Abstract
Previous reports have suggested that peroral delivery of antigens chemically coupled to non-toxic recombinant enterotoxin B subunits, such as the cholera toxin B subunit (CTB), induces tolerance to the antigen that may be abrogated by the toxic enzyme activity of intact enterotoxins, such as cholera toxin (CT). The aim of this study was to examine the immunogenicity of a genetically coupled protein composed of the saliva-binding region (SBR) of the Streptococcus mutans surface antigen AgI/II and the non-toxic A2 and B subunits of CT (SBR-CTA2/B) compared with that of recombinant SBR admixed with CT (SBR + CT) and SBR chemically coupled to recombinant CTB (SBR-CTB) following peroral delivery by intragastric (i.g.) immunization. The results showed that i.g. immunization with SBR-CTA2/B, like SBR + CT, induced antigen-specific serum immunoglobulin G (IgG) and salivary IgA antibodies, and sensitized splenic T cells. Comparison studies with SBR-CTB produced serum IgG but not salivary IgA titres and failed to sensitize splenic cells. Immunization with SBR-CTA2/B via the intranasal route also primed for the recall of antigen-specific memory antibody responses 6 months later. These findings show that SBR-CTA2/B is an immunogenic, not tolerogenic, chimeric protein that can induce and recall antigen-specific memory responses upon mucosal immunization.
Keywords: cholera toxin, memory cells, mucosal immunity, Streptococcus
Introduction
The great majority of human infectious diseases are acquired through the mucosal surfaces such as the gastrointestinal, respiratory and genital tracts, where the mucosal secretory immune system is the first line of defence. Immunization at mucosal surfaces such as the gut mucosa by gastric intubation [the intragastric (i.g.) route] and the nasopharyngeal tissue by application of small volumes to the external nares [the intranasal (i.n.) route] induces humoral and cellular immune responses within the integrated mucosal immune system that are superior in some instances to those responses induced by systemic immunization alone. However, the delivery of soluble protein antigens to mucosal surfaces may induce systemic tolerance to the immunizing antigen instead of, or even concomitantly with, mucosal responses desired for immune protection against infection.1 Because tolerance is considered to be the default response of the mucosal immune system to some non-replicating or soluble antigens, the effective generation of mucosal immune responses usually requires the administration of adjuvants or delivery systems that overcome this tendency.1,2 Among numerous strategies proposed, the use of cholera toxin (CT) or other related heat-labile enterotoxins as mucosal adjuvants has been found to be most effective (reviewed in reference 2).
Cholera toxin consists of a single toxic A subunit (CTA1) joined to a pentamer B subunit (CTB) by the linker A2 subunit. High-affinity binding of the B subunit to the GM1 ganglioside receptors found on most mammalian cells is thought to be essential for adjuvant activity.3–5 The toxic A1 moiety renders the entire molecule of CT unsuitable for human use, but considerable attention has been devoted to non-toxic mutants or derivatives such as the GM1-binding B subunit as potential adjuvants.6–8
Whether CTB can serve as a mucosal adjuvant when mixed with antigens has been a matter of controversy, complicated by the degree of purity of the CTB used. Some investigators have shown that immunization via the i.g., i.n., intravaginal or transcutaneous route using intact enterotoxins such as CT, non-toxic enterotoxin B subunits such as CTB or non-toxic enterotoxin mutants induces mucosal and systemic immune responses to antigen across many species.9–15 Conversely, other researchers have reported that coupling of antigen to CTB induces a state of tolerance to the antigen16–18 that depends on the use of a non-toxic recombinant CTB and may be abrogated by the addition of small amounts of intact CT.16,19–21 Table 1 outlines the divergent immune responses observed in rodents following i.n. or i.g. immunization with various forms of enterotoxins and their subunits.
Table 1.
Immune responses to antigens following intragastric or intranasal immunization with different forms of enterotoxins and/or their subunits
| Immunogenicity to antigen following immunization? | ||
|---|---|---|
| Type of enterotoxin and/or enterotoxin subunit administered with antigen | Intragastric | Intranasal |
| Chemically coupled | No16,18 | Yes11,27,43 |
| recombinant enterotoxin B | ‘Split’ tolerance44 | |
| subunit | No17 | |
| Admixed recombinant | Yes25 | Yes26,27,52 |
| enterotoxin B subunit | No19 | |
| Enterotoxin B subunit + CT | Yes16,19–21,33,41,53 | Yes20,27 |
| Non-toxic enterotoxin | Yes6,54 | Yes6–8,26 |
| mutants | No19,42 | |
| Genetically coupled CTA2/B | Yes24,35 | Yes24 |
CT, cholera toxin; CTA2/B, non-toxic A2 and B subunits of CT.
Streptococcus mutans plays a pivotal role in dental caries and the surface antigen AgI/II mediates its adherence to the saliva-coated tooth surfaces. The functional domain of AgI/II responsible for initial adherence is located on the N-terminal end and is named the saliva-binding region (SBR).22,23 A recombinant chimeric immunogen consisting of the non-toxic A2 and B subunits of CT genetically coupled to SBR induces antigen-specific immune responses subsequent to i.g. or i.n. immunization.24
Although i.g. or i.n. immunization with SBR-CTA2/B can be effective at inducing antigen-specific immunity, each route has different inductive sites. The Peyer's patches and nasal-associated lymphoid tissue (NALT) are the presumed inductive sites of i.g. and i.n. immunization, respectively, and it is here and in the draining lymphoid tissue that antigen-specific memory cells would be expected to be found. The i.g. route has been used in several studies that have demonstrated tolerance induction to recombinant CTB-coupled antigens, and was therefore chosen to evaluate the immunogenicity of SBR-CTA2/B in these studies. Conversely, recombinant enterotoxin B subunits have been repeatedly used as effective adjuvants via the i.n. route,11,25–27 and i.n. immunization gives rise to equivalent immune responses with lower doses of immunogens than i.g. immunization;28,29 hence this study used the i.n. route of delivery to assess the ability of SBR-CTA2/B to prime for recall immune responses to antigen 6 months later.
Because of the controversy surrounding the ability of CTB-coupled proteins to induce immunity or tolerance using the i.g. route of delivery, the purpose of this study was first to evaluate the immunogenicity of genetically coupled SBR-CTA2/B in comparison to SBR admixed with CT (SBR + CT) and chemically coupled SBR-CTB using the i.g. route, and secondly to determine whether SBR-CTA2/B primes for the recall of immune responses to antigen 6 months later using i.n. delivery.
Materials and methods
Antigens and adjuvants
Chimeric protein SBR-CTA2/B was purified from periplasmic extracts using ammonium sulphate precipitation, size-exclusion and anion-exchange chromatography as previously described.24 A GM1 enzyme-linked immunosorbent assay (ELISA) probed with anti-SBR antibody (4A1.3A11) was used to detect SBR-CTA2/B in purified fractions and sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) was performed to test for the presence of the SBR, CTA2 and CTB subunits in purified chimeric protein.24 CT was purchased from List Biological Laboratories, Inc. (Campbell, CA). Purified SBR-his was provided by Terry D. Connell (University at Buffalo, Buffalo, NY) by methods described previously.30 Briefly, the plasmid expressing SBR was expressed in Escherichia coli and the protein was purified by metal chelation chromatography from cell lysates. S. mutans surface protein AgI/II was purified from culture supernatants as detailed previously.31
Recombinant CTB was purified from an E. coli clone (MTD-9)32 and chemical conjugate preparations of equimolar amounts of recombinant SBR and recombinant CTB (SBR-CTB) were made using a variation of methods described previously.33 Briefly, 2 mg of CTB in 1 ml of phosphate buffer was dialysed to remove the storage Tris buffer and then treated with 14 µl of N-succinimidyl-3-(2-pyridyldithio)propionate (SPDP) dissolved in anhydrous ethanol at 8 mg/ml. 1·5 mg of SBR in 2·5 ml of 0·1 m phosphate buffer, pH 7·6, was treated with 4·3 µg of SPDP at 8 mg/ml. SBR and CTB were treated at room temperature with SPDP for 30 min. A volume of 10 µl of ethanolamine was added to the CTB derivative and dialysed overnight against 0·01 m phosphate-buffered saline (PBS), pH 7·4. Absorption of these derivatives at 343 nm after reduction with dithiothreitol (DTT)34 was used to calculate the substitution ratio of moles of SBR or CTB:moles SPDP, which was routinely approximately 1 : 3. The SBR derivative was reduced with 5 mm DTT for 30 min, run through a Sephadex G-25 (PD-10) column (Amersham Biosciences, Uppsala, Sweden) in PBS and immediately added to the CTB derivative and incubated overnight at 4°. A343 was used to calculate SBR:CTB substitution ratios. A280 was used to estimate protein concentration. The sample was then passed through a Superose HR6 16/50 chromatography column (Amersham Biosciences) and the SBR-CTB conjugate, free SBR and CTB were identified as separate peaks. GM1 ELISA probed with anti-SBR antibody was used to confirm SBR-CTB affinity.
All proteins were tested for endotoxin content using the limulus amebocyte lysate endochrome assay (Charles River Endosafe, Charlestown, SC) whereby the concentration of endotoxin in the sample was determined by end-point chromagenic assay. The endotoxin levels in all of those used were less than the equivalent of 0·026 ng of E. coli lipopolysaccharide per µg of protein.
Animals and immunizations
Female BALB/c mice 6–8 weeks old were purchased from Harlan Sprague Dawley, Indianapolis, IN and housed at the University at Buffalo Laboratory Animal Facility in compliance with National Institutes of Health guidelines for animal care. The Institutional Animal Care and Use Committee approved all procedures used in this study. Mice were given food and water ad libitum.
The dose and schedule of i.g. or i.n. priming were based on previous titrations and studies.24,27,30,35 Groups of mice (n = 4–6) were immunized i.g. by gastric intubation 3 times at 10-day intervals with 100 µg of SBR-CTA2/B, 100 µg of SBR-CTB or 40 µg of SBR with or without 5 µg of CT in 400 µl of 0·35 m NaHCO3 buffer. Control animals were immunized with 5 µg of CT alone or sham-immunized with buffer using an identical schedule. Certain groups of animals were given subcutaneous (s.c.) systemic challenge immunizations at later time-points as described in the Results section, consisting of 50 µg of AgI/II in 250 µl of Alhydrogel (HCI Biosector, Frederikssund, Denmark). Intranasal immunizations using 25 µg of SBR-CTA2/B in 10 µl of PBS were administered 3 times at 10-day intervals and boosted 6 months later with a further one, two or three doses of SBR-CTA2/B at 10-day intervals.
Serum and saliva samples were collected before and after immunization as described in the Results section. Blood was collected from the tail vein and saliva was collected at the same time by stimulating secretion with 5 µg of carbachol injected intraperitoneally.33 Samples were stored at −80° until assayed.
Cell isolation and culture
Groups of mice (n = 4–6) were killed 8 days after final i.g., s.c. or i.n. immunization. Spleens were excised and passed through a 40-µm nylon cell strainer and red blood cells were lysed with ammonium chloride red blood cell lysis buffer.36 NALT, superficial cervical lymph nodes (CLNs) and iliac lymph nodes (ILNs) were also removed from animals immunized i.n. and tissue was passed through a nylon cell strainer. Cells were suspended in complete medium consisting of RPMI-1640 (Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum (Gibco), 2 mm l-glutamine, 10 mm Hepes, 0·05 m 2-mercaptoethanol, 100 µg/ml penicillin and 100 µg/ml streptomycin. Cells were suspended in trypan blue (Gibco) and viable cells were counted using a haemacytometer.
Single-cell suspensions were prepared in sterile complete medium and triplicate cultures were established in 96-well white-walled flat-bottom sterile microplates (Cambrex Bio Science, Rockland, ME). Microplates were set up in duplicate. Cultures were incubated at 37°, under 5% CO2 for 5 days in the presence of 5 µg/ml AgI/II, 2·5 µg/ml concanavalin A or complete medium only (unstimulated). Cell responses were evaluated by determining ATP concentration using the ViaLightTM HS kit (Cambrex Bio Science).37 According to the manufacturer, this method has the sensitivity to detect as few as 10 cells per microwell and, when compared with conventional methods using [3H] thymidine, has r-values of 0·99 or greater. On a previously optimized day of harvest (day 5) and following the manufacturer's instructions, the cultured cells were lysed and the addition of the enzyme luciferase catalysed the formation of light from cellular ATP and luciferin. The intensity of the emitted light, expressed as relative light units (RLUs), depended linearly on ATP concentration and was measured using a luminometer (Wallac, Turku, Finland). An ATP standard curve ranging from 5 × 10−7 to 7·8 × 10−9 m was prepared in duplicate on each assay plate. The luminometer output data in RLUs were interpolated as ATP concentration (m) using the standard curve. The mean ATP concentration was determined from triplicate cultures. This technique of measuring cell responses to antigen was compared with procedures that require [3H] thymidine. Briefly, splenic cells from mice immunized i.g. with SBR-CTA2/B were cultured in identical conditions in flat-bottomed 96-well plates. Approximately 8 hr before harvesting, the cells were pulsed with 0·5 µCi of [3H] thymidine and uptake was determined by liquid scintillation counting and expressed as mean counts per minute (c.p.m.) of replicate cultures. Cell proliferation was also expressed as a stimulation index (SI = mean c.p.m. stimulated cultures ÷ mean c.p.m. of unstimulated cultures). The same cells used to establish cultures that were later evaluated by [3H] thymidine uptake were also used to create identical cultures in 96-well white-walled plates. Responses to antigen in these cultures were expressed as the mean ATP concentration in replicate cultures.
Antibody responses
ELISA was used to determine AgI/II-specific antibodies in serum and saliva as well as total salivary IgA. As previously described,33 microtitre plates were coated with AgI/II, GM1 (Calbiochem, La Jolla, CA) and CT, or with anti-mouse IgA (Southern Biotechnology Associates, Inc., Birmingham, AL). Peroxidase-conjugated goat anti-mouse IgA and IgG (Southern Biotechnology Associates) and o-phenylenediamine dihydrochloride plus H2O2 were used to develop the assay. The colorimetric reaction was stopped with sulphuric acid and read using a VERSAmax microplate reader (Molecular Devices, Sunnyvale, CA). Unknown values were calculated from standard curves using SOFTmax PRO software (Molecular Devices). The geometric means and standard deviations (SDs) of groups were plotted as log-transformed data.
Statistics
Antibody responses using log-transformed data and cell responses to antigen were analysed using the paired and unpaired Student's t-test with GraphPad InStat (GraphPad, San Diego, CA). Differences between groups were considered significant when P < 0·05. The results are representative of duplicate experiments.
Results
Systemic and mucosal antibody responses to i.g. immunization
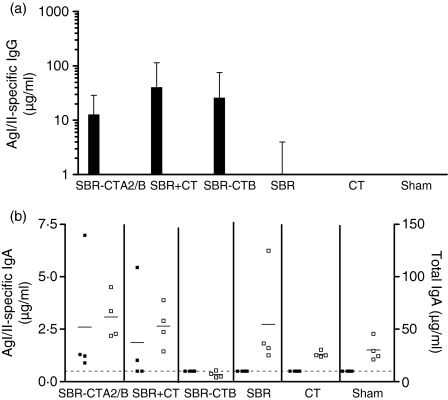
To evaluate the oral immunogenicity of SBR-CTA2/B, mice were immunized i.g. with three doses of SBR-CTA2/B at days 0, 10 and 20 and responses were compared with those obtained in mice immunized with an equimolar amount (40 µg) of SBR admixed with 5 µg of CT (SBR + CT) or 100 µg of chemically coupled SBR-CTB. Compared to mice sham-immunized or immunized with CT or SBR alone, mice immunized with SBR-CTA2/B, SBR + CT or SBR-CTB developed significant antigen-specific IgG antibodies in serum (P < 0·001) (Fig. 1a). There was no significant difference in the response amongst groups immunized with SBR-CTA2/B, SBR + CT or SBR-CTB (P > 0·05). In saliva (Fig. 1b), AgI/II-specific IgA antibodies were also detected in those groups immunized with SBR-CTA2/B or SBR + CT, but not in mice immunized with SBR-CTB, CT or SBR alone, or in sham-immunized controls. Compared to preimmune titres, significant AgI/II-specific IgA titres were detected in groups immunized with SBR-CTA2/B or SBR + CT (P < 0·05). Therefore, i.g. immunization with SBR-CTA2/B induced antibody titres in serum that were comparable to those from mice immunized with CT or SBR-CTB chemical conjugate as well as detectable salivary IgA titres.
Figure 1.
Surface antigen AgI/II-specific immunoglobulin G (IgG) in serum(a)and AgI/II-specific and total IgA levels in saliva(b)on day 27 following intragastric (i.g.) immunization of groups of mice (n = 4) on days 0, 10 and 20. Data are represented as geometric mean and standard deviation (a) or antibody responses from individual animals (b; closed symbols, specific IgA; open symbols, total IgA). The line denotes the lowest detectable limit of 0·5 µg/ml. SBR, saliva-binding region; CT, cholera toxin; SBR-CTA2/B, a genetically coupled protein composed of the SBR of the Streptococcus mutans surface antigen AgI/II and the non-toxic A2 and B subunits of CT; SBR+CT, recombinant SBR admixed with CT; SBR-CTB, SBR chemically coupled to the recombinant CT B subunit; Sham, buffer only.
Serum antibody responses to systemic challenge after i.g. priming
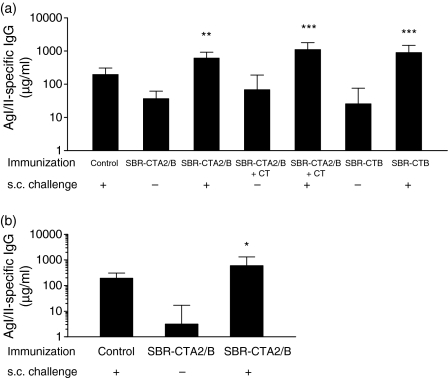
To determine whether mucosal priming with SBR-CTA2/B induced tolerance to subsequent systemic immunization, mice were immunized i.g. with 100 µg of SBR-CTA2/B on days 0, 10 and 20, and 2 weeks later were challenged s.c. with AgI/II. Control animals that had not been primed were similarly immunized s.c. with AgI/II. AgI/II-specific IgG responses in serum were significantly greater in those animals primed i.g. with SBR-CTA2/B and challenged with AgI/II s.c. (P < 0·01) (Fig. 2a). An additional group of mice that were primed with SBR-CTA2/B admixed with 5 µg of CT (SBR-CTA2/B + CT) also mounted a significant serum IgG response to challenge with AgI/II (P < 0·001) (Fig. 2a). However, the addition of CT to i.g. priming with SBR-CTA2/B did not enhance the serum IgG response either before or after systemic challenge. Another group i.g. primed with SBR-CTB showed similar significant AgI/II-specific titres in response to challenge (P < 0·001) (Fig. 2a). Thus, in each case, priming by i.g. immunization with SBR coupled (genetically or chemically) to CTB led to enhanced responses to AgI/II upon subsequent challenge without the need for coadministered CT.
Figure 2.
Surface antigen AgI/II-specific immunoglobulin G (IgG) in serum following intragastric (i.g.) immunization with or without systemic challenge. Groups (n = 4–6) were immunized on days 0, 10 and 20 with a genetically coupled protein composed of the saliva-binding region (SBR) of the Streptococcus mutans surface antigen AgI/II and the non-toxic A2 and B subunits of cholera toxin (CT) (SBR-CTA2/B), recombinant SBR admixed with CT (SBR-CTA2/B + CT) or SBR chemically coupled to the recombinant CT B subunit (SBR-CTB), and challenged with AgI/II subcutaneously (s.c.) 2 weeks(a)or 5 months(b)later. Control animals received AgI/II s.c. only. Samples were collected 7 days following the final i.g. or s.c. immunization. Results are represented as the geometric mean and standard deviation. *P < 0·05, **P < 0·01 and ***P < 0·001 compared with the corresponding group that was not challenged.
If the systemic challenge with AgI/II was delayed for 5 months after i.g. priming with SBR-CTA2/B, there was still a potent serum IgG response to AgI/II (P < 0·05) (Fig. 2b), even though the anti-AgI/II antibody levels in unchallenged mice had declined markedly in this interval. In all studies, immune responses to the systemic challenge with AgI/II were at least as great as those mounted in the s.c. immunized controls (Figs 2a and b). Thus, there was no evidence from these experiments that mucosal immunization with SBR coupled to CTB either chemically or genetically induced tolerance to AgI/II.
T-cell responses to AgI/II following i.g. immunization
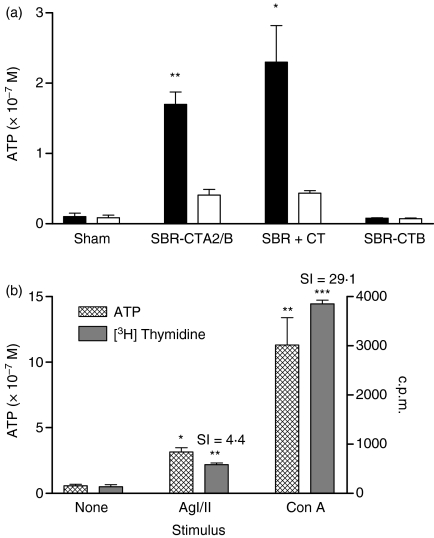
As i.g. immunization with SBR-CTA2/B induced antibody responses with no evidence for tolerance induction in the B-cell compartment, the question arose whether the T cells were tolerized by mucosal immunization as reported in other studies.38,39 To address this question, spleen cells from mice immunized with SBR-CTA2/B, SBR + CT or SBR-CTB were cultured in vitro with or without AgI/II, and T-cell responses were measured in terms of ATP production by metabolically active cells (Fig. 3a). Compared with splenic cultures treated with culture medium alone, cells from animals immunized with SBR-CTA2/B or SBR + CT responded significantly in the presence of AgI/II (P < 0·01 and P < 0·05, respectively). However, cells from control animals and those administered SBR-CTB showed no response to AgI/II (P > 0·05). Thus, mucosal immunization with SBR-CTA2/B appeared to sensitize T cells for an antigen-specific response, in a similar manner to SBR + CT but unlike SBR-CTB conjugate. All cell cultures showed strong responses of similar magnitude when stimulated with the mitogen concanavalin A (Con A) (data not shown).
Figure 3.
In vitro responses of splenic cells isolated from groups (n = 4) intragastrically (i.g.) immunized with a genetically coupled protein composed of the saliva-binding region (SBR) of the Streptococcus mutans surface antigen AgI/II and the non-toxic A2 and B subunits of cholera toxin (CT) (SBR-CTA2/B), recombinant SBR admixed with CT (SBR-CTA2/B + CT), SBR chemically coupled to the recombinant CT B subunit (SBR-CTB) or sham-immunized controls. Cell responses were quantified by assay of ATP produced in metabolically active cells cultured with surface antigen AgI/II (solid bars) or culture medium alone (open bars). *P < 0·05 and ***P < 0·001 relative to unstimulated cultures.(b)Comparison of ATP and [3H] thymidine uptake assays for measuring T-cell responses to stimulus. Responses of spleen cells to AgI/II and concanavalin A (Con A) were evaluated by ATP production (hatched bars) and [3H] thymidine uptake (solid bars). *P < 0·05 and **P < 0·01 relative to unstimulated cultures. SI, stimulation index.
In order to compare this assay for measuring T-cell responses with conventional methods of [3H] thymidine uptake, splenic cells from the same animals were cultured under identical conditions and later assayed by each method. Both assays showed significant T-cell responsiveness to stimulation with AgI/II (ATP P < 0·05; [3H] thymidine P < 0·01, SI = 4·4) and Con A (ATP P < 0·01; [3H] thymidine P < 0·001, SI = 29·1) (Fig. 3b) and the ATP assay was found to be a sensitive and reproducible system for determining T-cell responses.
Recalling systemic and mucosal memory following mucosal immunization with SBR-CTA2/B
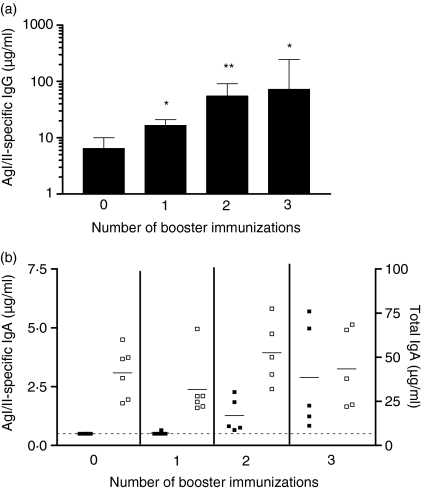
Groups of mice were immunized i.n. with 25 µg of SBR-CTA2/B on days 0, 10 and 20, rested for 6 months, and boosted i.n. with 25 µg of SBR-CTA2/B either 1, 2 or 3 times at 10-day intervals. Serum and saliva samples were collected 7 days after the final booster immunization in each case. A control group was immunized but not boosted and samples were collected from these animals at the 7-month time-point. Levels of AgI/II-specific IgG antibodies in serum (Fig. 4a) progressively increased with the number of booster doses and attained significance (P < 0·05) in comparison to pre-boost levels after one or more booster doses. At least two booster immunizations were required to reveal an increase in salivary IgA antibodies (Fig. 4b).
Figure 4.
Surface antigen AgI/II-specific immunoglobulin (IgG) in serum(a)and AgI/II-specific and total IgA in saliva(b)following one, two or three intranasal (i.n.) booster immunizations. Groups (n = 5–6) were primed i.n. with a genetically coupled protein composed of the saliva-binding region (SBR) of the Streptococcus mutans surface antigen AgI/II and the non-toxic A2 and B subunits of cholera toxin (CT) (SBR-CTA2/B) on days 0, 10 and 20 and rested for 6 months before boosting i.n. with SBR-CTA2/B 1, 2 or 3 times at 10-day intervals. Animals not boosted (zero boost) served as a control. Samples were collected 7 days after the final booster immunization or at the 7-month time-point for the group not boosted. Serum antibody data are represented as the geometric mean and standard deviation. *P < 0·05 and **P < 0·01 compared with animals not boosted (a). Salivary antibody responses are shown for individual animals (b; closed symbols, specific IgA; open symbols, total IgA). The line denotes the lowest detectable limit of 0·5 µg/ml.
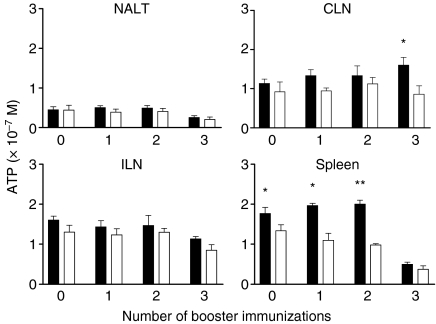
To determine whether Ag-specific T cells were present in local lymphoid tissues (NALT and CLNs) or in the spleen (as a central lymphoid organ) prior to or during recall (booster) immunization, mononuclear cells were isolated from these tissues and from the ILNs as a remote site for comparison, and cultured with AgI/II. Prior to boosting, cells capable of responding to AgI/II were found in the spleen, but not in the NALT, CLNs or ILNs (Fig. 5). AgI/II-responsive cells continued to be found in the spleen after one and two booster immunizations, and were present in CLNs but not other tissues after three booster doses (Fig. 5). It is unclear why such cells declined in the spleen after the third booster dose, but background response in medium only also declined in these cultures. However, these and all other cultures responded strongly to Con A (data not shown).
Figure 5.
Responses of cells isolated from the nasal-associated lymphoid tissue (NALT), cervical lymph nodes (CLNs), iliac lymph nodes (ILNs) and spleen following one, two or three booster immunizations. Animals not boosted (zero boost) were used as a control group. Animals were killed 8 days after final booster immunization or at the 7-month time-point for those not boosted. Cells were cultured with surface antigen AgI/II (solid bars) or culture medium alone (open bars), and proliferation was determined by quantifying cellular ATP concentrations from individual cultures. The significance of differences was evaluated between AgI/II-stimulated and unstimulated cultures. *P < 0·05; **P < 0·01.
Discussion
Our results clearly show that a protein antigen coupled to the recombinant B subunit of CT in the form of a genetically constructed chimeric protein is immunogenic via the i.g. route of administration without any requirement for intact toxin such as CT as a coadministered adjuvant. There was no evidence in our system for the induction of tolerance, in either B- or T-cell compartments, as immunization did not diminish the antibody response to parenteral challenge with AgI/II, or weaken the response of T cells cultured with AgI/II. This is in contrast to findings reported by others11,40 showing that mucosal immunization with antigens chemically conjugated to recombinant CTB induces profound tolerance to the subsequent parenteral administration of the same antigen. However, there are several differences between the two systems that may account for these divergent findings.
An important difference concerns the nature of the antigen–B subunit complex, i.e. recombinant chimeric protein versus chemical conjugate. In the former, a protein antigen is genetically fused to form a holotoxin-like chimeric molecule. Conversely, chemical conjugates yield covalent complexes of ill-defined and somewhat variable molecular structure. Even though the ganglioside-binding properties of both types of construct are preserved, as this is thought to be essential for uptake by the immune system, it is expected that the detailed molecular mechanisms of uptake and intracellular processing by antigen-presenting cells will be different. Chemical conjugates of AgI/II and CTB have been shown to be immunogenic upon i.g. administration but antibody responses were dependent on coadministration of intact CT as an adjuvant.33,41
In order to directly compare genetically coupled (SBR-CTA2/B) with chemically coupled proteins, we prepared a conjugate protein consisting of recombinant SBR and recombinant CTB (SBR-CTB). Interestingly, IgG antibody titres in serum following i.g. immunization with SBR-CTB were comparable to those from animals administered SBR-CTA2/B. However, unlike the chimeric protein, immunization with SBR-CTB conjugate did not induce detectable AgI/II-specific IgA titres in saliva, nor sensitized lymphoid cells in the spleen. These findings suggest that SBR-CTB is partially immunogenic when delivered via the i.g. route, although the response is restricted to circulating antibody. Conceivably, this limited antibody response to SBR-CTB is related to the lack of T-cell responses in the spleen. These results also indicate that the nature of the CTB and SBR coupling may contribute to its capacity to induce an immune response, at least under the conditions of immunization used in this study.
There has been considerable controversy over the requirement for toxic enzyme activity in the immunoenhancing property of the heat-labile enterotoxins such as CT and the closely related E. coli heat-labile toxin (LT). Despite an early finding to the contrary,42 mutants of either toxin lacking detectable toxicity or ADP-ribosyltransferase activities have been shown to possess adjuvant activity when coadministered with soluble antigens by mucosal routes.6,7 Furthermore, recombinant CTB or LTB can function as an adjuvant for some antigens administered by the i.n. route.27,43 Therefore, it is likely that other factors contribute to the outcome, i.e. active immunity or tolerance. Moreover, ‘split’ tolerance can occur after mucosal immunization in which mucosal IgA antibody responses develop concomitantly with systemic T-cell tolerance.38,39 It is notable that the T-cell compartment is much easier to tolerize than the B-cell compartment. Furthermore, i.n. immunization with conjugates of Schistosoma mansoni glutathione S-transferase and recombinant CTB has been shown to induce antibody responses and simultaneously to suppress inflammatory pathology.44
The route of mucosal immunization may also play a part. Intranasal immunization has usually been found to be more efficient than i.g. immunization in that equivalent responses are obtained with lower doses of immunogen. In part this may be a result of less degradation by digestive processes and more immediate access to the presumed inductive sites, these being NALT and Peyer's patches in the case of i.n and i.g. immunization, respectively. However, differences in the requirement for such factors as coupling of antigen to B subunit and dependence on holotoxin adjuvants29,33 suggest that there may be important differences in the way the two sites function to generate immune responses.
Mucosal immunization with SBR-CTA2/B also primed for anamnestic recall responses. This was observed in the recall of circulating IgG responses after systemic challenge with AgI/II and further examined in animals primed and boosted i.n. with SBR-CTA2/B. In these studies, one or two i.n. boosts were sufficient to recall serum IgG and salivary IgA antibody responses, respectively. This may be consistent with the finding that memory cells persisted in the spleen for 6 months prior to booster immunization and could be identified by their ability to respond to AgI/II in vitro. Similar results have also been reported using AgI/II and purified CTB conjugated proteins.45 Booster immunization did not further enhance splenic responses, which instead declined after three booster immunizations. This may have been a result of the development of regulatory cells that would suppress such responses to AgI/II46 and may indicate the development of ‘split’ tolerance. AgI/II-responsive cells could not be identified in NALT or CLNs 6 months after priming but before boosting. After i.n. boosting, a modest increase in AgI/II-specific proliferation was found in cells from the CLNs that drain the nasal mucosa, but not in the NALT or a remote lymph node such as the ILN. Thus, it appears that recall of mucosal memory responses depends upon a pool of memory cells that are not immediately available at the inductive sites but must be recruited from more central lymphoid organs such as the spleen or possibly the deeper CLNs as suggested by Wu et al.45,47
With respect to the properties of the antigen chosen for study, extensive experience has shown that AgI/II is a potent immunogen;48 this may influence the ability of CT or CTB to serve as an adjuvant or as a coupled delivery system for its SBR segment. Moreover, AgI/II is prone to induce T helper type 2 (Th2) responses in mice, such that attempts to use it in a model of delayed-type hypersensitivity that might be amenable to tolerization by mucosal immunization were unsuccessful (M. W. Russell, unpublished observations). Other studies on mucosal adjuvants have used such conventional antigens as ovalbumin, which in itself appears to be readily capable of inducing mucosal tolerance and has been widely used in studies of this phenomenon.49,50 The dose and scheduling of the antigen–CTB protein complex may also influence the induction of immunity. Possibly, the regimens followed by previous investigators that demonstrated tolerance induction using CTB–antigen conjugates skewed the immune response towards tolerance rather than immunity.16,51 The animal model in which studies are performed also affects the outcome. Mice are clearly responsive to mucosal immunization with SBR-CTA2/B by the i.g. and i.n. routes without additional CT as adjuvant (this study and reference 24), but in rats it was necessary to coadminister intact CT in order to obtain responses to SBR-CTA2/B given i.n.28
What influences the differences found in response to dissimilar antigens, the varied ability of CTB (or the B subunits of other heat-labile enterotoxins) to function as mucosal adjuvants, and the exact function of the toxic enzyme activity associated with the enterotoxin A subunit in different species remains incompletely understood. Indeed, it appears that the mechanisms that give rise to tolerance or immunity using enterotoxin B subunits are quite complex and depend on factors such as the type of antigen coupling, the purity of the B subunit, the nature of the antigen, previous priming with antigen, and T-cell or B-cell compartments. Nevertheless, while numerous factors interact to determine the outcome, enterotoxin B subunits such as CTB can be used effectively as mucosal adjuvants or genetically coupled delivery systems to enhance immunogenicity.
Acknowledgments
The authors would like to thank Mr Robert Nugent for his technical assistance in this project. This work was supported by US-PHS grant DE06746 from the National Institute of Dental and Craniofacial Research.
References
- 1.Garside P, Mowat AM. Oral tolerance. Semin Immunol. 2001;13:177–85. doi: 10.1006/smim.2001.0310. [DOI] [PubMed] [Google Scholar]
- 2.Russell MW. Mucosal immunity. In: Ellis RW, Brodeur BR, editors. New Bacterial Vaccines. New York: Kluwer/Plenum Publishers; 2003. pp. 63–79. [Google Scholar]
- 3.Elson CO, Holland SP, Dertzbaugh MT, Cuff CF, Anderson AO. Morphologic and functional alterations of mucosal T cells by cholera toxin and its B subunit. J Immunol. 1995;154:1032–40. [PubMed] [Google Scholar]
- 4.Nashar TO, Webb HM, Eaglestone S, Williams NA, Hirst TR. Potent immunogenicity of the B subunits of Escherichia coli heat-labile enterotoxin: receptor binding is essential and induces differential modulation of lymphocyte subsets. Proc Natl Acad Sci USA. 1996;93:226–30. doi: 10.1073/pnas.93.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.George-Chandy A, Eriksson K, Lebens M, Nordstrom I, Schon E, Holmgren J. Cholera toxin B subunit as a carrier molecule promotes antigen presentation and increases CD40 and CD86 expression on antigen-presenting cells. Infect Immun. 2001;69:5716–25. doi: 10.1128/IAI.69.9.5716-5725.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pizza M, Giuliani MM, Fontana MR, et al. Mucosal vaccines: non toxic derivatives of LT and CT as mucosal adjuvants. Vaccine. 2001;19:2534–41. doi: 10.1016/s0264-410x(00)00553-3. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez J, Wallerstrom G, Fredriksson M, Angstrom J, Holmgren J. Detoxification of cholera toxin without removal of its immunoadjuvanticity by the addition of (STa-related) peptides to the catalytic subunit. A potential new strategy to generate immunostimulants for vaccination. J Biol Chem. 2002;277:33369–77. doi: 10.1074/jbc.M112337200. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe I, Hagiwara Y, Kadowaki S, et al. Characterization of protective immune responses induced by nasal influenza vaccine containing mutant cholera toxin as a safe adjuvant (CT112K) Vaccine. 2002;20:3443–55. doi: 10.1016/s0264-410x(02)00351-1. [DOI] [PubMed] [Google Scholar]
- 9.Gockel CM, Bao S, Beagley KW. Transcutaneous immunization induces mucosal and systemic immunity: a potent method for targeting immunity to the female reproductive tract. Mol Immunol. 2000;37:537–44. doi: 10.1016/s0161-5890(00)00074-2. [DOI] [PubMed] [Google Scholar]
- 10.Johansson E, Rask C, Fredriksson M, Eriksson K, Czerkinsky C, Holmgren J. Antibodies and antibody-secreting cells in the female genital tract after vaginal and intranasal immunization with cholera toxin B subunit or conjugates. Infect Immun. 1998;66:514–20. doi: 10.1128/iai.66.2.514-520.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rask C, Fredriksson M, Lindblad M, Czerkinsky C, Holmgren J. Mucosal and systemic antibody responses after peroral or intranasal immunization: effects of conjugation to enterotoxin B subunits and/or of co-administration with free toxin as adjuvant. APMIS. 2000;108:178–86. doi: 10.1034/j.1600-0463.2000.d01-42.x. [DOI] [PubMed] [Google Scholar]
- 12.Shen X, Lagergard T, Yang Y, Lindblad M, Fredriksson M, Wallerstrom G, Holmgren J. Effect of pre-existing immunity for systemic and mucosal responses to intranasal immunization with group B Streptococcus type III capsular polysaccharide-cholera toxin B subunit conjugate. Vaccine. 2001;19:3360–8. doi: 10.1016/s0264-410x(00)00532-6. [DOI] [PubMed] [Google Scholar]
- 13.De Bernardis F, Boccanera M, Adriani D, Girolamo A, Cassone A. Intravaginal and intranasal immunizations are equally effective in inducing vaginal antibodies and conferring protection against vaginal candidiasis. Infect Immun. 2002;70:2725–9. doi: 10.1128/IAI.70.5.2725-2729.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arakawa T, Tsuboi T, Kishimoto A, et al. Serum antibodies induced by intranasal immunization of mice with Plasmodium vivax Pvs25 co-administered with cholera toxin completely block parasite transmission to mosquitoes. Vaccine. 2003;21:3143–8. doi: 10.1016/s0264-410x(03)00258-5. [DOI] [PubMed] [Google Scholar]
- 15.Russell MW, Moldoveanu Z, White PL, Sibert GJ, Mestecky J, Michalek SM. Salivary, nasal, genital, and systemic antibody responses in monkeys immunized intranasally with a bacterial protein antigen and the cholera toxin B subunit. Infect Immun. 1996;64:1272–83. doi: 10.1128/iai.64.4.1272-1283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun J, Holmgren J, Czerkinsky C. Cholera toxin B subunit: An efficient transmucosal carrier-delivery system for induction of peripheral immunological tolerance. Proc Natl Acad Sci USA. 1994;91:10795–9. doi: 10.1073/pnas.91.23.10795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tarkowski A, Sun J-B, Holmdahl R, Holmgren J, Czerkinsky C. Treatment of experimental autoimmune arthritis by nasal administration of a type II collagen-cholera toxoid conjugate vaccine. Arthritis Rheum. 1999;42:1628–34. doi: 10.1002/1529-0131(199908)42:8<1628::AID-ANR10>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 18.Peterson JS, Bregenholt S, Apostolopolous V, et al. Coupling of oral human or porcine insulin to the B subunit of cholera toxin (CTB) overcomes critical antigenic differences for prevention of type I diabetes. Clin Exp Immunol. 2003;134:38–45. doi: 10.1046/j.1365-2249.2003.02255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bagley KC, Abdelwahab SF, Tuskan RG, Lewis GK. An enzymatically active A domain is required for cholera-like enterotoxins to induce a long-lived blockade on the induction of oral tolerance: new method for screening mucosal adjuvants. Infect Immun. 2003;71:6850–6. doi: 10.1128/IAI.71.12.6850-6856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asanuma H, Aizawa C, Kurata T, Tamura S. IgA antibody-forming cell responses in the nasal-associated lymphoid tissue of mice vaccinated by intranasal, intravenous and/or subcutaneous administration. Vaccine. 1998;16:1257–62. doi: 10.1016/s0264-410x(98)00048-6. [DOI] [PubMed] [Google Scholar]
- 21.Hammond SA, Walwender D, Alving CR, Glenn GM. Transcutaneous immunization: T cell responses and boosting of existing immunity. Vaccine. 2001;19:2701–7. doi: 10.1016/s0264-410x(00)00506-5. [DOI] [PubMed] [Google Scholar]
- 22.Bleiweis AS, Oyston PCF, Brady LJ. Molecular, immunological, and functional characterization of the major surface adhesin of Streptococcus mutans. In: Ciardi JE, McGhee JR, Keith J, editors. Genetically Engineered Vaccines: Prospects for Oral Disease Prevention. New York: Plenum Publishing; 1992. pp. 229–41. [DOI] [PubMed] [Google Scholar]
- 23.Russell MW. Immunization against dental caries. Curr Opin Dent. 1992;2:72–80. [PubMed] [Google Scholar]
- 24.Hajishengallis G, Hollingshead SK, Koga T, Russell MW. Mucosal immunization with a bacterial protein antigen genetically coupled to cholera toxin A2/B subunits. J Immunol. 1995;154:4322–32. [PubMed] [Google Scholar]
- 25.Plant A, Williams R, Jackson ME, Williams NA. The B subunit of Escherichia coli heat labile enterotoxin abrogates oral tolerance, promoting predominantly Th2-type immune responses. Eur J Immunol. 2003;33:3186–95. doi: 10.1002/eji.200324154. [DOI] [PubMed] [Google Scholar]
- 26.Verweij WR, de Haan L, Holtrop M, Agsteribbe E, Brands R, van Scharrenburg GJ, Wilschut J. Mucosal immunoadjuvant activity of recombinant Escherichia coli heat-labile enterotoxin and its B subunit: induction of systemic IgG and secretory IgA responses in mice by intranasal immunization with influenza virus surface antigen. Vaccine. 1998;16:2069–76. doi: 10.1016/s0264-410x(98)00076-0. [DOI] [PubMed] [Google Scholar]
- 27.Wu HY, Russell MW. Induction of mucosal and systemic immune responses by intranasal immunization using recombinant cholera toxin B subunit as an adjuvant. Vaccine. 1998;16:286–92. doi: 10.1016/s0264-410x(97)00168-0. [DOI] [PubMed] [Google Scholar]
- 28.Hajishengallis G, Russell MW, Michalek SM. Comparison of an adherence domain and a structural region of Streptococcus mutans Antigen I/II in protective immunity against dental caries in rats after intranasal immunization. Infect Immun. 1998;66:1740–3. doi: 10.1128/iai.66.4.1740-1743.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu HY, Russell MW. Induction of mucosal immunity by intranasal application of a Streptococcal surface protein antigen with the cholera toxin B subunit. Infect Immun. 1993;61:314–22. doi: 10.1128/iai.61.1.314-322.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toida N, Hajishengallis G, Wu HY, Russell MW. Oral immunization with the saliva-binding region of Streptococcus mutans AgI/II genetically coupled to the cholera toxin B subunit elicits T-helper-cell responses in gut-associated lymphoid tissues. Infect Immun. 1997;65:909–15. doi: 10.1128/iai.65.3.909-915.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russell MW, Bergmeier LA, Zanders ED, Lehner T. Protein antigens of Streptococcus mutans: purification and properties of a double antigen and its protease-resistant component. Infect Immun. 1980;28:486–93. doi: 10.1128/iai.28.2.486-493.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dertzbaugh MT, Macrina FL. Plasmid vectors for constructing translational fusions to the B subunit of cholera toxin. Gene. 1989;82:335–42. doi: 10.1016/0378-1119(89)90060-7. [DOI] [PubMed] [Google Scholar]
- 33.Russell MW, Wu HY. Distribution, persistence, and recall of serum and salivary antibody responses to peroral immunization with protein Antigen I/II of Streptococcus mutans coupled to the cholera toxin B subunit. Infect Immun. 1991;59:4061–70. doi: 10.1128/iai.59.11.4061-4070.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carlsson J, Drevin H, Axen R. Protein thiolation and reversible protein-protein conjugation. N-Succinimidyl 3-(2-pyridyldithio) propionate, a new heterobifunctional reagent. Biochem J. 1978;173:723–37. doi: 10.1042/bj1730723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hajishengallis G, Michalek SM, Russell MW. Persistence of serum and salivary antibody responses after oral immunization with a bacterial protein antigen genetically linked to the A2/B subunits of cholera toxin. Infect Immun. 1996;64:665–7. doi: 10.1128/iai.64.2.665-667.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gockel CM, Bao S, Holland MK, Beagley KW. Influence of the murine oestrous cycle on the induction of mucosal immunity. Am J Reprod Immunol. 2003;50:369–79. doi: 10.1034/j.1600-0897.2003.00097.x. [DOI] [PubMed] [Google Scholar]
- 37.Dexter SJ, Camara M, Davies M, Shakesheff KM. Development of a bioluminescence ATP assay to quantify mammalian and bacterial cell numbers from a mixed population. Biomaterials. 2003;24:27–34. doi: 10.1016/s0142-9612(02)00239-9. [DOI] [PubMed] [Google Scholar]
- 38.Husby S, Mestecky J, Moldoveanu Z, Holland S, Elson CO. Oral tolerance in humans. T cell but not B cell tolerance after antigen feeding. J Immunol. 1994;152:4663–70. [PubMed] [Google Scholar]
- 39.Challacombe SJ, Tomasi TB. Systemic tolerance and secretory immunity after oral immunization. J Exp Med. 1980;152:1459–72. doi: 10.1084/jem.152.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bregenholt S, Wang M, Wolfe T, Hughes A, Baerentzen L, Dyrberg T, von Herrath MG, Petersen JS. The cholera toxin B subunit is a mucosal adjuvant for oral tolerance induction in type 1 diabetes. Scand J Immunol. 2003;57:432–8. doi: 10.1046/j.1365-3083.2003.01248.x. [DOI] [PubMed] [Google Scholar]
- 41.Czerkinsky C, Russell MW, Lycke N, Lindblad M, Holmgren J. Oral administration of Streptococcal antigen coupled to cholera toxin B subunit evokes strong antibody responses in salivary glands and extramucosal tissues. Infect Immun. 1989;57:1072–7. doi: 10.1128/iai.57.4.1072-1077.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lycke N, Tsuji T, Holmgren J. The adjuvant effect of Vibrio cholerae and Escherichia coli heat-labile enterotoxins is linked to their ADP-ribosyltransferase activity. Eur J Immunol. 1992;22:2277–81. doi: 10.1002/eji.1830220915. [DOI] [PubMed] [Google Scholar]
- 43.de Haan L, Verweij W, Agsteribbe E, Wilschut J. The role of ADP-ribosylation and G (M1)-binding activity in the mucosal immunogenicity and adjuvanticity of the Escherichia coli heat-labile enterotoxin and Vibrio cholerae cholera toxin. Immunol Cell Biol. 1998;76:270–9. doi: 10.1046/j.1440-1711.1998.00745.x. [DOI] [PubMed] [Google Scholar]
- 44.Sun JB, Mielcarek N, Lakew M, Grzych JM, Capron A, Holmgren J, Czerkinsky C. Intranasal administration of a Schistosoma mansoni glutathione S-transferase-cholera toxoid conjugate vaccine evokes antiparasitic and antipathological immunity in mice. J Immunol. 1999;163:1045–52. [PubMed] [Google Scholar]
- 45.Wu HY, Nikolova EB, Beagley KW, Eldridge JH, Russell MW. Development of antibody-secreting cells and antigen-specific T cells in cervical lymph nodes after intranasal immunization. Infect Immun. 1997;65:227–35. doi: 10.1128/iai.65.1.227-235.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walker LSK. CD4+CD25+ Treg: divide and rule? Immunology. 2004;111:129–37. doi: 10.1111/j.0019-2805.2003.01788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu HY, Russell MW. Nasal lymphoid tissue, intranasal immunization, and compartmentalization of the common mucosal immune system. Immunol Res. 1997;16:187–201. doi: 10.1007/BF02786362. [DOI] [PubMed] [Google Scholar]
- 48.Russell MW, Czerkinsky C, Moldoveanu Z. Detection and specificity of antibodies secreted by spleen cells in mice immunized with Streptococcus mutans. Infect Immun. 1986;53:317–23. doi: 10.1128/iai.53.2.317-323.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bruce MG, Ferguson A. Oral tolerance to ovalbumin in mice: studies of chemically modified and biologically filtered antigen. Immunology. 1986;57:627–30. [PMC free article] [PubMed] [Google Scholar]
- 50.Miller SD, Hanson DG. Inhibition of specific immune responses by feeding protein antigens. IV. Evidence for tolerance and specific active suppression of cell-mediated immune responses to ovalbumin. J Immunol. 1979;123:2344–50. [PubMed] [Google Scholar]
- 51.Ploix C, Bergerot I, Durand A, Czerkinsky C, Holmgren J, Thivolet C. Oral administration of cholera toxin B-insulin conjugates protects NOD mice from autoimmune diabetes by inducing CD4+ regulatory T-cells. Diabetes. 1999;48:2150–6. doi: 10.2337/diabetes.48.11.2150. [DOI] [PubMed] [Google Scholar]
- 52.Wang J, Li L, Zheng J, et al. Highly efficient expression, purification of recombinant LTB protein and its activity against mucosal immunoadjuvant by nasal immunization. Chin Med J. 2003;116:1115–7. [PubMed] [Google Scholar]
- 53.Wilson AD, Clarke CJ, Stokes CR. Whole cholera toxin and B subunit act synergistically as an adjuvant for the mucosal immune response of mice to keyhole limpet haemocyanin. Scand J Immunol. 1990;31:443–51. doi: 10.1111/j.1365-3083.1990.tb02791.x. [DOI] [PubMed] [Google Scholar]
- 54.Ryan ET, Crean TI, John M, Butterton JR, Clements JD, Calderwood SB. In vivo expression and immunoadjuvancy of a mutant of heat-labile enterotoxin of Escherichia coliin vaccine and vector strains of Vibrio cholerae. Infect Immun. 1999;67:1694–701. doi: 10.1128/iai.67.4.1694-1701.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]