Abstract
Naïve and memory B-lymphocyte populations are activated by CD154 interaction through cell-surface CD40. This interaction plays an important role in the regulation of the humoral immune response, and increasing evidence indicates that fine variation in CD40 binding influences B lymphocytes, macrophages and dendritic cells in murine models. Here we have investigated whether and how variations in the intensity of the CD40–CD154 interaction could contribute to differential regulation of human B-lymphocyte populations. Proliferation and differentiation of B lymphocytes were monitored in response to graded levels of CD40 stimulation in the presence of interleukin (IL)-2, IL-4 and IL-10. Our results show that the level of CD154 binding to CD40 on B lymphocytes can directly influence the evolution of CD19+ CD27– and CD19+ CD27+ cell populations. Furthermore, proliferation, global expansion of CD19+ cells and emergence of CD38++ CD138+ cells, as well as immunoglobulin G (IgG) and IgM secretion, were affected by the level of exposure of B lymphocytes to CD154. These results suggest that the CD40–CD154 interaction is more like a rheostat than an on/off switch, and its variation of intensity may play a role in the regulation of B-lymphocyte activation following the primary and/or secondary humoral immune response.
Keywords: human B lymphocytes, CD27, CD40–CD154, CD138
Introduction
Peripheral B lymphocytes are classified as naïve and memory B lymphocytes, representing about 60 and 40%, respectively, of the total B-cell pool. Previous studies have used membrane immunoglobulin D (IgD) expression to distinguish naïve and memory B lymphocytes, assuming that only IgD– cells are memory B lymphocytes.1,2 The recent finding that the expression of CD27 is restricted to memory B lymphocytes in the periphery3 has enabled these two populations to be distinguished; in fact, we now know that some memory B cells can be IgD positive. Naïve and memory B lymphocytes are both present in lymphoid tissues and, particularly after a primary immune response, in the mantle and marginal zones of lymphoid follicles.4–6 In germinal centres, the memory B-lymphocyte response to antigen (Ag) is faster than the naïve cell response, because of the intrinsic characteristics of memory B lymphocytes,7–10 their higher affinity for Ag epitopes11 and the presence of cytokines, follicular dendritic cells and memory T lymphocytes in the follicular microenvironment.6,11–13 Thus, memory B lymphocytes could have the capacity to populate niches faster than naïve B lymphocytes, as described for several immunoregulatory mechanisms.12,14,15
The binding of CD40 to CD154 expressed on activated T lymphocytes plays a central role in B-lymphocyte activation (reviewed in references 16 and 17). Naïve and memory T lymphocytes, which are believed to interact with their respective B-lymphocyte counterparts,18,19 differentially respond to activation. Following antigenic challenge, both T-lymphocyte subpopulations express high levels of CD154 within hours,20,21 but only memory T lymphocytes have preformed CD154 molecules, which can be targeted to the cell membrane within 5–15 min after cellular activation. These observations suggest that stimulation of B lymphocytes through CD40 binding could depend on the maturation status of the interacting T lymphocytes.
We previously reported that strong CD154 stimulation in the presence of interleukin (IL)-4 alone could result in distinct responses of naïve and memory B lymphocytes. In fact, naïve B lymphocytes were able to proliferate and further differentiate to secrete immunoglobulins, while memory cells were only able to differentiate. This result contrasted with the findings of other studies showing that memory (IgD– or CD27+ cells) B lymphocytes were able to proliferate following CD40 stimulation in the presence of IL-4. As these groups, including ours, used a single level of CD154 stimulation, we hypothesized that these different sources of CD154 could result in variation of CD40 stimulation and thus differences in B lymphocyte outcome. Indeed, various forms of CD40 ligand used for in vitro stimulation of B lymphocytes, such as membrane-associated CD154, soluble trimeric, dimeric or monomeric CD154 proteins or anti-CD40 antibodies, produce distinct functional responses (reviewed in reference 22). Even anti-CD40 monoclonal antibodies targeting different epitopes on CD40 molecules may give rise to distinct responses.23,24 The extent of CD40 binding using anti-CD40 or membrane-associated CD154 affects the cellular response of human hybridomas as well as murine B lymphocytes and B cell lines.25–28 Finally, it was recently reported that differences in the intensity of the CD40–CD154 interaction lead to functional differences in dendritic cells and macrophages.29,30 Collectively, these studies suggest that variation in CD40 binding could result in important differences in B-lymphocyte outcome.
We have therefore investigated whether fine variations in the CD154 interaction intensity could further influence the responses and homeostasis of B-lymphocyte subpopulations.
First, we found that a low level of CD40 stimulation allowed the maintenance of a relatively high proportion of CD27+ B lymphocytes. However, increasing CD154 binding exerted negative effects on CD27+ B-lymphocyte populations, while positively influencing CD27– cell populations. We also found that low CD40 stimulation increased IgG secretion by up to 5-fold, and led to a reversion of the IgG/IgM secretion ratio. In addition, a high proportion of B lymphocytes (up to 20%) differentiated towards plasma cells, as demonstrated by coexpression of CD138 and CD38, when less than 5000 CD154 molecules were available per B cell. Finally, we report that the density of CD154 expressed on the CD154+ cell lines could also influence the balance between CD27+ and CD27– B-lymphocyte populations. Overall, our results suggest that fine-tuning of the CD40–CD154 interaction could be a significant parameter in the regulation of B-lymphocyte physiology during the primary and secondary humoral immune responses.
Materials and methods
Isolation of human peripheral B lymphocytes
Blood samples were collected from healthy individuals, after informed consent had been obtained, in heparinized tubes (Vacutainer; BD Labware, Franklin Lakes, NJ), pooled and diluted with 1 volume of phosphate-buffered saline (PBS) (10 mm potassium/sodium phosphate buffer, 136 mm NaCl, pH 7.4, and Dulbecco's PBS; Invitrogen, Grand Island, NY). Peripheral blood mononuclear cells (PBMCs) were prepared by density centrifugation over Ficoll-Paque (Amersham Biosciences, Baie D'Urfé, Canada). B lymphocytes were purified by negative selection using the StemSep CD19 mixture according to the manufacturer's instructions (Stem Cell Technologies, Vancouver, Canada). Purified human B lymphocytes were >95% CD19+, as determined by flow cytometry.
Preparation of L4.5high and L4.5low cells and quantification of CD154 molecules per cell
L4.5, a genetically modified L929 cell line (CCL-1; American Type Culture Collection, Manassas, VA) expressing CD154,31 shows heterogeneous levels of CD154 expression.32 We have therefore used a phycoerythrin (PE)-conjugated hamster anti-mouse CD154 antibody (Ab) (Serotec, Oxford, UK) and a FACScalibur cytometer equipped with a low-speed sorter (BD Biosciences, Mountain View, CA) to sort two populations, respectively, expressing high and low levels of CD154. The numbers of CD154 molecules expressed on the parental L4.5 cell line (21 000 ± 20%) and the sorted L4.5high (47 000 ± 8%) and L4.5low (9000 ± 10%) populations were estimated using PE anti-CD154 and the QuantiBRITETM system, according to the manufacturer's instructions (BD Biosciences). For the CD154 dose–response experiments, the estimated number of CD154 molecules per L4.5high and L4.5low cell enabled to obtain 1000, 2000, 5000, 10000, and 25000 CD154 molecules per B lymphocyte. The expression of CD154 by L4.5high and L4.5low cells was found to be stable (± 15%) for the duration of the experiments described herein. All L4.5 cell lines were mycoplasma-free, as determined with the Mycotect kit (Invitrogen).
Culture of human B lymphocytes
Purified B lymphocytes (3.75 × 105 cells/ml) were seeded in Primaria plates (BD Labware) in the presence of L4.5 cells that had been gamma-irradiated with 75 Gy (7500 rad). The specificity of the response induced by L4.5 has been previously confirmed, as mock-transfected L929 cells are unable to activate B lymphocytes in the presence of cytokines. Gamma-irradiated L929, L4.5, L4.5high and L4.5low cells were seeded at a density ranging from 0.15 to 0.75 × 105 cells/cm2. Human B lymphocytes were cultured in Iscove's modified Dulbecco's medium (IMDM) supplemented with 10% heat-inactivated ultra-low IgG fetal calf serum (FCS), 10 µg/ml of insulin, 5.5 µg/ml of transferrin, 6.7 ng/ml of sodium selenite, antibiotics (all from Invitrogen), and 50 U/ml of IL-2, 25 U/ml of IL-10 (PeproTech, Rocky Hill, NJ) and/or 100 U/ml of IL-4 (R & D Systems, Minneapolis, MN). Cultures were fed by replacing half of the culture medium every 2–3 days, while irradiated L929 and/or L4.5 cells were renewed every 4–5 days. The ratios of L4.5 cells, as well as the numbers of CD154 molecules per B lymphocyte, were kept constant throughout the culture period. Cell counts and viability were evaluated in triplicate by Trypan blue dye exclusion. Cultured B lymphocytes were always >96% CD19+, and, unless specified otherwise, viability was >90%.
Flow cytometry analysis
Allophycocyanin-, peridinin chlorophyll protein (PerCP)-cyanin 5.5- or fluorescein isothiocyanate (FITC)-conjugated anti-CD19, PE-conjugated anti-CD27, allophycocyanin-conjugated anti-CD38, PE-conjugated anti-CD138, FITC-conjugated anti-IgG, and allophycocyanin-, PerCP-cyanin 5.5-, PE- and FITC-conjugated isotype controls were used in double, triple or quadruple staining procedures. All Abs were IgG1 mouse mAbs obtained from BD Biosciences. All staining was carried out using 1 µg of each Ab for 1 × 106 cells at 4°. Cells were fixed with 2% paraformaldehyde. In all analyses, >95% of the cells were double-negative when using isotype-matched control Abs. Regions containing dead cells were delineated using 7-amino-actinomycin D staining, following the manufacturer's instructions (BD Biosciences). Analyses were performed by gating 10 000 cells, with a FACSCalibur Flow cytometer and the CellQuest Pro software (BD Biosciences).
Quantification of IgG and IgM secretion
IgG and IgM concentrations were determined by a standard enzyme-linked immunosorbent assay (ELISA) using 96-well plates and plastic-adsorbed affinity-purified goat Abs specific to human γ- and µ-chains, respectively. Captured IgG and IgM were revealed with peroxidase-conjugated goat anti-human immunoglobulin Abs. All Abs were from Jackson Laboratories (Mississauga, ON, Canada). Tetramethylbenzidine (Scy Tek, Logan, UT) was used as substrate and optical densities (ODs) were measured at 450 nm, with reference at 630 nm.
Western blot analysis of tyrosine phosphorylation patterns
B lymphocytes were stimulated for either 3 or 72 hr in the presence of IL-2, IL-4 and IL-10, and either L4.5low cells (1000 and 5000 CD154 molecules per B lymphocyte) or L4.5high cells (25 000 CD154 molecules per B lymphocyte). B-cell pellets were prepared using CD19 Dynabeads (Dynal Biotech, Lake Success, NY). CD154–CD40-induced tyrosine phosphorylation was evaluated as described elsewhere.33 Briefly, protein extracts were prepared by incubating cell pellets at 4° in lysis buffer containing 20 mm Tris-HCl, pH 8.0, 137 mm NaCl, 10%[weight/volume (w/v)] glycerol, 1% (w/v) phenylmethylsulphonyl fluoride (PMSF), 2 µm pepstatin A, 4 µg/ml of aprotinin and 1 mm sodium orthovanadate.33,34 Protein content was determined using Bio-Rad Protein Assay (Bio-Rad Laboratories, Mississauga, Canada), and 20 µg was subjected to sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) on a 10% polyacrylamide gel. Prestained SDS-PAGE standards (Bio-Rad Laboratories), ranging from 20 to 206 kDa, were run in parallel. The gel was transferred onto a Hybond ECL nitrocellulose membrane (Amersham Biosciences). Tyrosine-phosphorylated proteins were probed using a mouse 4G10 anti-phosphotyrosine mAb (Upstate Biotechnology, Charlottesville, VA). A peroxidase-conjugated goat anti-mouse IgG (Jackson Laboratories) was used as a secondary reagent. The blot was revealed using the enhanced chemiluminescence (ECL) detection system, according to the manufacturer's instructions (Amersham Biosciences). As a loading control experiment, the same blot was subsequently stripped using Restore Western Blot stripping buffer (Pierce Biotechnology, Rockford, IL), and then probed with a polyclonal rabbit anti-actin Abs (Sigma Canada, Oakville, ON, Canada) whose binding was revealed with peroxidase-conjugated goat anti-rabbit IgG and the ECL system, as described above.
Results
The number of CD154-expressing L4.5 cells exerts differential effects on B-lymphocyte populations
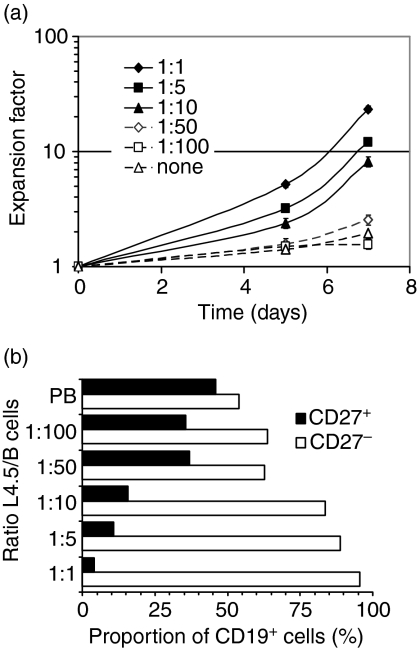
We previously reported that CD40 stimulation by CD154-expressing L4.5 cells enhances naïve B-lymphocyte proliferation and long-term differentiation, whereas memory B lymphocytes undergo differentiation and die within 7 days.35 We relied upon the same in vitro system to investigate the effect of variations in the number of CD154+ cells on B-lymphocyte growth and differentiation. One, five, 10, 50 and 100 B lymphocytes were seeded per L4.5 cell in the presence of IL-4, and cell growth was monitored for 7 days (Fig. 1). B-lymphocyte seeding was identical and kept constant in all five conditions; therefore, the number of L4.5 cells was adjusted to obtain the desired ratios. B lymphocytes stimulated in the presence of IL-4 alone, without CD154-expressing cells, did not expand and died within the first few days of culture (Fig. 1a, ‘none’). We observed that, 24 hr post-seeding, up to 30 B lymphocytes were able to interact with a single L4.5 cell (data not shown). Thus, the seeding of 50 and 100 B lymphocytes per L4.5 cell probably corresponded to oversaturation in our assay conditions.
Figure 1.
A low level of CD154 stimulation allowed maintenance of memory B lymphocytes. CD19-purified human peripheral blood B lymphocytes (3.75 × 105 cells/ml) containing 40% CD27+ cells were stimulated in the presence of variable numbers of gamma-irradiated CD154+ L4.5 cells in complete medium containing 100 U/ml interleukin (IL)-4 for 7 days. Ratios corresponding to 1–100 B lymphocytes per L4.5 cell were obtained by varying the number of L4.5 cells while keeping constant the seeding number of B lymphocytes. (a) Expansion factors were evaluated using viable cell counts performed in triplicate at the indicated times. Error bars may be smaller than the symbols. (b) On day 5, the proportions of CD19+ CD27+ and CD19+ CD27– cells were determined by flow cytometry, using phycoerythrin (PE)-conjugated anti-CD27 and peridinin chlorophyll protein (PerCP)-cyanin 5.5-conjugated anti-CD19 antibodies. PB, phenotype of peripheral blood B cells. Viable cells were ≥ 95% CD19+ throughout the 7 days of stimulation. These results are representative of two independent experiments.
Expansion of CD19+ cells was indirectly proportional to the ratio of B lymphocytes to L4.5 cells (Fig. 1a). No expansion occurred when >10 B lymphocytes were seeded per L4.5 cell, and, in these conditions, viability showed a marked decline on day 14 (∼ 70%; data not shown). However, decreasing the CD154 interaction from 1 to 10 B cells per L4.5 cell led to a proportional decline in B lymphocyte proliferation, although L4.5 cells are large enough to bind up to 30 B cells.
On day 5, phenotypic analysis of cells showed that the proportions of CD27– and CD27+ B-lymphocyte populations were influenced by the numbers of CD154-expressing L4.5 cells (Fig. 1b). Ratios of 1, 5 and 10 B lymphocytes per L4.5 cell favour CD27– B lymphocytes, which represented 96, 89 and 84% of the total population of CD19+ cells, respectively. In contrast, when >10 B lymphocytes were seeded per L4.5, the proportions of CD27– and CD27+ B lymphocytes were both maintained at levels similar to those found in the starting initial peripheral blood populations: approximately 60% naïve B lymphocytes and 40% memory cells (Fig. 1b). This may be a result of the combined effects of increased cell death and low proliferation, as in these conditions less than 200–500 CD154 molecules are available per B cell, as shown in Table 1. Ig secretion was similarly low after 7 days of stimulation for all conditions tested (1 : 1 to 1 : 100), yielding 14 ± 5 ng/ml for IgM and 56 ± 13 ng/ml for IgG (data not shown).
Table 1.
The number of CD154-expressing cells influenced B-lymphocyte differentiation and proliferation
| Proportion of positive cells3 (%) | Ig secretion4(ng/106 cells/hr; mean ± SD) | |||||||
|---|---|---|---|---|---|---|---|---|
| L4.5:B cell ratio1 | Number of CD154/B cell1 | Expansion of CD19+ cells2(× 106) | Viability2(%) | CD27+ | CD38+ | IgG | IgM | IgG:IgM ratio |
| 1 : 2 | 8000–13000 | 333 | 96 | 5.7 | 1.2 | 34 ± 2 | 47 ± 3 | 0.72 |
| 1 : 5 | 3000–5000 | 839 | 96 | 21.2 | 9.2 | 62 ± 8 | 26 ± 2 | 2.4 |
| 1 : 10 | 1700–3000 | 634 | 95 | 25.9 | 14.3 | 70 ± 6 | 12 ± 1 | 5.8 |
| 1 : 25 | 700–1000 | 189 | 89 | 31.3 | 31.2 | 181 ± 36 | 16 ± 1 | 11.3 |
| 1 : 50 | 200–500 | 56 | 82 | 35.4 | 34.6 | 223 ± 8 | 25 ± 2 | 8.9 |
| 1 : 100 | <250 | 4 | 75 | 38.7 | 43.4 | – | – | – |
These results are representative of two independent experiments. Ig, immunoglobulin; SD, standard deviation.
CD19-purified human peripheral B lymphocytes containing 31% CD27+ cells (375 000 cells/ml) were stimulated in the presence of variable numbers of CD154+ L4.5 cells and in the presence of interleukin (IL)-2, IL-4 and IL-10. Ranges of CD154 molecules shown are based on an average of 21 000 ± 25% molecules per L4.5 cell. Parental L929 cells were added as needed to adjust the total number of L cells (L4.5 + L929) to 500 000 cells/well.
Viability and B-cell expansion were evaluated on day 14.
The percentage of CD19+ CD27+ (CD27+) and CD19+ CD27++ CD38+ (CD38+) cells were determined on day 9.
IgG and IgM secretion from day 14–15 was measured by enzyme-linked immunosorbent assay (ELISA).
Similar results were obtained when L929 parental cells (CD154–) were added to keep the number of L cells per well (5 × 105) constant. These results suggest that CD27– and CD27+ B lymphocytes are differentially sensitive to CD154 stimulation in conditions of low numbers of B lymphocytes (1, 5 or 10) per L4.5 cell. Although the addition of IL-4 by itself seemed insufficient to induce global expansion of B lymphocytes, lowering the level of CD154 stimulation allowed at least maintenance of a higher proportion of CD27+ B lymphocytes.
The number of CD154+ cells influences B-lymphocyte expansion and differentiation
The loss of CD27+ B lymphocytes in the presence of IL-4 alone indicates that other cytokines are needed to maintain this population in the culture. Some studies have reported that IL-10 alone or in combination with IL-2 or IL-4 allows the proliferation of memory B lymphocytes.8,36,37 We have previously observed that addition of IL-10 alone or in the presence of IL-235 or IL-10 and IL-4 to high levels of CD40 stimulation is not favourable to expansion of CD27+ B cells (unpublished observations). We have recently reported that a mixture of IL-2, IL-4 and IL-10 allows proliferation and differentiation of sorted CD27– naïve and CD27+ memory B lymphocytes.38 A combination of IL-2, IL-4 and IL-10 was therefore used to examine the influence of the number of L4.5 cells (CD154+ cells) on B-lymphocyte expansion and differentiation (Table 1). As in experiments reported in Fig. 1, the number of L4.5 cells ranged from 5000 to 500 000 cells/well. Therefore, L929 parental cells (CD154–) were seeded so as to keep a constant number of 500 000 L cells/well. In these conditions, any observed differences between the conditions assayed would be related to the presence of variable numbers of CD154-expressing cells, rather than non-specific interactions between B lymphocytes and variable numbers of L cells. As observed in previous experiments (Fig. 1), expansion of CD19+ cells was directly related to the number of L4.5 cells, and the proportion of CD19+ CD27++ CD38+ cells was higher when the numbers of L4.5 cells were relatively low. Increased expression of CD38 on these cells also indicated their commitment to differentiation.39 Furthermore, in these conditions, IgG secretion was increased, while IgM secretion was reduced, as shown by reversal of the IgG/IgM ratio (Table 1). We therefore conclude that the number of CD154+ L4.5 cells directly influences B-lymphocyte proliferation and differentiation in the presence of IL-2, IL-4 and IL-10.
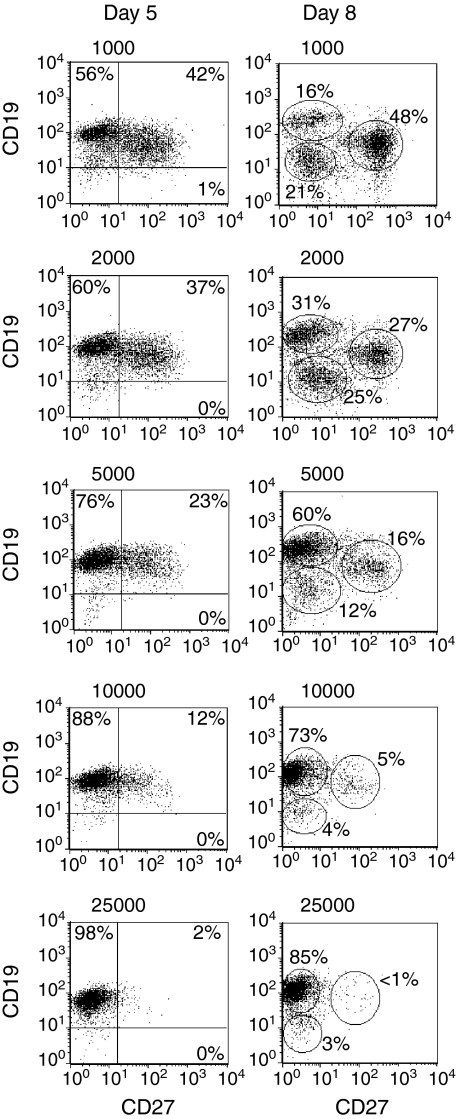
B-lymphocyte expansion and differentiation are influenced by the number of CD154 molecules
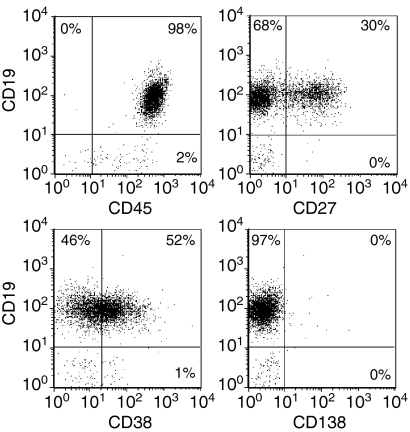
As shown in Fig. 2, the phenotype of our CD19+ purified B lymphocytes is typical of that of peripheral B lymphocytes.40–42 This purified population, representative of all our experimental samples, was used in subsequent experiments measuring the effect of the amount of CD154 on B-lymphocyte proliferation and differentiation. Sorted L4.5high and L4.5low cell populations were used to obtain a range of 1000–25 000 CD154 molecules per B lymphocyte stimulated in the presence of IL-2, IL-4 and IL-10 (Fig. 3 and Table 2). To obtain the desired number of CD154 molecules per B cell, ratios of 2, 5 or 10 B lymphocytes per L4.5high or L4.5low cell were used. The range was equivalent to 50 000–150 000 L4.5 cells/well, without additional L929 cells, as the number of CD154– cells does not have any effect on B lymphocytes (Fig. 1 and Table 1). As observed in Fig. 1 and Table 1, B-lymphocyte expansion and differentiation and Ig secretion were all influenced by the number of CD154 molecules available per B lymphocyte, but not by the relative number of L4.5 cells. On day 8, phenotypic analysis of the resulting cells revealed three distinct populations, namely CD19+ CD27–, CD19++ CD27– and CD19+ CD27++ (Fig. 3; ellipsoid regions). The CD19+ CD27– and CD19+CD27++ populations were also characterized by re-expression of CD38 (Table 2). On days 5 and 8, the relative proportion of CD19+ CD27++ cells was inversely related to the number of CD154 molecules to which B lymphocytes were exposed. We also determined the proportion of cells expressing CD138, a plasma cell marker.43 On day 13, in the presence of <5000 CD154 molecules per B lymphocyte, up to 20% of cells were CD19+ CD38++ CD138+, whereas in all other conditions this population represented <3% of the cells (Table 2). Finally, as observed above, when variable numbers of L4.5 cells were seeded (Table 1), IgG secretion and IgM secretion were reciprocally related to the number of CD154 molecules per B lymphocyte. This increase in IgG secretion and decrease in IgM secretion as CD40 stimulation is reduced suggests that class switch recombination is increased in B lymphocytes. These results further indicate that, in the presence of IL-2, IL-4 and IL-10, the amount of CD154 molecules per B lymphocyte can directly influence B-lymphocyte proliferation and differentiation.
Figure 2.
Purified B lymphocytes showing normal peripheral phenotypes. CD19-purified B lymphocytes were analysed for CD19, CD27, CD38 and CD138 expression following negative selection, as described in the Materials and methods. These results are representative of more than three independent experiments.
Figure 3.
The number of CD154 molecules per B lymphocyte influenced the evolution of CD19+ CD27– and CD19+ CD27+ populations. CD19-purified B lymphocytes were stimulated in the presence of cytokines, and irradiated L4.5low or L4.5high cells were adjusted to obtain 1000, 2000, 5000 (L4.5low), 10 000 and 25 000 (L4.5high) CD154 molecules per B lymphocyte. In all cases, the number of B cells varied from 2 to 10 per L4.5 cell (L4.5high or L4.5low). The expression of CD19 and CD27 was determined on days 5 and 8 as described in Fig. 1. On day 8, ellipsoid regions highlight three distinct populations, namely CD19++ CD27–, CD19low CD27– and CD19+ CD27++. For day 5 data, numbers indicate the percentages of cells in the indicated quadrants, whereas for day 8 data, they refer to the percentages of cells in each region. These results are representative of three independent experiments.
Table 2.
Expansion and differentiation of B lymphocytes are inversely related to the number of CD154 molecules per B lymphocyte.
| Proportion of positive cells (%)3 | Ig secretion (ng/106 cells/hr; mean ± SD)4 | ||||||
|---|---|---|---|---|---|---|---|
| Number of CD154 molecules/B cell1 | Expansion of CD19+ cells2(× 106 cells) | CD19+CD38+ | CD27++CD38+ | CD38++CD138+ | IgG | IgM | IgG:IgM |
| 1000 | 65 | 75 | 54 | 20 | 342 ± 48 | 12 ± 0.1 | 28 |
| 2000 | 267 | 57 | 32 | 8 | 178 ± 22 | 9 ± 0.1 | 20 |
| 5000 | 526 | 20 | 15 | 3 | 99 ± 6 | 13 ± 1 | 8 |
| 10000 | 686 | 3 | 2 | 1 | 50 ± 6 | 14 ± 1 | 4 |
| 25000 | 328 | < 1 | < 1 | 1 | 82 ± 6 | 90 ± 6 | 1 |
These results are representative of three independent experiments. Ig, immunoglobulin; SD, standard deviation.
CD19-purified peripheral B lymphocytes containing 30% CD27+ cells (375 000 cells/ml) were stimulated in the presence of interleukin (IL)-2, IL-4 and IL-10, along with CD154+ L4.5 cells adjusted so as to obtain the indicated numbers of CD154 molecules per B cell. L4.5low cells were used for 1000, 2000 and 5000 and L4.5high for 10 000 and 25 000 CD154 molecules per B cell.
B-cell expansion was evaluated on day 14.
The percentages of CD19+ CD38+ and CD27++ CD38+ cells were determined on day 8, and the percentage of CD38++ CD138+ cells was determined on day 13.
IgG and IgM secretion from day 14–15 was measured by enzyme-linked immunosorbent assay (ELISA).
B-lymphocyte populations are influenced by CD154 density
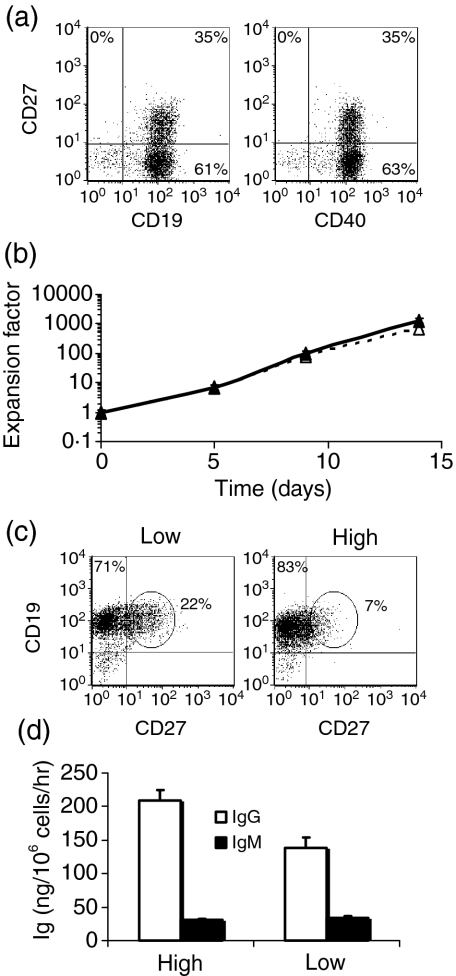
We sought to determine whether the 5-fold difference in the density of CD154 expression on sorted L4.5high and L4.5low cells could further modulate B-lymphocyte expansion and differentiation. To this end, we determined and tested the respective numbers of L4.5high and L4.5low cells needed to have ∼5000 CD154 molecules per B lymphocyte (Fig. 4), while B lymphocyte seeding was maintained at a constant level. Purified B lymphocytes were analysed for their expression of CD19, CD27 and CD40 (Fig. 4a). CD40 expression was equivalent in both CD27+ and CD27– populations. Expansion was similar for both conditions using L4.5high and L4.5low cells (Fig. 4b). Conversely, the proportion of CD19+ CD27++ (Fig. 4c, ellipsoid regions) was 4-fold higher when L4.5low cells were used (Fig. 4c). Finally, IgM secretion was unaltered by CD154 density, whereas IgG secretion was 1.3- to 2-fold higher in the presence of L4.5high cells (Fig. 4d). However, we observed no significant differences when L4.5high and L4.5low cell densities were adjusted to give 1000 or 2000 CD154 molecules per B lymphocyte (data not shown). Given that the number of CD154 molecules was equivalent in the two conditions, these results suggest that not only the number but also the density of CD154 expressed on L4.5 cells can influence, albeit to a lesser extent, B-lymphocyte expansion and differentiation, and Ig secretion.
Figure 4.
The density of CD154 expression on L4.5 cells influenced B lymphocytes. CD19-purified peripheral blood human B lymphocytes, containing 35% CD27+, were stimulated in the presence of cytokines and either irradiated L4.5high (High) or irradiated L4.5low (Low) cells. In both cases, the number of L4.5 cells (L4.5high or L4.5low) was adjusted to obtain ∼5000 CD154 molecules per B lymphocyte, yielding ratios of 10 and 2 B cells per L4.5high and L4.5low cell, respectively. Expansion, expression of CD19 and CD27, and immunoglobulin G (IgG) and IgM secretion were determined as described in Figs 1 and 3 and Table 1. (a) Levels of CD19, CD27 and CD40 expression were determined on purified B lymphocytes. (b) The expansion of B lymphocytes in the presence of L4.5low (dashed lines) or L4.5high (solid lines) cells is shown. Error bars for triplicate experiments may be smaller than the symbols. (c) CD19 and CD27 expression on day 5, as determined by flow cytometry. (d) IgG and IgM secretion, as determined on day 14. These results are representative of three independent experiments.
Increased CD154 stimulation enhances tyrosine phosphorylation
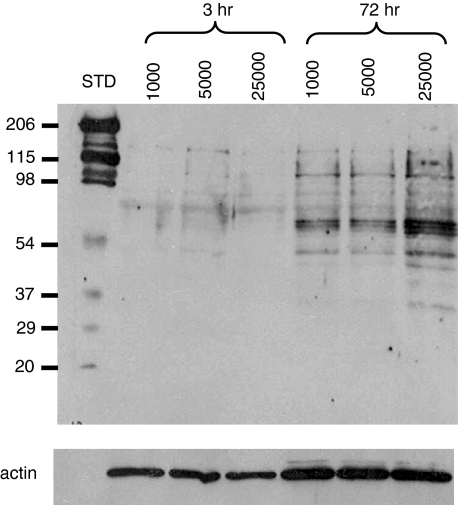
The binding of CD40 induces phosphorylation of several substrates by tyrosine kinases (reviewed in 15), such as the Janus kinase (JAK), Ras/extracellular signal-regulated kinase (Ras/ERK) or phosphatidylinositol 3-kinase (PI3K) pathways. To test whether the number of CD154 molecules per B lymphocyte alters signal transduction, we analysed phosphotyrosine proteins in B lymphocytes stimulated for either 3 or 72 hr in the presence of 1000, 5000 and 25 000 CD154 molecules per B cell (Fig. 5). After 3 hr, no differences were observed amongst the three CD154 intensities. However, after 72 hr, the overall level of tyrosine phosphorylation was clearly higher when B lymphocytes were exposed to 25 000 CD154 molecules per B cell. These results support the notion that increasing the number of CD154 molecules results in enhanced CD40 stimulation, which in turn can up-regulate directly or indirectly intracellular tyrosine phosphorylation and eventually result in variations in B-lymphocyte activation.
Figure 5.
An increased level of CD154 stimulation can yield higher tyrosine phosphorylation. CD19+ B lymphocytes, containing 42% CD27+ cells, were stimulated as described in Fig. 3 with 1000, 5000 and 25 000 CD154 molecules per B lymphocyte. After 3 and 72 hr, B lymphocytes were selected using CD19-positive selection with magnetic beads, protein extracts were prepared, and tyrosine phosphorylation patterns were determined using 4G10 antiphosphotyrosine monoclonal antibodies. A peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) was used as the secondary reagent, as described in Materials and methods. The blot was stripped and re-probed with an anti-actin antibody. These results are representative of two independent experiments. STD, molecular weight protein standard.
Discussion
CD40 stimulation in the presence of IL-4 has been shown to efficiently stimulate human B lymphocytes to proliferate and differentiate. In contrast, the specific responses of naïve and memory B lymphocytes to CD40 stimulation were found to vary amongst studies. In addition, we also observed that naïve and memory B lymphocytes can be differentially activated following a strong and persistent CD40 stimulation by CD154+ cells in the presence of IL-4.35 The results presented here may reconcile these observations, as it was found that B-lymphocyte populations could indeed differentially respond to fine variation in CD154 interaction in the presence of IL-4 alone or in combination with IL-2 and IL-10.
Overall, we observed that important changes in proliferation, Ig secretion, and evolution of B-lymphocyte populations, such as CD19+ CD27++ CD38++ and CD19+ CD38++ CD138+ cells, gradually occurred as the number of CD154 molecules per B lymphocyte was modulated over a 25-fold range (Figs 1 and 2 and Tables 1 and 2). Notably, the maintenance of CD27+ B-lymphocyte populations was favoured when relatively low levels and/or low densities of CD154 were available per B lymphocyte. However, both CD27+ and CD27– B lymphocytes were maintained in these limiting conditions, indicating that this low level of stimulation could be minimally sufficient to support cell viability for a few days in the presence of cytokines.
The possibility that L929 cells could influence B lymphocytes44 was tested by varying the number of L4.5 cells from very high ratios (1 B cell per L4.5 cell) to very low ratios (100 B cells per L4.5 cell). We did not observe any effects that could be attributed to non-specific factors provided by L cells, indicating that variations in proliferation and differentiation solely related to the number of CD154 molecules.
Although all B-lymphocyte populations were exposed to identical sources and levels of CD40 binding for identical times, CD27+ and CD27– population responses were sensitive to CD154 gradients. In the light of our previous observations, we used CD27 expression to distinguish B-lymphocyte subpopulations after short-term stimulation (≤ 5 days), as a fraction of naïve B lymphocytes (30%) can express low levels of CD27 only after long-term stimulation (≥ 9 days).35 Although the utilization of CD27 as a marker for memory B cells is still accepted,10 we cannot exclude the possibility that naïve and memory B lymphocytes might be able to, respectively, acquire or modulate CD27 expression.45 Therefore, if we consider that CD27 expression is not absolutely specific to memory B lymphocytes in this system, it is clear that the evolution of the CD27+ and CD27– cell populations was different between days 5 and 14, suggesting that we are dealing with distinct evolution of B lymphocyte subsets. In addition, although the global amount of Ig secretion was similar despite a 10-fold difference in the level of CD40 stimulation, the highest ratio of IgG to IgM secretion following low CD40 stimulation suggests that class switch recombination was increased among B lymphocytes, as previously observed,35 and thus further suggests increased differentiation within these populations.
Several studies have reported that CD40 exhibits an unexpected level of complexity, as exemplified by the expression of distinct isoforms,24,46 modulation of the expression kinetics47 and the formation of CD40/CD40 homodimers.48 However, in the periphery, all B-lymphocyte populations express similar levels of CD40 on their surface, suggesting that naïve and memory B lymphocytes could possibly diverge with respect to CD40 expression as activation proceeds. Whether naïve and memory B lymphocytes differ with regard to CD40 expression and activity remains to be investigated.
The observed differential responses of naïve and memory B lymphocytes to graded levels of CD154 could also be relevant to homotypic interaction among these B-cell populations or to competition for stimulatory molecules.49 We are currently investigating whether cytokines or B cell–B cell interactions, such as between CD27 and CD70,50 could be involved in homeostatic fluctuations following high and low CD40 stimulation. Competition involves the intrinsic characteristics of the cells, as reported for cytokines, IgG and rapid cycling.7,8,51 Cells capable of preferentially responding to either high or low levels of CD154, expressed on T lymphocytes or, as in our model, by L4.5 cells, could be favoured if there is competition. Therefore, some intrinsic characteristics linked to CD40 threshold activation could come into play during the cellular response to immune stimulation. Indeed, it has been reported that the density of CD154 on activated T cells influences Ig secretion and CD38 expression on human B lymphocytes.52 The main conclusion of the latter study is that complex bidirectional interactions between B and T lymphocytes is related to signal intensities from CD40 and CD154, which are dependent on both B and T lymphocyte activation status.52 In the present work, we have focused on a single axis of this interaction. Nevertheless, we were able to observe striking effects on B-lymphocyte proliferation, differentiation and Ig secretion.
Reports that variations in the intensity of CD40 stimulation influence Ig secretion and proliferation of human hybridomas25 and murine cell lines27 have been published. These studies relied upon antibodies specific to CD40 or soluble membrane fractions expressing CD154. High numbers of CD154 molecules were shown to inhibit the generation of plasma cells and IgG secretion of tonsil IgD– CD38– memory B lymphocytes without affecting IgD+ CD38– cells37 which are now known to include CD27+ cells. The design of this study differs markedly from ours, as these observations were made using two-step cultures where the cells were first exposed to high levels of CD154 stimulation, followed by a range of ∼104 to 2 × 105 CD154 molecules per B cell. Our results extend these findings by demonstrating that fine variations in the intensity of the CD40–CD154 interaction (103 to 2.5 × 104 molecules per B cell), at relatively low levels, can strongly and directly influence peripheral B-lymphocyte proliferation, Ig secretion, and differentiation towards plasma cells.
CD40 binding strength has been recently linked to the activation of distinct signalling pathways in murine macrophages and dendritic cells.29,30 These two studies reported that strong CD40 stimulation induced p38MAPK phosphorylation, whereas weak stimulation resulted in ERK1/2 phosphorylation. Interestingly, increased concentrations of CD40-specific antibodies, FcCD40L fusion protein or soluble CD40L were able to induce p38MAPK phosphorylation, whereas lower concentrations induced phosphorylation of ERK1/2.29 These results suggest that the CD40–CD154 interaction could act as a rheostat operating as a two-way switch on the respective signalling pathways. These reports29,30 and the results described herein suggest that the signalling pathways activated in response to strong or weak CD40 stimulation could target distinct B-lymphocyte populations, resulting in distinct overall responses.
Acknowledgments
We thank Nicolas Dupuis, Ing. and Drs André Darveau and Walid Mourad for inspiring discussion and helpful suggestions. We also thank Marguerite Massinga Loembé and Jessie F. Fecteau for assistance with tyrosine phosphorylation assays and Nathalie Dussault for B-cell culture. We are grateful to Dr Maryse St-Louis for her constructive comments on the manuscript and to Jean-François Leblanc for editing. Finally, we are grateful to all participants in this study and to Claudine Côté for the co-ordination of blood sample collection.
References
- 1.Black SJ, van der Loo W, Loken MR, Herzenberg LA. Expression of IgD by murine lymphocytes. Loss of surface IgD indicates maturation of memory B cells. J Exp Med. 1978;147:984–96. doi: 10.1084/jem.147.4.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayakawa K, Ishii R, Yamasaki K, Kishimoto T, Hardy RR. Isolation of high-affinity memory B cells: phycoerythrin as a probe for antigen-binding cells. Proc Natl Acad Sci USA. 1987;84:1379–82. doi: 10.1073/pnas.84.5.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig) M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188:1679–89. doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sagaert X, De Wolf-Peeters C. Classification of B-cells according to their differentiation status, their micro-anatomical localisation and their developmental lineage. Immunol Lett. 2003;90:179–86. doi: 10.1016/j.imlet.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 5.McHeyzer-Williams MG. Immune response decisions at the single cell level. Semin Immunol. 1997;9:219–27. doi: 10.1006/smim.1997.0079. [DOI] [PubMed] [Google Scholar]
- 6.Vitetta ES, Berton MT, Burger C, Kepron M, Lee WT, Yin XM. Memory B and T cells. Ann Rev Immunol. 1991;9:193–217. doi: 10.1146/annurev.iy.09.040191.001205. [DOI] [PubMed] [Google Scholar]
- 7.Martin SW, Goodnow CC. Burst-enhancing role of the IgG membrane tail as a molecular determinant of memory. Nat Immunol. 2002;3:182–8. doi: 10.1038/ni752. [DOI] [PubMed] [Google Scholar]
- 8.Tangye SG, Avery DT, Deenick EK, Hodgkin PD. Intrinsic differences in the proliferation of naïve and memory human B cells as a mechanism for enhanced secondary immune responses. J Immunol. 2003;170:686–94. doi: 10.4049/jimmunol.170.2.686. [DOI] [PubMed] [Google Scholar]
- 9.Tangye SG, Avery DT, Hodgkin PD. A division-linked mechanism for the rapid generation of Ig-secreting cells from human memory B cells. J Immunol. 2003;170:261–9. doi: 10.4049/jimmunol.170.1.261. [DOI] [PubMed] [Google Scholar]
- 10.Tangye SG, Hodgkin PD. Divide and conquer: the importance of cell division in regulating B-cell responses. Immunology. 2004;112:509–20. doi: 10.1111/j.1365-2567.2004.01950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McHeyzer-Williams MG, McHeyzer-Williams LJ, Fanelli Panus J, Bikah G, Pogue-Caley RR, Driver DJ, Eisenbraun MD. Antigen-specific immunity. Th cell-dependent B cell responses. Immunol Res. 2000;22:223–36. doi: 10.1385/IR:22:2-3:223. [DOI] [PubMed] [Google Scholar]
- 12.Agenes F, Rosado MM, Freitas AA. Peripheral B cell survival. Cell Mol Life Sci. 2000;57:1220–8. doi: 10.1007/PL00000761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray D. Immunological memory. Ann Rev Immunol. 1993;11:49–77. doi: 10.1146/annurev.iy.11.040193.000405. [DOI] [PubMed] [Google Scholar]
- 14.Cyster JG, Hartley SB, Goodnow CC. Competition for follicular niches excludes self-reactive cells from the recirculating B-cell repertoire. Nature. 1994;371:389–95. doi: 10.1038/371389a0. [DOI] [PubMed] [Google Scholar]
- 15.Manz R, Arce S, Cassese G, Hauser A, Hiepe F, Radbruch A. Humoral immunity and long-lived plasma cells. Curr Op Immunol. 2002;14:517–21. doi: 10.1016/s0952-7915(02)00356-4. [DOI] [PubMed] [Google Scholar]
- 16.Banchereau J, Bazan F, Blanchard D, et al. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- 17.van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol. 2000;67:2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 18.Manser T. Textbook germinal centers? J Immunol. 2004;172:3369–75. doi: 10.4049/jimmunol.172.6.3369. [DOI] [PubMed] [Google Scholar]
- 19.McHeyzer-Williams MG. B cells as effectors. Curr Opin Immunol. 2003;15:354–61. doi: 10.1016/s0952-7915(03)00046-3. [DOI] [PubMed] [Google Scholar]
- 20.Graf D, Muller S, Korthauer U, van Kooten C, Weise C, Kroczek RA. A soluble form of TRAP (CD40 ligand) is rapidly released after T cell activation. Eur J Immunol. 1995;25:1749–54. doi: 10.1002/eji.1830250639. [DOI] [PubMed] [Google Scholar]
- 21.Roy M, Waldschmidt T, Aruffo A, Ledbetter JA, Noelle RJ. The regulation of the expression of gp39, the CD40 ligand, on normal and cloned CD4+ T cells. J Immunol. 1993;151:2497–510. [PubMed] [Google Scholar]
- 22.Fanslow WC, Srinivasan S, Paxton R, Gibson MG, Spriggs MK, Armitage RJ. Structural characteristics of CD40 ligand that determine biological function. Semin Immunol. 1994;6:267–78. doi: 10.1006/smim.1994.1035. [DOI] [PubMed] [Google Scholar]
- 23.Barr TA, Heath AW. Functional activity of CD40 antibodies correlates to the position of binding relative to CD154. Immunology. 2001;102:39–43. doi: 10.1046/j.1365-2567.2001.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakata N, Hamelmann E, Siadak AW, Terada N, Gerwins P, Aruffo A, Johnson GL, Gelfand EW. Differential regulation of CD40-mediated human B cell responses by antibodies directed against different CD40 epitopes. Cell Immunol. 2000;201:109–23. doi: 10.1006/cimm.2000.1645. [DOI] [PubMed] [Google Scholar]
- 25.Bergman MC, Attrep JF, Grammer AC, Lipsky PE. Ligation of CD40 influences the function of human Ig-secreting B cell hybridomas both positively and negatively. J Immunol. 1996;156:3118–22. [PubMed] [Google Scholar]
- 26.Santos-Argumedo L, Alvarez-Maya I, Romero-Ramirez H, Flores-Romo L. Enforced and prolonged CD40 ligand expression triggers autoantibody production in vivo. Eur J Immunol. 2001;31:3484–92. doi: 10.1002/1521-4141(200112)31:12<3484::aid-immu3484>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 27.Randall TD, Heath AW, Santos-Argumedo L, Howard MC, Weissman IL, Lund FE. Arrest of B lymphocyte terminal differentiation by CD40 signaling: mechanism for lack of antibody-secreting cells in germinal centers. Immunity. 1998;8:733–42. doi: 10.1016/s1074-7613(00)80578-6. [DOI] [PubMed] [Google Scholar]
- 28.Qi CH, Zheng L, Zhou X, et al. Cross-linking of CD40 using anti-CD40 antibody, 5C11, has different effects on XG2 multiple myeloma cells. Immunol Lett. 2004;93:151–8. doi: 10.1016/j.imlet.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Mathur RK, Awasthi A, Wadhone P, Ramanamurthy B, Saha B. Reciprocal CD40 signals through p38MAPK and ERK-1/2 induce counteracting immune responses. Nat Med. 2004;10:540–4. doi: 10.1038/nm1045. [DOI] [PubMed] [Google Scholar]
- 30.Luft T, Maraskovsky E, Schnurr M, et al. Tuning the volume of the immune response. Strength and persistence of stimulation determine migration and cytokine secretion of dendritic cells. Blood. 2004;104:1066–74. doi: 10.1182/blood-2003-12-4146. [DOI] [PubMed] [Google Scholar]
- 31.Néron S, Pelletier A, Chevrier MC, Monier G, Lemieux R, Darveau A. Induction of LFA-1 independent human B cell proliferation and differentiation by binding of CD40 with its ligand. Immunol Invest. 1996;25:79–89. doi: 10.3109/08820139609059292. [DOI] [PubMed] [Google Scholar]
- 32.Roy A, Krzykwa E, Lemieux R, Néron S. Increased efficiency of gamma-irradiated versus mitomycin C-treated feeder cells for the expansion of normal human cells in long-term cultures. J Hematother Stem Cell Res. 2001;10:873–80. doi: 10.1089/152581601317210962. [DOI] [PubMed] [Google Scholar]
- 33.Loembe MM, Lamoureux J, Deslauriers N, Darveau A, Delage R. Lack of CD40-dependent B-cell proliferation in B lymphocytes isolated from patients with persistent polyclonal B-cell lymphocytosis. Br J Haematol. 2001;113:699–705. doi: 10.1046/j.1365-2141.2001.02806.x. [DOI] [PubMed] [Google Scholar]
- 34.Lapointe R, Lemieux R, Olivier M, Darveau A. Tyrosine kinase and cAMP-dependent protein kinase activities in CD40-activated human B lymphocytes. Eur J Immunol. 1996;26:2376–82. doi: 10.1002/eji.1830261016. [DOI] [PubMed] [Google Scholar]
- 35.Fecteau JF, Néron S. CD40 stimulation of human peripheral B lymphocytes: distinct response from naïve and memory cells. J Immunol. 2003;171:4621–9. doi: 10.4049/jimmunol.171.9.4621. [DOI] [PubMed] [Google Scholar]
- 36.Blanchard D, Gaillard C, Hermann P, Banchereau J. Role of CD40 antigen and interleukin-2 in T cell-dependent human B lymphocyte growth. Eur J Immunol. 1994;24:330–5. doi: 10.1002/eji.1830240209. [DOI] [PubMed] [Google Scholar]
- 37.Arpin C, Banchereau J, Liu YJ. Memory B cells are biased towards terminal differentiation: a strategy that may prevent repertoire freezing. J Exp Med. 1997;186:931–40. doi: 10.1084/jem.186.6.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fecteau JF, Néron S. Characterization of naïve and memory B cell differentiation toward plasma cells following low CD40 stimulation. In: Monduzzi, editor. Immunology 2004. Collection of Free Papers Presented at the 12th International Congress of Immunology and 4th Annual Conference of FOCIS (Montreal, Canada, July 18–23, 2004) Bologna, Italy: Medimond Srl; 2004. pp. 303–7. [Google Scholar]
- 39.Shubinsky G, Schlesinger M. The CD38 lymphocyte differentiation marker: new insight into its ectoenzymatic activity and its role as a signal transducer. Immunity. 1997;7:315–24. doi: 10.1016/s1074-7613(00)80353-2. [DOI] [PubMed] [Google Scholar]
- 40.Deneys V, Mazzon AM, Marques JL, Benoit H, De Bruyere M. Reference values for peripheral blood B-lymphocyte subpopulations: a basis for multiparametric immunophenotyping of abnormal lymphocytes. J Immunol Meth. 2001;253:23–36. doi: 10.1016/s0022-1759(01)00338-6. [DOI] [PubMed] [Google Scholar]
- 41.Horst A, Hunzelmann N, Arce S, et al. Detection and characterization of plasma cells in peripheral blood: correlation of IgE+ plasma cell frequency with IgE serum titre. Clin Exp Immunol. 2002;130:370–8. doi: 10.1046/j.1365-2249.2002.02025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuppers R, Klein U, Hansmann ML, Rajewsky K. Cellular origin of human B-cell lymphomas. N Engl J Med. 1999;341:1520–9. doi: 10.1056/NEJM199911113412007. [DOI] [PubMed] [Google Scholar]
- 43.Calame KL, Lin KI, Tunyaplin C. Regulatory mechanisms that determine the development and function of plasma cells. Ann Rev Immunol. 2003;21:205–30. doi: 10.1146/annurev.immunol.21.120601.141138. [DOI] [PubMed] [Google Scholar]
- 44.Jeoung EJ, Lee IY, Choi JS, Cheon IS, Kang G, Choe J. Fibroblasts enhance the in vitro survival of human memory and naive B cells by maintaining intracellular levels of glutathione. Mol Cells. 2004;17:430–7. [PubMed] [Google Scholar]
- 45.Gagro A, Toellner KM, Grafton G, et al. Naive and memory B cells respond differentially to T-dependent signaling but display an equal potential for differentiation toward the centroblast-restricted CD77/globotriaosylceramide phenotype. Eur J Immunol. 2003;33:1889–98. doi: 10.1002/eji.200323357. [DOI] [PubMed] [Google Scholar]
- 46.Tone M, Tone Y, Fairchild PJ, Wykes M, Waldmann H. Regulation of CD40 function by its isoforms generated through alternative splicing. Proc Natl Acad Sci USA. 2003;98:1751–6. doi: 10.1073/pnas.98.4.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Contin C, Pitard V, Itai T, Nagata S, Moreau JF, Dechanet-Merville J. Membrane-anchored CD40 is processed by the tumor necrosis factor-alpha-converting enzyme. Implications for CD40 signaling. J Biol Chem. 2001;278:32801–9. doi: 10.1074/jbc.M209993200. [DOI] [PubMed] [Google Scholar]
- 48.Reyes-Moreno C, Girouard J, Lapointe R, Darveau A, Mourad W. CD40/CD40 homodimers are required for CD40-induced phosphatidylinositol 3-kinase-dependent expression of B7.2 by human B lymphocytes. J Biol Chem. 2004;279:7799–806. doi: 10.1074/jbc.M313168200. [DOI] [PubMed] [Google Scholar]
- 49.Gaudin E, Rosado M, Agenes F, McLean A, Freitas AA. B-cell homeostasis, competition, resources, and positive selection by self-antigens. Immunol Rev. 2004;197:102–15. doi: 10.1111/j.0105-2896.2004.0095.x. [DOI] [PubMed] [Google Scholar]
- 50.Lens SM, de Jong R, Hooibrink B, Koopman G, Pals ST, van Oers MH, van Lier RA. Phenotype and function of human B cells expressing CD70 (CD27 ligand) Eur J Immunol. 1996;26:2964–71. doi: 10.1002/eji.1830261223. [DOI] [PubMed] [Google Scholar]
- 51.Duddy ME, Alter A, Bar-Or A. Distinct profiles of human B cell effector cytokines: a role in immune regulation? J Immunol. 2004;172:3422–7. doi: 10.4049/jimmunol.172.6.3422. [DOI] [PubMed] [Google Scholar]
- 52.Miyashita T, McIlraith MJ, Grammer AC, Miura Y, Attrep JF, Shimaoka Y, Lipsky PE. Bidirectional regulation of human B cell responses by CD40–CD40 ligand interactions. J Immunol. 1997;158:4620–33. [PubMed] [Google Scholar]