD) mice but is unable to transfer disease to either adult NOD or NOD scid recipients. Transfer of diabetes to adult recipients by BDC-2·5 is only accomplished by cotransfer of CD8+ T cells from a diabetic donor. To understand why this CD4+ T cell clone is able to mediate diabetes in neonatal but not the adult recipients we examined the ability of the clone to traffic in the different recipients. Our studies showed that MAdCAM-1 has a very different expression pattern in the neonatal and adult pancreas. Blockade of this addressin prevents the clone from transferring diabetes to neonatal mice, suggesting that the differential pancreatic expression of MAdCAM-1 in neonatal and adult pancreas provides an explanation of the differences in diabetes development.
Keywords: BDC-2·5, CD8, diabetes, MAdCAM-1, neonates
Introduction
The spontaneous development of diabetes in non-obese diabetic (NOD) mice has been shown to require the functional activity of both CD4+ and CD8+ T cells. Successful transfer of Type 1 diabetes by T cells from a NOD donor mouse to neonatal or lymphopenic recipients has also been shown to require both subsets of T cells.1–4 However, despite using both CD4+ and CD8+ T cells the efficiency of the transfer of diabetes to young recipients has been found to fall when the recipients are greater than 3 weeks of age. This inability to transfer diabetes into intact recipients over the age of 3 weeks has been attributed to the presence of regulatory T cells. Recent studies have emphasized the importance of a neonatal window of lymphopenia,5 suggesting the possibility that the efficiency of diabetes transfer into mice less than 3 weeks of age is due to their lymphopenic status.
The NOD-derived islet-reactive CD4+ T cell clone, BDC-2·5, is able to transfer diabetes to neonatal NODs but fails to transfer disease to either adult NOD or NOD scid recipients. This clone is, however, able to transfer diabetes to adult NOD scid recipients if CD8+ splenic T cells from a diabetic donor are transferred with it.6 As this mirrors the requirements for disease transfer in NOD mice, we initiated studies using the BDC-2·5 clone to try to understand the role of CD8+ T cells in the development of Type 1 diabetes. We find that the requirement for CD8+ T cells for diabetes induction by the BDC-2·5 T cell clone is restricted to adult mice. We show furthermore that the success of neonatal disease induction with this T cell clone is dependent on the expression of MAdCAM-1, highlighting the importance of trafficking in diabetes development.
Materials and methods
Animals
NOD and NOD scid mice were maintained in the Biological Services facility of the Department of Pathology at the University of Cambridge. They received standard laboratory food and water ad libitum. NOD scid mice were maintained in micro-isolator cages with filtered air and handled under sterile conditions in a laminar flow hood. All animal experiments were approved by the Ethical Review Committee of the University of Cambridge.
Antibodies for in vivo treatment
The hybridomas YTS191·1.2 (anti-CD4), YTS156 (anti-CD8α), YTS169 (anti-CD8β) and YTH34·5 (a rat IgG2a isotype control) were obtained from Professor Herman Waldmann (Oxford, UK). MECA-367 (anti-MAdCAM-1) [American Type Culture Collection (ATCC) no. HB-9478] was obtained from ATCC. All hybridomas were grown in our own laboratory in hollow fibre cartridges. Antibodies were purified by precipitation with 50% saturated ammonium sulphate and dialysed extensively against phosphate buffered saline (PBS). An estimate of total protein was determined from the OD280. Antibody concentrations were determined by an anti-rat immunoglobulin enzyme-linked immunosorbent assay (ELISA). The endotoxin levels were < 1 EU/mg protein and the preparations were stored at −20° until use.
In vivo antibody treatment
For experiments involving the addition of splenic CD8+ T cells to the clone transfer in adult recipients, spontaneously diabetic female donors were treated with three intraperitoneal (i.p.) injections, on alternate days, of 1 mg of a depleting anti-CD4 antibody (YTS191) to provide CD8+ T cells for the transfers. CD4+ T cells were from diabetic female donors treated with three i.p. injections on alternate days of 1 mg of a depleting anti-CD8 antibody (YTS169). To deplete CD8+ T cells from neonates, groups of mice were treated i.p. with 500 µg YTS156 and YTS169 on days − 1, 1 and 3 of the clone transfer. To block MAdCAM-1, MECA 367 (500 µg) was given to neonates on days 0, 2 and 4 of the clone transfer. All injections of the clone or antibody were given i.p.
Antibodies for immunohistochemistry
Some of the hybridoma lines used for immunohistochemistry were grown under standard tissue culture conditions and spent supernatants were harvested. Anti-CD3 (KT3) and anti-CD4 (KT4) were from Dr K. Tomonari (Japan), anti-CD8 (YTS105) was from Professor H. Waldmann (Oxford, UK) and anti-ICAM-1 (YNI/1·7.4) (ATCC No. CRL-1878) and anti-MAdCAM-1 (MECA-367) (ATCC no. HB-9478) were obtained from ATCC. Unless stated otherwise, all other antibodies were obtained from Pharmingen (Becton Dickinson UK Ltd, Oxford, UK).
Immunohistochemistry
Tissues were removed at sacrifice and snap-frozen in isopentane. Five-micrometre cryostat sections were air-dried and fixed in acetone for 10 min. Air-dried sections were stored at −80°.
Pancreatic β cells were detected by preblocking sections with 20% normal mouse serum (NMS) followed by incubation with guinea pig anti-porcine insulin (Dako, High Wycombe, UK) in 10% NMS and detected by rhodaminated goat anti-guinea-pig IgG (ICN Pharmaceuticals, Thame, UK) in 10% NMS. Adhesion molecules and CD3 were detected using the appropriate monoclonal antibodies and visualised with fluorescein isothiocyanate (FITC) goat ant-irat Ig (Serotec, Kidlington, UK).
Propagation of the BDC-2·5 clone
The diabetogenic T cell clone was derived from the spleen and lymph nodes of newly diabetic female mice, as described previously.7 Cultures were re-stimulated every 2 weeks by combining 1 × 106 T cells, 2·5 × 107 antigen-presenting cells (APC) (irradiated NOD spleen cells), 10 µg β cell membrane antigen8 and human recombinant interleukin (IL)-2 (1000 units) in 20 ml of culture medium (IMDM) supplemented with 10% fetal bovine serum. To expand cultures for the disease transfer experiments, the 20 ml volume containing the 4-day culture was transferred to a larger flask with 50 ml of new culture medium, containing more IL-2 (3500 units) for a further 4 days. T cells were washed and resuspended in sterile PBS for injection.
Disease transfer by the BDC-2·5 clone
For each experiment, litters of young NOD or NOD scid mice (4–11 days old) were injected i.p. with 1 × 107 T cells. Recipient mice were tested daily from day 4 onwards for glucose in the urine using Diastix (Bayer Diagnostics, Basingstoke, UK). In clone transfer studies in adult mice, recipients were 8 weeks of age and cells were injected intravenously (i.v.).
Flow cytometric analysis
Immunofluorescent staining and flow cytometric analysis were used to look for the presence of α4 and β7 integrins on the BDC-2·5 clone. MAC 219 (a rat IgG2a isotype control) was obtained from Dr Geoff Butcher (Babraham, UK) and was used to assess background staining. Anti-α4 integrin was detected with the clone R1-2 and anti-β7 integrin with the clone M293, both from Pharmingen (Becton Dickinson UK Ltd, Oxford, UK). After incubation with the purified antibodies, biotinylated rabbit anti-rat IgG (Vector, Burlingame, CA) was added followed by streptavidin–phycoerythrin (PE) (Pharmingen, Becton Dickinson). Cells were analysed using FACScan and CELLQuestTM software (Becton Dickinson).
Statistics
Statistical analysis was performed with GraphPad Prism using a Kaplan–Meier survival curve and log-rank test. Results were considered significant if P-values were ≤ 0·05.
Results
CD8+ T cells are required for BDC-2·5-induced diabetes in adult NOD scid but not in neonates
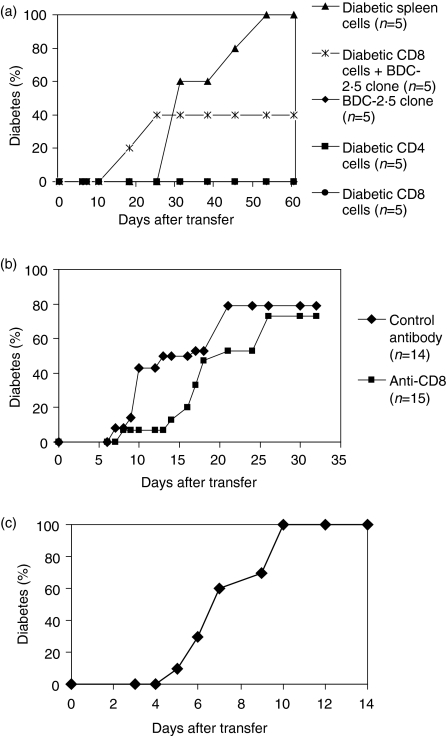
The CD4+ T cell clone, BDC-2·5, has been shown to transfer diabetes to neonatal NOD mice but requires the presence of CD8+ T cells to transfer diabetes to adult NOD scid recipients (Fig. 1a).6 Neither the CD4-depleted nor CD8-depleted spleen cells from a diabetic donor were able to transfer diabetes on their own. This suggested that CD8+ T cells may play a functional role in the induction of beta cell destruction. We decided to see if CD8+ T cells had a role in the induction of beta cell destruction in neonatal NOD mice. When neonatal NOD recipients were depleted of CD8+ T cells by in vivo antibody treatment, BDC-2·5 was still able to transfer diabetes (Fig. 1b). There was no significant difference between the controls and the anti-CD8-treated mice (P = 0·30). The lack of a requirement for CD8+ T cells in this neonatal transfer was confirmed by the ability of the clone to transfer diabetes to neonatal NOD scid recipients (Fig. 1c). These differences between neonatal and adult transfer are not attributable to the different routes of T cell transfer in the recipients, as the same results are seen when T cells are injected i.p. in the adult, the route employed when injecting neonatal recipients (data not shown). These observations have been reproduced several times.
Figure 1.
(a) The BDC-2·5 clone cannot cause diabetes in adult non-obese diabetic (NOD) scid recipients in the absence of CD8+ T cells. The BDC-2·5 T cell clone was transferred to 8-week-old male NOD scid recipients, either alone or with cells from a diabetic donor: CD8+ T cells (CD4 depleted) or CD4+ T cells (CD8 depleted). Control mice received spleen cells, CD4+ T cells or CD8+ T cells all from diabetic donors. This result was reproduced several times in two different laboratories.(b) The BDC-2·5 clone initiates diabetes in neonatal NOD mice in the absence of CD8+ T cells. BDC-2·5 cells (1 × 107) were transferred into 7-day-old NOD recipients, some of which received the clone alone (n = 14) and others (n = 15) were treated with depleting anti-CD8 antibodies. Analysis of spleens and lymph nodes of the treated mice showed an absence of CD8+ T cells (data not shown).(c) The BDC-2·5 clone initiates diabetes in neonatal NOD.scid mice. BDC-2·5 clone cells (1 × 107) were injected intraperitoneally into neonatal NOD scid recipients (n = 8).
BDC-2·5 shows differential trafficking in neonatal and adult NOD mice
In the absence of CD8+ T cells, the BDC-2·5 clone cells are undetectable in the pancreas of the adult recipient, suggesting that they completely fail to traffic to that site. On the other hand, the cells can be detected in the pancreas within 24 hr following clone transfer into a neonatal NOD recipient.9 This was a reproducible finding.
MAdCAM-1 is expressed at high levels in the neonatal pancreas
As there were clear differences between clone access to the adult and neonatal pancreas, this suggested trafficking differences into the pancreas. Addressin expression in adult and neonatal pancreas was therefore examined.
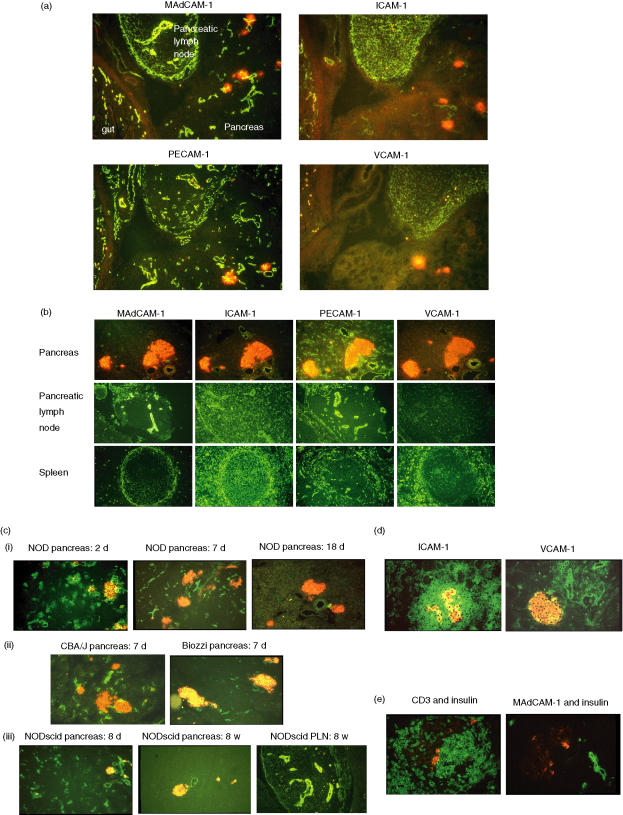
Immunohistochemical analysis of the neonatal and adult NOD mouse pancreas for expression of addressins revealed differences in the expression patterns in the two age groups. Sections of pancreas and adjacent tissues were taken at different time-points after birth and analysed by immunohistochemistry for expression of MAdCAM-1, PECAM-1, ICAM-1 and VCAM-1. Although both the lymph node adjacent to the pancreas and the gut showed high levels of expression of MAdCAM-1, PECAM-1 and ICAM-1, VCAM-1 was detected only in the lymph node. In the neonatal pancreas, MAdCAM-1 and PECAM-1 were both expressed clearly while ICAM-1 was expressed at a lower level and VCAM-1 expression was absent (Fig. 2a). Changes in this neonatal pattern of expression were noted by 18 days of age. Although PECAM-1 and ICAM-1 were expressed at levels comparable to that seen in neonatal pancreas, the expression of MAdCAM-1 was much reduced in the pancreas but was still expressed in the pancreatic draining lymph node (Fig. 2b). VCAM-1 expression remained undetectable in the pancreas. All the adhesion molecules could be detected in the marginal zone of the spleen (Fig. 2b). Further examination of pancreatic MAdCAM-1 expression showed a gradual diminution of expression from day 2 until 18 days after birth. In 7-day-old mice, identical patterns of pancreatic expression of MAdCAM-1 were seen in Biozzi and CBA/J mice to those in NOD mice (Fig. 2c). This suggests that early high levels of expression of MAdCAM-1, followed by its disappearance around 18 days of age, is a normal sequence of events and is not restricted to autoimmune-prone mice. The observation that comparable results were seen in both NOD and NOD scid mice demonstrated that this profile of MAdCAM-1 expression is independent of the presence of lymphocytes (Fig. 2c). High-level expression of MAdCAM-1 remains in the pancreatic draining lymph nodes (Fig. 2b,c). High expression levels of both ICAM-1 and VCAM-1 are seen in the neonatal pancreas when diabetes is induced by transfer of the T cell clone BDC2·5 (Fig. 2d). Although there is little MAdCAM-1 expression in the adult pancreas it is known to be induced during inflammation.10 However, this pattern of induced expression in the adult pancreas is seen at focal sites of inflammation and contrasts with that seen in the normal neonatal pancreas where expression is distributed evenly throughout the pancreas on the microvasculature. Such expression of MAdCAM-1 was visible at 26 days of age in a NOD recipient of the BDC-2·5 clone (16 days after injection) (Fig. 2e), although its more restricted appearance in the islet area, rather than uniformly throughout the pancreas on the microvasculature, was more like that seen in an adult NOD with insulitis.10,11
Figure 2.
A variety of tissues from young mice were snap-frozen and sections stained for insulin (red fluorescence) and adhesion molecules or CD3 (green fluorescence). All photomicrographs were taken using a × 20 objective lens. (a) Expression of MAdCAM-1, PECAM-1, ICAM-1 and VCAM-1 in 4-day-old non-obese diabetic (NOD) mice. Serial sections from a 4-day-old NOD mouse showing pancreas, pancreatic lymph node and gut in each section were stained with antibodies to detect expression of MAdCAM-1, PECAM-1, ICAM-1 and VCAM-1 as indicated. (b) Expression of MAdCAM-1, PECAM-1, ICAM-1 and VCAM-1 in 18-day-old NOD mice. Serial sections of pancreas, lymph node and spleen from an 18-day-old NOD mouse were stained for expression of adhesion molecules as indicated. (c) Expression of MAdCAM-1 at different ages and in different strains. Sections of pancreas, spleen or lymph node were stained with anti-MAdCAM-1 antibody. Sections stained were of (i) pancreas from 2-day-, 7-day- and 18-day-old NOD mice; (ii) pancreas from 7-day-old CBA/J and Biozzi mice; (iii) pancreas from 8-day-old and 8-week-old NOD scid mice, pancreatic lymph node from an 8-week-old NOD scid mouse. (d) ICAM-1 and VCAM-1 are up-regulated after infiltration of the BDC-2·5 clone. Sections of pancreas from a mouse that was injected intraperitoneally (i.p.) with BDC-2·5 clone cells (1 × 107) at 7 days of age. The pancreas was taken 3 days later and sections stained for expression of ICAM-1 and VCAM-1. (e) MAdCAM-1 was visible at 26 days of age in a NOD recipient of the BDC-2·5 clone. BDC-2·5 clone cells (1 × 107) were injected i.p. into NOD recipients aged 9 days. Sixteen days after injection of the clone the pancreas was removed, processed for histology and sections stained for expression of MAdCAM-1.
MAdCAM-1 plays a role in diabetes induction by BDC-2·5
The ability of the clone to transfer diabetes to neonatal recipients is restricted to the first 2 weeks of life. We have shown that although MAdCAM-1 expression remains high in the pancreatic lymph nodes of adult mice, in the pancreas itself it is restricted to this narrow neonatal time window. We therefore determined whether the BDC-2·5 T cell clone requires MAdCAM-1 to access the pancreas. First we tested the BDC-2·5 clone for the presence of integrins which might interact with MAdCAM-1. We confirmed the presence of α4 and β7 integrins as well as the conformational epitope α4β7 (LPAM) recognized by the antibody DATK32 (Fig. 3). CD62L, which is also known to interact with MAdCAM-1, was absent from these cells (data not shown).
Figure 3.
Expression of α4 and β7 integrins on the BDC-2·5 clone. BDC-2·5 clone cells were stained for expression of α4 and β7 integrins as well as the conformational epitope α4β7 (LPAM) using antibodies, as indicated in Materials and methods.
We then administered anti-MAdCAM-1 antibody (MECA-367) to groups of neonatal recipients of the clone and found this to consistently significantly reduce the development of Type 1 diabetes compared to recipient groups that received control antibody. Figure 4 shows the pooled data from several experiments demonstrating the significant prevention of diabetes by MAdCAM-1 blockade (P < 0·0001). This shows that expression of MAdCAM-1 is important for T cell trafficking into the pancreas during the neonatal period.
Figure 4.
MAdCAM-1 blockade prevents BDC-2·5 clone transfer of diabetes to neonatal recipients. BDC-2·5 clone cells (1 × 107) were injected intraperitoneally into neonatal non-obese diabetic (NOD) scid recipients. Anti-MAdCAM-1 (MECA 367) (500 µg) was given to one group on days 0, 2 and 4. The other group received control antibody.
Discussion
The spontaneous onset of Type 1 diabetes in NOD mice requires the presence of both CD4+ and CD8+ T cells. This requirement for both T cell populations is also seen when diabetes is transferred by splenic T cells from a diabetic donor to either neonatal or irradiated adult NOD recipients. It has been suggested that CD8+ T cells are required for the priming and activation of islet-specific CD4+ T cells, as insulitis does not occur in their absence.12 The observation that the CD4+ T cell clone BDC-2·5 also requires the presence of CD8+ T cells to transfer Type 1 diabetes to adult recipients prompted us to examine the requirement for CD8+ T cells in the transfer of diabetes by the T cell clone to neonatal recipients. Our finding that CD8-depleted or NOD scid neonatal recipients developed Type 1 diabetes rapidly on BDC-2·5 clone transfer means that the CD8+ T cell requirement is age-dependent. The inability to induce diabetes in adult NOD scid mice in the absence of CD8+ T cells suggests that successful transfer in a neonatal mouse is not attributable to the simple ability to expand homeostatically in the neonatal lymphopenic environment.5 There are data suggesting that trafficking might be different in the neonatal period, permitting T cell access to peripheral tissues.13 Experiments of Hanninen and colleagues had suggested a role for MAdCAM-1 at different points during the progression towards diabetes onset.14 This addressin was proposed to be used initially to access the site of T cell priming and ultimately to access the pancreas. The expression of MAdCAM-1 on the pancreatic draining lymph nodes remains the same in both neonates and adult NOD mice. Our analysis, however, of addressin expression in the pancreas of neonates and adults revealed a marked difference in MAdCAM-1 expression. The pancreas is derived embryonically from the foregut and may have retained MAdCAM-1 expression neonatally as a consequence of its origin. MAdCAM-1 expression is reduced markedly by day 18 unless inflammation is present. Its expression remains high in transgenic mice expressing interferon (IFN)-γ in the pancreatic β cells15 and MAdCAM-1 expression is increased in the islet area when an inflammatory insulitis develops prior to diabetes onset in NOD mice.10,16
Many of the T cells accumulating in the early infiltrates in NOD mice were found to express α4β7.17 BDC-2·5 cells have also been shown to express this integrin (Fig. 3).18 As this T cell clone is already primed and expresses α4β7, it is enabled to access the neonatal pancreas by interaction with MAdCAM-1. The ability of MAdCAM-1 blockade to inhibit diabetes development following T cell clone transfer to neonatal mice confirms its involvement in the trafficking of the clone. In contrast to the neonate, MAdCAM-1 expression was almost undetectable in the adult pancreas in the absence of inflammation. This suggests that at least one possible way by which CD8+ T cells aid diabetes onset in adult recipients might be through facilitating clone trafficking to the pancreas.
MAdCAM-1 has been shown to be involved in the spontaneous development of Type 1 diabetes in NOD mice, as either antibody to this addressin or to its integrin ligand prevents disease onset.14,19,20 In addition to its role in facilitating diabetes onset, it is possible that neonatal expression of MAdCAM-1 may be important for the development of peripheral tolerance. Under normal circumstances it might be important for naïve T cells to traffic into peripheral tissues early in neonatal life. The T cells may then be exposed to self-antigens in a non-inflammatory environment which would lead to peripheral self tolerance either through anergy or the development of regulatory T cells.13
Acknowledgments
We would like to thank the Wellcome Trust and NIH award DK50561 for supporting the research of Anne Cooke and Kathryn Haskins, respectively. We are also grateful to Dr P. Kilshaw for discussion of addressins in the work carried out in this manuscript.
References
- 1.Bendelac A, Carnaud C, Boitard C, Bach JF. Syngeneic transfer of autoimmune diabetes from diabetic NOD mice to healthy neonates. Requirement for both L3T4+ and Lyt-2+ T cells. J Exp Med. 1987;166:823–32. doi: 10.1084/jem.166.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bedossa P, Bendelac A, Bach JF, Carnaud C. Syngeneic T cell transfer of diabetes into NOD newborn mice: in situ studies of the autoimmune steps leading to insulin-producing cell destruction. Eur J Immunol. 1989;19:1947–51. doi: 10.1002/eji.1830191028. [DOI] [PubMed] [Google Scholar]
- 3.Miller BJ, Appel MC, O'Neil JJ, Wicker LS. Both the Lyt-2+ and L3T4+ T cell subsets are required for the transfer of diabetes in nonobese diabetic mice. J Immunol. 1988;140:52–8. [PubMed] [Google Scholar]
- 4.Varey AM, Hutchings P, O'Reilly L, Hussell T, Waldmann H, Simpson E, Cooke A. The development of insulin-dependent diabetes mellitus in non-obese diabetic mice: the role of CD4+ and CD8+ T cells. Biochem Soc Trans. 1991;19:187–91. doi: 10.1042/bst0190187. [DOI] [PubMed] [Google Scholar]
- 5.Min B, McHugh R, Sempowski GD, Mackall C, Foucras G, Paul WE. Neonates support lymphopenia-induced proliferation. Immunity. 2003;18:131–40. doi: 10.1016/s1074-7613(02)00508-3. [DOI] [PubMed] [Google Scholar]
- 6.Peterson JD, Haskins K. Transfer of diabetes in the NOD-scid mouse by CD4 T-cell clones. Differential requirement for CD8 T-cells. Diabetes. 1996;45:328–36. doi: 10.2337/diab.45.3.328. [DOI] [PubMed] [Google Scholar]
- 7.Haskins K, Portas M, Bradley B, Wegmann D, Lafferty K. T-lymphocyte clone specific for pancreatic islet antigen. Diabetes. 1988;37:1444–8. doi: 10.2337/diab.37.10.1444. [DOI] [PubMed] [Google Scholar]
- 8.Bergman B, Haskins K. Islet-specific T-cell clones from the NOD mouse respond to beta-granule antigen. Diabetes. 1994;43:197–203. doi: 10.2337/diab.43.2.197. [DOI] [PubMed] [Google Scholar]
- 9.Phillips JM, Harach SZ, Parish NM, Fehervari Z, Haskins K, Cooke A. Nondepleting anti-CD4 has an immediate action on diabetogenic effector cells, halting their destruction of pancreatic beta cells. J Immunol. 2000;165:1949–55. doi: 10.4049/jimmunol.165.4.1949. [DOI] [PubMed] [Google Scholar]
- 10.Hanninen A, Taylor C, Streeter PR, Stark LS, Sarte JM, Shizuru JA, Simell O, Michie SA. Vascular addressins are induced on islet vessels during insulitis in nonobese diabetic mice and are involved in lymphoid cell binding to islet endothelium. J Clin Invest. 1993;92:2509–15. doi: 10.1172/JCI116859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faveeuw C, Gagnerault MC, Lepault F. Modifications of the expression of homing and adhesion molecules in infiltrated islets of Langerhans in NOD mice. Adv Exp Med Biol. 1994;355:137–42. doi: 10.1007/978-1-4615-2492-2_23. [DOI] [PubMed] [Google Scholar]
- 12.Wang B, Gonzalez A, Benoist C, Mathis D. The role of CD8+ T cells in the initiation of insulin-dependent diabetes mellitus. Eur J Immunol. 1996;26:1762–9. doi: 10.1002/eji.1830260815. [DOI] [PubMed] [Google Scholar]
- 13.Alferink J, Tafuri A, Vestweber D, Hallmann R, Hammerling GJ, Arnold B. Control of neonatal tolerance to tissue antigens by peripheral T cell trafficking. Science. 1998;282:1338–41. doi: 10.1126/science.282.5392.1338. [DOI] [PubMed] [Google Scholar]
- 14.Hanninen A, Jaakkola I, Jalkanen S. Mucosal addressin is required for the development of diabetes in nonobese diabetic mice. J Immunol. 1998;160:6018–25. [PubMed] [Google Scholar]
- 15.Lee MS, Sarvetnick N. Induction of vascular addressins and adhesion molecules in the pancreas of IFN-gamma transgenic mice. J Immunol. 1994;152:4597–603. [PubMed] [Google Scholar]
- 16.Faveeuw C, Gagnerault MC, Lepault F. Expression of homing and adhesion molecules in infiltrated islets of Langerhans and salivary glands of nonobese diabetic mice. J Immunol. 1994;152:5969–78. [PubMed] [Google Scholar]
- 17.Hanninen A, Salmi M, Simell O, Jalkanen S. Mucosa-associated (beta 7-integrinhigh) lymphocytes accumulate early in the pancreas of NOD mice and show aberrant recirculation behavior. Diabetes. 1996;45:1173–80. doi: 10.2337/diab.45.9.1173. [DOI] [PubMed] [Google Scholar]
- 18.Dobbs C, Haskins K. Comparison of a T cell clone and of T cells from a TCR transgenic mouse: TCR transgenic T cells specific for self-antigen are atypical. J Immunol. 2001;166:2495–504. doi: 10.4049/jimmunol.166.4.2495. [DOI] [PubMed] [Google Scholar]
- 19.Michie SA, Sytwu HK, McDevitt JO, Yang XD. The roles of alpha 4-integrins in the development of insulin-dependent diabetes mellitus. Curr Top Microbiol Immunol. 1998;231:65–83. doi: 10.1007/978-3-642-71987-5_5. [DOI] [PubMed] [Google Scholar]
- 20.Yang XD, Sytwu HK, McDevitt HO, Michie SA. Involvement of beta 7 integrin and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in the development of diabetes in obese diabetic mice. Diabetes. 1997;46:1542–7. doi: 10.2337/diacare.46.10.1542. [DOI] [PubMed] [Google Scholar]