Abstract
Leukotriene B4 (LTB4) and cysteinyl leukotrienes (CysLTs) are known as potent mediators of inflammation, whereas their role in the regulation of adaptive immunity remains poorly characterized. Dendritic cells (DCs) are specialized antigen-presenting cells, uniquely capable to initiate primary immune responses. We have found that zymosan, but not lipopolysaccharide (LPS) stimulates murine bone marrow-derived dendritic cells (BM-DCs) to produce large amounts of CysLTs and LTB4 from endogenous substrates. A selective inhibitor of leukotriene synthesis MK886 as well as an antagonist of the high affinity LTB4 receptor (BLT1) U-75302 slightly inhibited zymosan-, but not LPS-stimulated interleukin (IL)-10 release from BM-DCs. In contrast, U-75302 increased zymosan-stimulated release of IL-12 p40 by ∼23%. Pre-treatment with transforming growth factor-β1 enhanced both stimulated leukotriene synthesis and the inhibitory effect of U-75302 and MK886 on IL-10 release from DCs. Consistent with the effects of leukotriene antagonists, exogenous LTB4 enhanced LPS-stimulated IL-10 release by ∼39% and inhibited IL-12 p40 release by ∼22%. Both effects were mediated by the BLT1 receptor. Ligands of the high affinity CysLTs receptor (CysLT1), MK-571 and LTD4 had little or no effect on cytokine release. Agonists of the nuclear LTB4 receptor peroxisome proliferator-activated receptor-α, 8(S)-hydroxyeicosatetraenoic acid and 5,8,11,14-eicosatetraynoic acid, inhibited release of both IL-12 p40 and IL-10. Our results indicate that both autocrine and paracrine leukotrienes may modulate cytokine release from DCs, in a manner that is consistent with previously reported T helper 2-polarizing effects of leukotrienes.
Keywords: dendritic cells, leukotriene, IL-10, IL-12, IL-6
Introduction
Leukotrienes are potent lipid mediators, synthesized in activated leucocytes from membrane phospholipid-liberated arachidonic acid. Leukotriene synthesis is initiated by a two-step conversion of free arachidonic acid into an unstable epoxide intermediate, leukotriene A4 (LTA4). This reaction is catalyzed by 5-lipoxygenase (5-LOX) in the presence of the accessory 5-LOX-activating protein (FLAP). Further metabolism of LTA4 gives rise to two types of leukotrienes, playing disparate roles in inflammatory responses. LTA4 hydrolase transforms LTA4 into LTB4, whereas LTC4 synthase couples LTA4 with glutathione to form LTC4 (Fig. 1). LTC4, together with its partial degradation products – LTD4 and LTE4, are collectively termed cysteinyl leukotrienes (CysLTs). Expression of 5-LOX and LTC4 synthase, and therefore leukotriene synthesis, is essentially limited to the cells of myeloid origin (mast cells, eosinophils, basophils, monocytes-macrophages, and (in the case of LTB4) neutrophils). In contrast, LTA4 hydrolase is expressed in several cell types.1,2
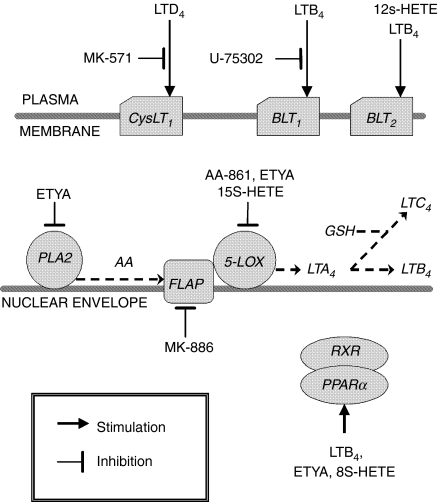
Figure 1.
Cellular targets of pharmacological agents used in the presented study. AA, arachidonic acid; PLA2, phospholipase A2; RXR, retinoid X receptor.
Leukotrienes are potent mediators of inflammation, and their excessive production is associated with pathological manifestations of various inflammatory disorders, such as asthma, arthritis, psoriasis, ulcerative colitis and ischaemic reperfusion injury. CysLTs produce vasodilatation, increased permeability of postcapillary venules, bronchoconstriction, and mucus secretion, whereas LTB4 acts as a strong chemoattractant for neutrophils and eosinophils, stimulating their adherence to endothelial cells, degranulation and generation of reactive oxygen species.1,3 Two types of CysLTs receptors and three types of LTB4 receptors have been identified to date. Both CysLTs receptors, CysLT1 and CysLT2, are seven transmembrane-spanning G protein-coupled receptors, increasing [Ca2+]i and antagonizing cAMP elevation produced by other receptors. Besides this, little is known about the signalling triggered by leukotriene receptors at a mechanistic level. LTD4 is a more potent agonist of CysLT1 than LTC4, whereas LTD4and LTC4 bind and activate CysLT2 with similar potencies.4,5 Also, two homologous high affinity LTB4 receptors, BLT1 and BLT2, belong to the family of G protein-coupled receptors and mediate increases of [Ca2+]i and inhibition of adenyl cyclase. The high affinity BLT1 receptor is expressed mainly in leucocytes, whereas the expression of BLT2 is ubiquitous. BLT2 binds LTB4 with lower affinity than BLT1, but, unlike BLT1, may also mediate responses to 12/15-LOX-derived 12- and 15-hydroxyeicosanoids.6,7 Nevertheless, analysis of BLT1-deficient mice suggests that BLT1 is the major receptor mediating pro-inflammatory effects of LTB4.8,9 The third receptor for LTB4 is the nuclear peroxisome proliferator-activated receptor-α (PPARα). In contrast to the membrane receptors, PPARα appears to be linked to anti-inflammatory effects because PPARα–/– mice exhibit prolonged inflammation in response to LTB4 or arachidonic acid, but not to phorbol esters.10
Unlike their well-established role in inflammation, involvement of leukotrienes in the regulation of different aspects of adaptive immunity, including functions of antigen-presenting cells, remains controversial.1,8,11,12 Dendritic cells (DCs) are specialized antigen-presenting cells, furnished with the unique ability to initiate primary immune responses. Expression of 5-LOX has been detected in different populations of DCs.2,13 For instance, Langerhans cells are the major, and most likely the sole, 5-LOX pathway-expressing cells in the normal human epidermis.2 In human DCs, expression of 5-LOX was induced during their in vitro differentiation from CD34+ haematopoietic progenitors. The expression was down-regulated by interleukin (IL)-4, whereas transforming growth factor-β1 (TGFβ1) up-regulated 5-LOX expression as well as counteracted down-regulation by IL-4.13,14 5-FLAP, but not 5-LOX expression was reportedly down-regulated by IL-10 in murine bone marrow-derived DCs (BM-DCs).15
CysLTs were found to regulate DC migration from the skin to lymph nodes, apparently by promoting both mobilization of DCs from the epidermis and their chemotaxis towards chemokine CCL19.16 According to our knowledge, the role of leukotrienes in the regulation of cytokine release from DCs has been investigated in only three recent studies, reporting three qualitatively different effects. Harizi et al.17 found no effect of LTB4 or the 5-LOX inhibitor/antioxidant (nor-dihydrogurinic acid) on either spontaneous or lipopolysaccharide (LPS)-stimulated IL-12 p70 and IL-10 release from murine bone-marrow-derived DCs (BM-DCs). In contrast, Machida et al.18 have reported that LTD4 increases IL-10 release, whereas antagonists of CysLT1 receptors increase IL-12 p40 release from murine BM-DCs pulsed with Dermatophagoides farinae. Finally, Okunishi et al.19 reported that splenic dendritic cells, isolated from mice treated with a CysLT1 antagonist, release significantly less of both IL-10 and IL-12 p70 upon ex vivo stimulation with LPS.
In the presented study, the capacity of murine BM-DCs to synthesize leukotrienes from endogenous substrates was tested. The potential role of endogenous and exogenous (paracrine) leukotrienes in the regulation of cytokine release from DCs was also assessed with the use of exogenous leukotrienes, specific inhibitors of their synthesis and receptor-selective ligands.
Materials and methods
Reagents
Fluorescein isothiocyanate (FITC)-conjugated hamster anti-mouse CD11c (clone HL3) as well as phycoeritrin-conjugated rat anti-mouse CD86 (clone GL1) and I-Ak (clone 11-5.2) monoclonal antibodies (mAbs) were obtained from PharMingen (San Diego, CA). A23187 (50 mm) and indometacin (50 mm) were purchased from Sigma (St. Louis, MO), 5,8,11,14-eicosatetraynoic acid (ETYA; 20 mm), AA-861 (50 mm), MK-886 (10 mm) from Biomol Research Laboratories (Plymouth Meeting, PA) and MK-571 (20 mm) from Cayman (Ann Arbor, MI). Stock solutions of these drugs were prepared in dimethyl sulphoxide (DMSO) at concentrations indicated in brackets and stored at −80°. 15(S)-hydroxyeicosatetraenoic acid (15S-HETE), 8S-HETE (0·31 mm), U-75302 (10 mm), LTB4 (2 mm) and LTD4 (0·2 mm) were provided by Cayman as ethanol solutions. Drugs were thawed and diluted in culture medium just before addition to cell cultures.
Zymosan from Saccharomyces cerevisiae (Sigma) was suspended in 0·9% NaCl and boiled for 10 min. Following centrifugation, the pellet was opsonized in normal mouse serum by incubation for 30 min at 37°. After washing three times, aliquots of opsonized zymosan (OZ) suspension in RPMI-1640 medium (4 mg/ml) were stored at −80°. Unopsonized zymosan (ZYM) was prepared in a similar manner as OZ except that the opsonization step was omitted. All chemical reagents not otherwise specified were obtained from Sigma.
Preparation of cells
BM-DCs were propagated from bone marrow cells of CBA mice in the presence of granulocyte–macrophage colony-stimulating factor (GM-CSF) according to the method developed by Inaba et al.20 with some modifications. In brief, bone marrow cells were flushed out from the femurs and grown at the starting density of 3 × 105/ml in Iscove's modified Dulbecco's medium (Gibco BRL, Rockville, MD) supplemented with 5 mg/ml apo-transsferrin, 50 nm 2-mercaptoethanol, 10% fetal calf serum (FCS, Gibco BRL), and 10% X-63 cell line supernatant as a source of GM-CSF. After 2 days, the medium, containing most of the non-adherent cells, was removed and replaced with the fresh medium. When indicated, the cultures were additionally supplemented on the 5th day with 5 ng/ml of recombinant human TGF-β1 (eBioscience, San Diego, CA). Non-adherent BM-DCs were recovered on the 7th day of culture, and either used directly in experiments or purified by positive selection with the use of CD11c (N418) MicroBeads and 25 MS Separation Columns (Miltenyi Biotec, Bergisch Gladbach, Germany), according to the manufacturer's instructions. Bulk (BM-DCs) or purified DCs (purDCs) were washed once and re-suspended in RPMI-1640 medium (Gibco BRL), containing antibiotics and 5% FCS (FCS-RPMI medium), plated in 24-or 96-well tissue culture plates and treated as described below.
Flow cytometry
BM-DCs or purDCs (1 × 106) were preincubated with 50 µg/ml mouse Fc block antibody (clone 2.4G2, own source from hybridoma) for 20 min at 4° and then labelled for 30 min at 4° with 10 µg/ml of fluorochrome-conjugated mAbs in 100 µl of phosphate-buffered saline (PBS) containing 2% FCS and 0·05% NaN3. After washing two times, the labelled cells were analysed with the use of an Ortho CytoronAbsolute flow cytometer (Ortho-Clinical Diagnostics, Rochester, NY).
Leukotriene release
BM-DCs or purDCs were washed once and re-suspended in serum-free RPMI-1640 medium at a final density of 1 × 106/ml. Eicosanoid formation was stimulated by 40 min incubation with zymosan (0·2 or 0·5 mg/ml), LPS from Escherichia coli (200 ng/ml) or A23187 (5 µm) +/0 phorbol myristate acetate (PMA, 100 nm). When used, pharmacological inhibitors were added 20 min before OZ. Supernatants were collected and immediately frozen at −80°. CysLTs and LTB4 concentrations in the supernatants were assayed with the use of monoclonal antibody/enzyme immunoassay kits purchased from Cayman, according to manufacturer's instructions.
Cell viability
The effect of treatments on cell viability was assessed by measuring lactic dehydrogenase (LDH) activity in culture supernatants with the use of the LDH Cytotoxity Detection Kit (Takara Biomedicals, Shiga, Japan) and the CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega, Madison, WI), according to manufacturer's instructions.
Cytokine release
BM-DCs or purDCs (5 × 105/ml) were preincubated for 30 min with the FLAP inhibitor MK-886, leukotrienes (LTB4, LTD4) or leukotriene antagonists (MK-571, U-75302; Fig. 1), and subsequently stimulated with LPS (200 ng/ml) or OZ (0·2 mg/ml) for 22 hr. Control cells were treated with equivalent concentrations of solvents, ethanol or DMSO. At the concentrations used, neither DMSO (up to 0·1%), nor ethanol (up to 0·5%) affected cell viability or cytokine release (data not shown). Cell viability, as measured in the LDH release or MTS reduction tests, was also unaffected by the drugs (data not shown).
Cytokine concentrations (tumour necrosis factor-α (TNF-α), IL-6, IL-12p40 and IL-12p70) in culture supernatants were determined by capture enzyme-linked immunosorbent assays (ELISAs), as described previously21 with the use of matched pairs of capture and secondary mAbs purchased from PharMingen. Cytokines were quantified relative to a standard curve representing a range of dilutions of recombinant cytokines. The concentrations of IL-10 were determined with a commercial kit (OptEIA, PharMingen), according to the manufacturer's instruction.
Data analysis
After assessing homogeneity of variances with the F-test, means were compared with Student's t-test for single comparisons. anova was used for multiple comparisons, with the assumption that P-values <0·05 indicate statistically significant differences (Graph-Pad Prism software, San Diego, CA). In dose–response experiments, the values of maximal effect and concentrations producing half-maximal effect (EC50) were determined by non-linear regression curve fitting with the Graph-Pad Prism software.
Results
OZ, but not LPS stimulates the release of leukotrienes from BM-DCs
BM-DCs stimulated for 40 min with OZ or with the calcium ionophore A23187 (stimuli known to trigger leukotriene release in other cell types22,23) released large amounts of CysLTs (Fig. 2a) and LTB4 (Fig. 2b). Co-treatment with PMA, an activator of protein kinase C which augments leukotriene production in other cell types24 also enhanced A23187-stimulated leukotriene release in BM-DCs. In contrast, LPS did not stimulate release of leukotrienes (Fig. 2a, b).
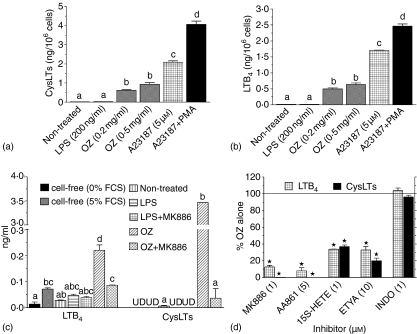
Figure 2.
Leukotriene production by BM-DCs. BM-DCs were plated at 1 × 106/ml in serum-free RPMI-1640 medium (a, b, d) or at 5 × 105/ml in FCS–RPMI (c) and treated for 40 min (a, b, d) or for 22 hr (c) with indicated stimuli. Leukotriene concentrations in supernatants were measured with EIAs. Graphs present results of single experiments (mean ±SEM), performed in triplicates, representative of four (a, b) or two (c, d) such experiments. On graphs a–c bars not sharing letters above error bars represent values significantly different from each other, according to anova. On graph D * marks statistically significant inhibition (P < 0·05 in one-sample t-test). UD – undetectable.
We also detected neither spontaneous nor LPS-stimulated LTB4 production from endogenous arachidonic acid in the 22-hr culture supernatants of BM-DCs. This was not affected by the blockade21 of prostaglandin synthesis with indometacin (Fig. 2c). Quite to the contrary, more LTB4 immunoreactivity was present in cell-free than in cell-containing medium incubated under the same conditions. This may be explained by cell-mediated degradation of serum-derived immunoreactive products. Moreover, unlike those stimulated by OZ, levels of LTB4 immunoreactivity in medium of LPS-stimulated cells were not affected by the specific inhibitor of leukotriene synthesis MK-88625 consistent with a non-specific nature of LTB4 immunoreactivity in medium of LPS-stimulated cells (Fig. 2c).
In contrast, effects of pharmacological inhibitors (Fig. 1) confirmed the involvement of the 5-LOX-FLAP complex in leukotriene synthesis in OZ-stimulated BM-DCs. Leukotriene release was inhibited by 5-LOX/FLAP-selective inhibitors: MK-886, 15S-HETE26 and AA-86127 as well as by a general inhibitor of arachidonate metabolism, ETYA (Fig. 2c, d). However, it was not inhibited by the cyclooxygenase-selective inhibitor indometacin.28
Endogenous leukotrienes modulate cytokine release from OZ-stimulated, but not from LPS-stimulated BM-DCs
LPS (200 ng/ml) and OZ (0·2 mg/ml) differed in their effects on cytokine release from BM-DCs. Cells treated with OZ released ∼6·5 times more IL-10 (P < 0·0001), ∼2·8 times more TNF-α (P = 0·0001), and ∼1·7 times more IL-6 (P = 0·03) than cells treated with LPS (Fig. 3a, b). On the other hand, LPS stimulated ∼2·1-fold higher IL-12 p40 release (P = 0·04) than OZ (Fig. 3b).
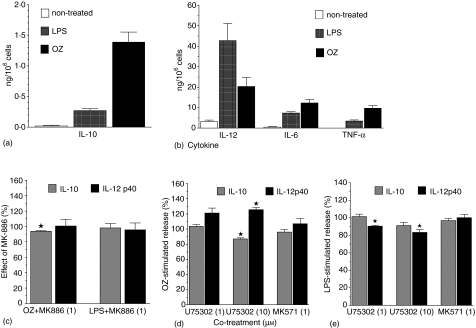
Figure 3.
LPS- or OZ-stimulated cytokine release (a, b) and its modulation by MK886 (c), U75302, or MK-571 (d, e). BM-DCs (5 × 105/ml) were preincubated for 20 min with indicated concentrations of MK886, U75302, MK-571 or with solvents and then treated for 22 hr with OZ (0·2 mg/ml) or LPS (200 ng/ml). TNF-α, IL-6, IL-12 p40 and IL-10 levels were measured in culture supernatants with ELISAs. Results are means ± SEM from 4 to 10 independent experiments, each performed in three to four replicates. Results on graphs C-E are presented as percentages of controls stimulated with LPS or OZ only. *Significant effect (P < 0·05 in one-sample t-test).
Inhibition of endogenous leukotriene production with 1 µm MK-886 inhibited slightly, but significantly (by 6·3 ± 0·92%, n = 5, P = 0·002), OZ-stimulated release of IL-10, while having no effect on cytokine release in the absence of leukotriene synthesis in LPS-stimulated BM-DCs (Fig. 3c). Like MK-886, a selective antagonist of BLT1, U-753026 at 10 µm inhibited the release of IL-10 stimulated by OZ (by 13·1 ± 1·68%, n = 7, P = 0·0002, Fig. 3d), but not by LPS (Fig. 3e). U75302 additionally enhanced by 25·6 ± 2·94% (n = 5, P = 0·001) IL-12 p40 release from OZ-stimulated BM-DCs (Fig. 3d). U-75302 had the opposite effect on IL-12 p40 release stimulated by LPS (Fig. 3e). The latter effect might be explained by partial agonistic properties of this drug7 displayed in the absence of endogenous LTB4 release as in LPS-stimulated BM-DCs (see below).
MK-571 is a specific antagonist of CysLT1– the major receptor mediating pro-inflammatory effects of cysLTs in mice.5,29 As shown on Fig. 3(d, e), MK-571 had no effect on either LPS- or OZ-stimulated release of cytokine from BM-DCs.
Taken together, the above results suggest that IL-12 and IL-10 release from OZ-stimulated BM-DCs is modulated by endogenous LTB4 acting through BLT1 receptor, but not by CysLTs acting through CysLT1 receptor (Fig. 1). In particular, endogenous LTB4 seems partially responsible for the higher IL-10 and lower IL-12 p40 release from OZ-stimulated as compared to LPS-stimulated DCs.
DCs themselves produce leukotrienes in a mixed culture of bone marrow-derived cells
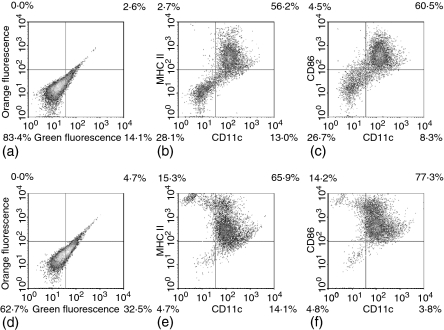
Previously, synthesis of CysLTs has been demonstrated in a mixed culture of bone marrow-derived cells18 known to contain several other cell types which are capable of high output leukotriene synthesis, such as macrophages and granulocytes.14,20 Indeed, flow cytometric analysis confirmed that BM-DCs constitute heterogeneous population of cells (Fig. 4a–c). The percentage of DCs, identified as double positive CD11c+/I-Ak+ or CD11c+/CD86+ cells30,31 ranged from 34 to 55% in four different BM-DC cultures (Fig. 4a–c and data not shown). However, due to weak labelling (Fig. 4d–f) and high autofluorescence of a fraction of cells (Fig. 4a, c), this assessment seems to be an underestimation.
Figure 4.
Flow cytometric analysis of surface phenotype of cells before (a–c) and after (d–f) positive selection on anti-CD11c mAb-coated magnetic beads. Cells were labelled with FITC- or phycoerythrin-conjugated mAbs and analysed by flow cytometry, as described under Materials and methods. (a and d) Autofluorescence; (b and e) CD11c versus major histocompatibility complex-II; (c and f) CD11c versus CD86.
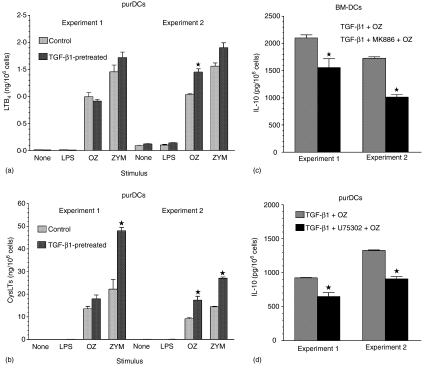
In order to confirm that DCs themselves produce leukotrienes, we studied leukotriene production in DCs purified by positive selection on anti-CD11c mAb-coated magnetic beads (puDCs, Fig. 4d–f). Upon stimulation with OZ or ZYM (0·2 mg/ml), but not LPS, purDCs produced high amounts of both LTB4(Fig. 5a) and CysLTs (Fig. 5b). Whereas OZ stimulated similar production of LTB4 in purDCs (1·02 ± 0·020 ng/106 cells, n = 2) and BM-DCs (0·86 ± 0·203, n = 3), purDCs produced about seven times more CysLTs (11·35 ±2·195) than BM-DCs (1·64 ± 0·527 ng/106 cells; Fig. 2a, b and Fig. 5a, b). These results show that CysLTs are produced mainly by DCs, whereas other cell types seem to contribute significantly to LTB4 production in the mixed culture of BM-DCs.
Figure 5.
Leukotriene production (a, b) and effect of 1 µm MK886 (c) or 10 µm U75302 (d) on OZ-stimulated IL-10 production by BM-DCs (c) or by purified DCs (a, b, and d), obtained from cultures supplemented or not in the 5th day with TGF-β1 (5 ng/ml). DCs were isolated by positive selection on anti-CD11c mAb-coated magnetic beads and leukotriene release and OZ-stimulated IL-10 production were assessed as described under Materials and methods. Graphs present means ± SEM of duplicates (a, b and d) or triplicates (c), obtained in two independent experiments.
TGF-β1 pretreatment enhances both leukotriene production and the inhibitory effect of leukotriene antagonists on IL-10 production
It has been reported that in human DCs, differentiated in vitro from CD34+ haematopoietic progenitors, TGF-β1 produces a functional up-regulation of 5-LOX.14 We have also found that murine purDCs, if differentiated for the final 2 days in the presence of TGF-β1, produce increased amounts of leukotrienes in response to OZ or ZYM than control cells (Fig. 5a, b). A stronger effect of TGFβ1 on CysLTs (Fig. 5b) than LTB4 (Fig. 5a) production suggests that, except for 5-LOX, TGF-β1 might also up-regulate LTC4 synthase expression or activity in DCs.
Increases of zymosan-stimulated leukotriene release in TGF-β1-pretreated cells were accompanied by enhanced effects of the leukotriene antagonists MK886 and U75302 on OZ-stimulated IL-10 release. In TGFβ1-pretreated BM-DCs, MK886 inhibited OZ-stimulated IL-10 release by 33·6 ± 7·75% (n = 2, Fig. 5c) as compared to a 6·3 ±0·92% (n = 5) inhibition in cells not treated with TGF-β1 (Fig. 3c). A BLT1 antagonist, U75302, inhibited OZ-stimulated IL-10 production in BM-DCs (Fig. 3d) by 13·1 ± 1·68% (n = 7) and in TGF-β1-pretreated, purDCs (Fig. 5d) by 30·1 ± 0·95% (n = 2).
Effects of exogenous leukotrienes on cytokine release from DCs
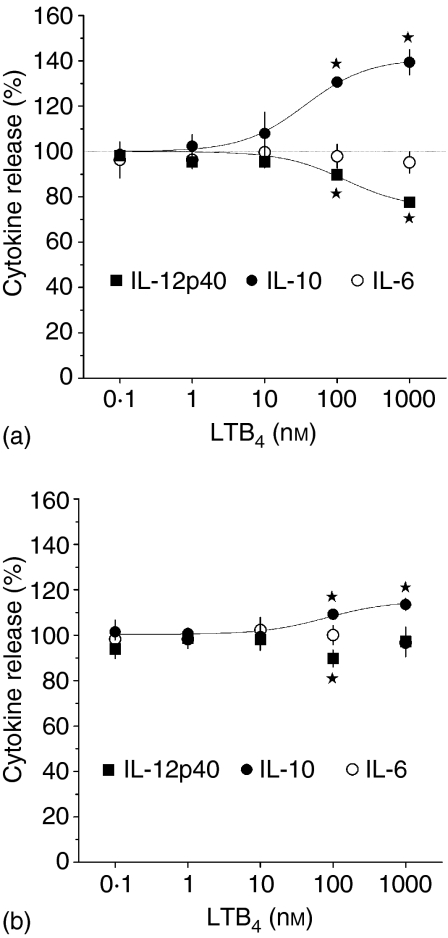
Exogenous LTB4 dose-dependently increased IL-10 release and decreased IL-12 p40 release from LPS-stimulated BM-DCs by 39 ± 5·5% (n = 7, P = 0·0004) and 22 ± 1·5% (n = 5, P = 0·0001), respectively (using 1 µm of LTB4). The EC50 values of these effects were 36 ± 10·3 nm, and 130 ± 21·6 nm, respectively (Fig. 6a). Exogenous LTD4 turned out to be a less potent regulator of cytokine release in BM-DCs than LTB4, producing only 14 ± 2·6% (n = 3, P = 0·03) increases of IL-10 release at 1 µm (Fig. 6b). Neither LTB4 nor LTD4 affected IL-6 release (Fig. 6).
Figure 6.
Effects of exogenous LTB4(a)or LTD4(b)on LPS-stimulated IL-6, IL-10 and IL-12 p40 release. BM-DCs (5 × 105/ml) were preincubated for 20 min with indicated concentrations of leukotrienes and then treated for 22 h with LPS (200 ng/ml). IL-6, IL-12 p40 and IL-10 levels were measured in culture supernatants with ELISAs. Results are presented as percentages of LPS-only stimulated controls and are means ± SEM from four to seven experiments, each performed in three to four replicates. *Significant effect (P < 0·05 in one-sample t-test).
Exogenous LTB4 increased OZ-stimulated IL-10 release to a lesser extent than LPS-stimulated release (38 ± 7·8% versus 21 ± 1·8% stimulation, P = 0·07), which is consistent with the involvement of endogenous LTB4 in autoregulation of OZ-stimulated, but not LPS-stimulated IL-10 production in BM-DCs (Fig. 7a, b).
Figure 7.
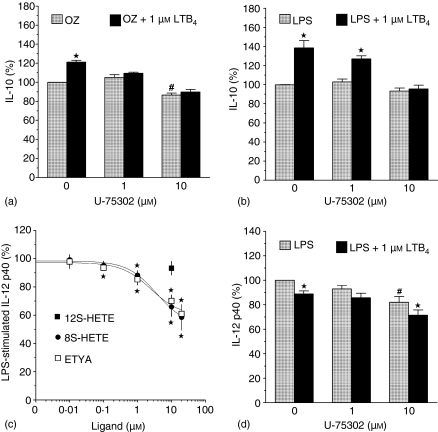
Effect of U75302 (a, b, d), 8S-HETE, 12S-HETE, or ETYA (c) on OZ+/0 LTB4-stimulated (a) or LPS+/0 LTB4-stimulated (b–d) IL-10 and IL-12 p40 production in cultures of BM-DCs. BM-DCs were preincubated for 30 min with U75302, followed by 20 min preincubation with LTB4, 8S-HETE, 12S-HETE, or ETYA, and then treated for 22 hr with LPS or OZ. IL-12 p40 and IL-10 levels were measured in culture supernatants with ELISAs. Results, presented as percentages of controls treated with LPS or OZ only, are means ± SEM from three to five experiments, each performed in two to four replicates. #Significant effect of U75302. *Significant effect of LTB4, 8S-HETE, or ETYA (P < 0·05 in one-sample t-test).
The BLT1 receptor mediates the effects of exogenous LTB4 on cytokine release
In order to identify the receptors mediating the effects of exogenous LTB4 on cytokine release from BM-DCs, we studied the effects of available agonists or antagonists of three known LTB4 receptors: BLT1, BLT2 and PPARα (Fig. 1). 12S-HETE has been shown to be a selective agonist of the BLT2 over the BLT1 receptor, as 10 µm 12S-HETE stimulated calcium mobilization in CHO cells transfected with BLT2 as potently as equimolar LTB4.7 However, at this concentration 12S-HETE had no effect on LPS-stimulated IL-12 release from BM-DCs (Fig. 7c). In contrast, LPS-stimulated IL-12 p40 release was dose-dependently inhibited, by up to ∼40%, by the activators of nuclear PPARα receptors ETYA and 8S-HETE32 (EC50 values of 2·5 ± 0·63 µm and 3·7 ± 0·51 µm, respectively (Fig. 7c)). Together with the fact that the nuclear envelope is the main location of leukotriene synthesis15,33 these results suggest a potential role of PPARα in regulating cytokine release from BM-DCs upon ligation with endogenous LTB4. However, two lines of evidence suggest that the effects of exogenous LTB4 on IL-10 release are mediated exclusively through its membrane receptor, BLT1. First, a selective antagonist of BLT1 receptor, U75302, completely reversed the stimulation of IL-10 release by LTB4 (Fig. 7a, b). Second, PPARα agonists (10 µm 8S-HETE and ETYA) inhibited LPS-stimulated IL-10 release by 20 ± 9·3% (n = 3, P = 0·17), and 9·3 ± 2·02% (n = 5, P = 0·01), respectively (data not shown).
Concomitant use of U-75302 and LTB4 allowed us to verify the hypothesis that the effect of U75302 on LPS-stimulated IL-12 p40 release results from its partial agonism at the murine BLT1 receptor. Indeed, consistent with such a mechanism, a lower concentration of U-75302 (1 µm) partially reversed the LTB4-mediated inhibition of IL-12 p40 release, whereas a higher concentration (10 µm) acted additively with LTB4 (Fig. 7d).
Discussion
Only a few cell types, mainly of myeloid origin (mast cells, basophils, macrophages, neutrophils and eosinophils) has been shown to express 5-LOX and be capable of LTB4 synthesis. Synthesis of CysLTs seems to be even more restricted. For instance, neutrophils express 5-LOX, but not LTC4 synthase and, consequently, synthesize LTB4, but not CysLTs.5 DCs were previously found to express 5-LOX and synthesize LTB4 upon stimulation with calcium ionophores.13–15 Recently, Machida et al.18 reported expression of mRNA for LTC4 synthase in murine BM-DCs and the accumulation of LTD4 and LTC4 in medium during propagation of DCs from mixed culture of BM cells. This accumulation was two- to threefold higher in BM-DCs cultures pulsed with D. farinae. In our study, we observed high levels of both LTB4 and CysLTs synthesis from endogenous substrates in purified DCs that were triggered not only by a calcium ionophore, but also by a more physiological stimulus – zymosan – whereas non-stimulated cells released only small quantities of leukotrienes. The identities of leukotriene detected with the immunoassays were confirmed in our study by inhibiting their generation with selective inhibitors of the 5-LOX–FLAP complex.
LPS has been reported to be unable to stimulate leukotriene release from different cell types.22,23,25 We have found that LPS is also not able to stimulate leukotriene release from BM-DCs. Our results thus contrast with reports by Harizi et al.17,34 These authors reported spontaneous generation of LTB4 by murine BM-DCs cultured in medium containing 10% FCS. This production was increased by LPS treatment and further enhanced by cotreatment with cyclooxygenase inhibitors. In another study by these authors, 5 µm A23187 increased this high basal LTB4 accumulation by less then twofold.15 The presence of IL-4 during propagation of DCs from bone marrow cells in the latter study does not seem to account for the discrepancy because we have found that a two day treatment with IL-4 (10 ng/ml) caused ∼40% suppression, rather than stimulation, of leukotriene release from DCs upon subsequent stimulation with OZ (data not shown). In contrast, our results confirm earlier reports35–37 that this ‘spontaneous LTB4 release’ in FCS-containing medium may be ascribed, at least in part, to nonspecific generation of LTB4 immunoreactivities from serum components. In such a case, LPS might increase accumulation of serum-derived LTB4 immunoreactivity by inhibiting cell-mediated degradation of these products (Fig. 2c).
In addition, our results concerning involvement of LTB4 in the regulation of IL-10 and IL-12 release from BM-DCs differ from those reported by Harizi et al.17 BM-DCs used by these authors were reported to release spontaneously large quantities of IL-12 p70 and this release was paradoxically inhibited by LPS, but not affected by LTB4 or a 5-LOX inhibitor/antioxidant (nor-dihydroguarinic acid). Neither LTB4 nor nor-dihydroguarinic acid affected spontaneous or LPS-stimulated IL-10 production in the latter study.17 In our hands, non-stimulated DCs do not release detectable quantities of IL-12 p70, whereas LPS treatment stimulates a low, but detectable, release of IL-12 p70 in BM-DC from BALB/c21 but not CBA mice (the present study, data not shown). Lack of or low production of IL-12 p70 in DCs in response to LPS alone is consistent with other reports (review in 38). LPS alone, as well as OZ, did however, stimulate high levels of IL-12 p40, IL-10 and IL-6 release from DCs. Exogenous LTB4 decreased LPS-stimulated IL-12 p40 release, increased IL-10, and had no effect on IL-6 release. These LTB4 effects were mediated through the BLT1 receptor, as indicated by U75302-exerted antagonism. Moreover, effects of the leukotriene synthesis inhibitor and the selective antagonist of leukotriene BLT1 receptor suggest that endogenous LTB4 participates in autocrine/paracrine regulation of OZ-stimulated, but not LPS-stimulated, cytokine release. This is consistent with the lack of LTB4 production in LPS-stimulated cells.
Exogenous LTD4 had a weaker effect on cytokine release from LPS-stimulated BM-DCs than LTB4, increasing IL-10 release only slightly. Thus, our results reproduce in part the recent report by Machida et al.18 These authors reported that LTD4 increased IL-10 release, whereas antagonists of CysLT receptors increased IL-12 p40 release from murine BM-DCs pulsed with extract of the mite D. farinae. The lack of effect of the CysLT1 antagonist in our study might be caused by the very different culture conditions as well as by the different kinds of stimuli used. In this context it is noteworthy that nonstimulated BM-DCs in the study of Machida et al.18 neither expressed detectable levels of CysLT1 mRNA nor responded to exogenous LTD4, unless they were pulsed with D. farinae.
In contrast to our study and that by Machida et al.18 Okunishi et al.19 suggested in their recent report that CysLTs exert a generalized stimulatory effect on DC activation and functions. Their conclusions were based solely on generalized inhibitory effects of CysLT1 antagonists administered in vivo on assessed ex vivo functions of splenic DCs, including suppression of LPS-stimulated release of both IL-10 and IL-12 p70. One may question, however, the CysLT1-specificity of reported effects.39 For instance, PPARs are known to be activated by different CysLTs antagonists40–42 and to produce a generalized suppressive effect on activation of immune cells.43 Involvement of PPARs, rather than CysLT1, in those in vivo effects of CysLT1 antagonists would be consistent with our finding that two activators of PPARα, 8S-HETE and ETYA, inhibited release of both IL-12 and IL-10 from LPS-stimulated BM-DCs.
In conclusion, we have demonstrated that, unlike previously suggested15,17,34 high-output leukotriene production in murine DCs is triggered by the same stimuli that are effective in other leukotriene-producing cell types.22–25 Moreover, we have found that both endogenous and exogenous LTB4 is capable of modulating IL-10 and IL-12, but not IL-6 release from BM-DCs, acting through BLT1 receptor. Endogenous LTB4 seems partially responsible for higher IL-10 and lower IL-12 p40 production in OZ-stimulated as compared to LPS-stimulated DCs. In similar systems, effects of leukotrienes on cytokine release were smaller than effects of the second major class of eicosanoids – prostaglandins.21 Nevertheless, despite similarly modest effect of CysLTs on IL-10 and IL-12 p40 release from BM-DCs in the study of Machida et al.18 CysLTs-pretreated BM-DCs had a strongly enhanced capacity to initiate T helper 2-type immune responses following adoptive transfer into the lungs. The effects of TGF-β1 pretreatment reported here suggest that leukotrienes may play particularly important role in the biology of Langerhans cells, whose differentiation is driven by TGF-β1.44,45
Acknowledgments
We wish to thank Dr Timothy Sulahian (Harvard School of Public Health, Boston, MA, USA) for editorial assistance. This work was supported by the State Committee for Scientific Research of Poland.
References
- 1.Byrum RS, Goulet JL, Snouwaert JN, Griffiths RJ, Koller BH. Determination of the contribution of cysteinyl leukotrienes and leukotriene B4 in acute inflammatory responses using 5-lipoxygenase- and leukotriene A4 hydrolase-deficient mice. J Immunol. 1999;163:6810–9. [PubMed] [Google Scholar]
- 2.Spanbroek R, Stark H-J, Janßen-Timmen U, et al. 5-Lipoxygenase expression in Langerhans cells of normal human epidermis. Proc Natl Acad Sci USA. 1998;95:663–8. doi: 10.1073/pnas.95.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henderson WR., Jr The role of leukotrienes in inflammation. Ann Intern Med. 1994;121:684–97. doi: 10.7326/0003-4819-121-9-199411010-00010. [DOI] [PubMed] [Google Scholar]
- 4.Hui Y, Funk CD. Cysteinyl leukotriene receptors. Biochem Pharmacol. 2002;64:1549–57. doi: 10.1016/s0006-2952(02)01357-6. [DOI] [PubMed] [Google Scholar]
- 5.Maekawa A, Kanaoka Y, Lam BK, Austen KF. Identification in mice of two isoforms of the cysteinyl leukotriene 1 receptor that result from alternative splicing. Proc Natl Acad Sci USA. 2001;98:2256–61. doi: 10.1073/pnas.041624398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yokomizo T, Kato K, Terawaki K, Izumi T, Shimizu T. A second leukotriene B4 receptor, BLT2: a new therapeutic target in inflammation and immunological disorders. J Exp Med. 2000;192:421–31. doi: 10.1084/jem.192.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yokomizo T, Kato K, Hagiya H, Izumi T, Shimizu T. Hydroxyeicosanoids bind to and activate the low affinity leukotriene B4 receptor, BLT2. J Biol Chem. 2001;276:12454–9. doi: 10.1074/jbc.M011361200. [DOI] [PubMed] [Google Scholar]
- 8.Haribabu B, Verghese MW, Steeber DA, Sellars DD, Bock CB, Snyderman R. Targeted disruption of the leukotriene B4 receptor in mice reveals its role in inflammation and platelet-activating factor-induced anaphylaxis. J Exp Med. 2000;192:433–8. doi: 10.1084/jem.192.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tager AM, Dufour JH, Goodarzi K, Bercury SD, von Andrian UH, Luster AD. BLTR mediates leukotriene B4-induced chemotaxis and adhesion and plays a dominant role in eosinophil accumulation in a murine model of peritonitis. J Exp Med. 2000;192:439–6. doi: 10.1084/jem.192.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devchand PR, Keller H, Peters JM, Vazquez M, Gonzalez FJ, Wahli W. The PPARα-leukotriene B4 pathway to inflammation control. Nature. 1996;384:39–43. doi: 10.1038/384039a0. [DOI] [PubMed] [Google Scholar]
- 11.Goulet JL, Snouwaert JN, Latour AM, Coffman TM, Koller BH. Altered inflammatory responses in leukotriene-deficient mice. Proc Natl Acad Sci USA. 1994;91:12852–6. doi: 10.1073/pnas.91.26.12852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morita H, Takeda K, Yagita H, Okumura K. Immunosuppressive effect of leukotriene B4 receptor antagonist in vitro. Biochem Biophys Res Commun. 1999;264:321–6. doi: 10.1006/bbrc.1999.1523. [DOI] [PubMed] [Google Scholar]
- 13.Spanbroek R, Hildner M, Steinhilber D, Fusenig N, Yoneda K, Rådmark O, Samuelsson B, Habenicht AJ. 5-Lipoxygenase expression in dendritic cells generated from CD34 (+) hematopoietic progenitors and in lymphoid organs. Blood. 2000;96:3857–65. [PubMed] [Google Scholar]
- 14.Spanbroek R, Hildner M, Köhler A, et al. IL-4 determines eicosanoid formation in dendritic cells by down-regulation of 5-lipoxygenase and up-regulation of 15-lipoxygenase 1 expression. Proc Natl Acad Sci USA. 2001;98:5152–7. doi: 10.1073/pnas.091076998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harizi H, Juzan M, Pitard V, Moreau J-F, Gualde N. Prostaglandins inhibit 5-lipoxygenase-activating protein expression and leukotriene B4 production from dendritic cells via an IL-10-dependent mechanism. J Immunol. 2002;169:139–46. doi: 10.4049/jimmunol.170.1.139. [DOI] [PubMed] [Google Scholar]
- 16.Robbiani DF, Finch RA, Jager D, Muller WA, Sartorelli AC, Randolph GJ. The leukotriene C4 transporter MRP1 regulates CCL19 (MIP-3β, ELC)-dependent mobilization of dendritic cells to lymph nodes. Cell. 2000;103:757–68. doi: 10.1016/s0092-8674(00)00179-3. [DOI] [PubMed] [Google Scholar]
- 17.Harizi H, Juzan M, Pitard V, Moreau J-F, Gualde N. Cyclooxygenase-2-issued prostaglandin E2 enhances the production of endogenous IL-10, which down-regulates dendritic cell functions. J Immunol. 2002;168:2255–63. doi: 10.4049/jimmunol.168.5.2255. [DOI] [PubMed] [Google Scholar]
- 18.Machida I, Matsuse H, Kondo Y, et al. Cysteinyl leukotrienes regulate dendritic cell functions in a murine model of asthma. J Immunol. 2004;172:1833–8. doi: 10.4049/jimmunol.172.3.1833. [DOI] [PubMed] [Google Scholar]
- 19.Okunishi K, Dohi M, Nakagome K, Tanaka R, Yamamoto K. A novel role of cysteinyl leukotrienes to promote dendritic cell activation in the antigen-induced immune responses in the lung. J Immunol. 2004;173:6393–402. doi: 10.4049/jimmunol.173.10.6393. [DOI] [PubMed] [Google Scholar]
- 20.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jozefowski S, Bobek M, Marcinkiewicz J. Exogenous but not endogenous prostanoids regulate cytokine secretion from murine bone marrow dendritic cells. EP2, DP, and IP but not EP1, EP3, and FP prostanoid receptors are involved. Int Immunopharmacol. 2003;3:865–78. doi: 10.1016/S1567-5769(03)00072-9. [DOI] [PubMed] [Google Scholar]
- 22.Brown GP, Monick MM, Hunninghake GW. Human alveolar macrophage arachidonic acid metabolism. Am J Physiol. 1988;254:C809–15. doi: 10.1152/ajpcell.1988.254.6.C809. [DOI] [PubMed] [Google Scholar]
- 23.Doerfler ME, Danner RL, Shelhamer JH, Parrillo JE. Bacterial lipopolysaccharides prime human neutrophils for enhanced production of leukotriene B4. J Clin Invest. 1989;83:970–7. doi: 10.1172/JCI113983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wertz O, Klemm J, Samuelsson B, Radmark O. Phorbol ester up-regulates capacities for nuclear translocation and phosphorylation of 5-lipoxygenase in Mono Mac 6 cells and human polymorphonuclear leukocytes. Blood. 2001;97:2487–95. doi: 10.1182/blood.v97.8.2487. [DOI] [PubMed] [Google Scholar]
- 25.Parkar BA, McCormick ME, Foster SJ. Leukotrienes do not regulate interleukin 1 production by activated macrophages. Biochem Biophys Res Commun. 1990;169:422–9. doi: 10.1016/0006-291x(90)90348-q. [DOI] [PubMed] [Google Scholar]
- 26.Chang J, Lamb B, Marinari L, Kreft AF, Lewis AJ. Modulation by hydroxyeicosatetraenoic acids (HETEs) of arachidonic acid metabolism in mouse resident peritoneal macrophages. Eur J Pharmacol. 1985;107:215–22. doi: 10.1016/0014-2999(85)90061-5. [DOI] [PubMed] [Google Scholar]
- 27.Mita H, Yui Y, Shida T. Effect of AA-861, a 5-lipoxygenase inhibitor, on leukotriene synthesis in human polymorphonuclear leukocytes and on cyclooxygenase and 12-lipoxygenase activities in human platelets. Allergy. 1986;41:493–8. doi: 10.1111/j.1398-9995.1986.tb00334.x. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell JA, Akarasereenont P, Thiemermann C, Flower RJ, Vane JR. Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc Natl Acad Sci USA. 1994;90:11693–7. doi: 10.1073/pnas.90.24.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maekawa A, Austen KF, Kanaoka Y. Targeted gene disruption reveals the role of cysteinyl leukotriene 1 receptor in the enhanced vascular permeability of mice undergoing acute inflammatory responses. J Biol Chem. 2002;277:20820–4. doi: 10.1074/jbc.M203163200. [DOI] [PubMed] [Google Scholar]
- 30.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 31.Nikolic T, Bunk M, Drexhage HA, Leenen PJ. Bone marrow precursors of nonobese diabetic mice develop into defective macrophage-like dendritic cells in vitro. J Immunol. 2004;173:4342–51. doi: 10.4049/jimmunol.173.7.4342. [DOI] [PubMed] [Google Scholar]
- 32.Yu K, Bayona W, Kallen CB, Harding HP, Ravera CP, McMahon G, Brown M, Lazar MA. Differential activation of peroxisome proliferator-activated receptors by eicosanoids. J Biol Chem. 1995;270:23975–83. doi: 10.1074/jbc.270.41.23975. [DOI] [PubMed] [Google Scholar]
- 33.Brock TG, McNish RW, Peters-Golden M. Translocation and leukotriene synthetic capacity of nuclear 5-lipoxygenase in rat basophilic leukemia cells and alveolar macrophages. J Biol Chem. 1995;270:21652–8. doi: 10.1074/jbc.270.37.21652. [DOI] [PubMed] [Google Scholar]
- 34.Harizi H, Gualde N. Dendritic cells produce eicosanoids which modulate generation and functions of antigen presenting cells. Prostaglandins Leukot Essent Fatty Acids. 2002;66:459–66. doi: 10.1054/plef.2002.0383. [DOI] [PubMed] [Google Scholar]
- 35.Metz SA, Rice MG, Robertson RP. Applications and limitations of measurement of 15-keto,13,14-dihydro-prostaglandin E2 in human blood by radioimmunoassay. Prostaglandins. 1979;17:839–61. doi: 10.1016/0090-6980(79)90057-1. [DOI] [PubMed] [Google Scholar]
- 36.Harrison KA, Murphy RC. Isoleukotrienes are biologically active free radical products of lipid peroxidation. J Biol Chem. 1995;270:17273–8. doi: 10.1074/jbc.270.29.17273. [DOI] [PubMed] [Google Scholar]
- 37.Patrignani P, Canete-Soler R. Biosynthesis, characterization and inhibition of leukotriene B4 in human whole blood. Prostaglandins. 1987;33:539–51. doi: 10.1016/0090-6980(87)90277-2. [DOI] [PubMed] [Google Scholar]
- 38.Abdi K. IL-12. The role of p40 versus p75. Scand J Immunol. 2002;56:1–11. doi: 10.1046/j.1365-3083.2002.01101.x. [DOI] [PubMed] [Google Scholar]
- 39.Ishinaga H, Takeuchi K, Kishioka C, Suzuki S, Basbaum C, Majima Y. Pranlukast inhibits NF-κB activation and MUC2 gene expression in cultured human epithelial cells. Pharmacology. 2004;73:89–96. doi: 10.1159/000081294. [DOI] [PubMed] [Google Scholar]
- 40.Grossman SJ, DeLuca JG, Zamboni RJ, Keenan KP, Patrick DH, Herold EG, van Zwieten MJ, Zacchei AG. Enantioselective induction of peroxisomal proliferation in CD-1 mice by leukotriene antagonists. Toxicol Appl Pharmacol. 1992;116:217–24. doi: 10.1016/0041-008x(92)90301-8. [DOI] [PubMed] [Google Scholar]
- 41.Kelley M, Groth-Watson A, Knoble D, Kornbrust D. Induction of peroxisomal enzymes by a tetrazole-substituted 2-quinolmethoxy leukotriene D4 antagonist. Fundam Appl Toxicol. 1994;23:298–303. doi: 10.1006/faat.1994.1107. [DOI] [PubMed] [Google Scholar]
- 42.Kojo H, Fukagawa M, Tajima K, Suzuki A, Fujimura T, Aramori I, Hayashi K, Nishimura S. Evaluation of human peroxisome proliferators-activated receptor (PPAR) subtype selectivity of a variety of anti-inflammatory drugs based on a novel assay for PPARδ (β) J Pharmacol Sci. 2003;93:347–55. doi: 10.1254/jphs.93.347. [DOI] [PubMed] [Google Scholar]
- 43.McMillian M, Nie AY, Parker JB, et al. Inverse gene expression patterns for macrophage activating hepatotoxicants and peroxisome proliferators in rat liver. Biochem Pharmacol. 2004;67:2141–65. doi: 10.1016/j.bcp.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 44.Borkowski TA, Latterio JJ, Farr AG, Udey MC. A role for endogenous transforming growth factor β1 in Langerhans cell biology: The skin of transforming growth factor β1 null mice is devoid of epidermal Langerhans cells. J Exp Med. 1996;184:2417–22. doi: 10.1084/jem.184.6.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geissmann F, Prost C, Monnet JP, Dy M, Brousse N, Hermine O. Transforming growth factor β1, in the presence of granulocyte/macrophage colony-stimulating factor and interleukin 4, induces differentiation of human peripheral blood monocytes into dendritic Langerhans cells. J Exp Med. 1998;187:961–6. doi: 10.1084/jem.187.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]