Abstract
We present here the analysis of fluid-phase endocytosis (FPE) in human blood monocytes and monocyte-derived dendritic cells (MDDC) facilitated by our serendipitous identification of rottlerin as an efficient inhibitor of dendritic cell FPE (IC50 of 0·4 µm). Rottlerin was found to be an excellent tool for FPE analysis: rapid-acting, irreversible and selective for FPE (as opposed to receptor-mediated endocytosis) at concentrations of 3 µm and below. The inhibitory effect was not due to toxicity or visible change in membrane ruffles, but affects on cytoskeletal reorganization were evident in MDDC treated with relevant rottlerin concentrations during adhesion. A marked increase in FPE was observed in 1 hr interleukin (IL)-4 and granulocyte macrophage-colony stimulating factor (GM-CSF)-stimulated monocytes. Moreover, rottlerin inhibited the augmented FPE of 1-day cytokine treated monocytes and their augmented ability to induce T cell proliferative responses to tetanus toxoid. We conclude that rottlerin is a useful tool for investigating FPE and its functional importance.
Keywords: antigen presentation, dendritic cells, fluid-phase endocytosis, GM-CSF, IL-4, monocytes, T lymphocytes
Introduction
Cells that present exogenous antigen to T cells must first endocytose that antigen. One specialized mode of antigen uptake is fluid-phase endocytosis (FPE). Macropinocytosis is a mode of FPE, which is considered to be a canonical feature of dendritic cells (DC). There are a number of immunologically relevant characteristics of DC macropinocytosis. It enables DC to concentrate soluble antigens non-specifically from a very large volume of extra cellular fluid, estimated to be 1000–1500 µm3, a volume that is close to the total cell volume per hr.1 The molecules internalized by macropinocytosis are delivered to compartments understood to be critical for antigen presentation: to MIICs implicated in presentation by major histocompatibility complex (MHC) class II1,2 and to endoplasmic reticulum via cytosol, a pathway implicated in MHC class I presentation.2,3 Finally, DC macropinocytosis is regulated actively during DC differentiation; efficient macropinocytosis is a constitutive property of most immature dendritic cells (thought to represent tissue dendritic cells), but this macropinocytosis is abruptly down-regulated as DC differentiates into mature DC (thought to represent the cell type which presents antigen in lymphoid tissue).4–6
The features of macropinocytosis described above suggest that macropinocytosis could be important for antigen presentation of DC to T cells. However, since receptor-mediated endocytosis (RME) is so efficient in concentrating and internalizing many antigens, it becomes important to critically test whether FPE actually plays a role in responses of interest. The importance of macropinocytosis in antigen presentation has been reported in studies using a number of agents that have been described to inhibit macropinocytosis, including: cytochalasin D, an inhibitor of actin polymerization; amiloride and amiloride analogues which inhibit Na+/H + exchange.7–9 However, these agents are neither as efficient nor as selective as one would like; this complicates interpretation of the role of macropinocytosis per se in studies in which agents such as these have been used to inhibit macropinocytic uptake (see Discussion). Identification of more selective inhibitors of different subtypes of fluid phase uptake will be important both for functional analysis of FPE and for understanding its molecular mechanisms.
Macropinocytosis occurs via characteristic large vesicles formed by closure of actin-based cell surface ruffles. The Rho family of GTPase are involved critically in regulating cytoskeletal organization and two members of this family have been reported to have important regulatory roles in regulating macropinocytosis.10,11 However, a coherent understanding of their involvement is not yet available.
The protein kinase C (PKC) family of serine threonine kinases is another family of signalling proteins involved frequently in regulating membrane-proximal cellular processes, cytoskeletal regulation and endocytic process like phagocytosis.12,13 PKC has also been implicated in stimulated fluid pinocytosis as Ro 31–8220, a highly selective PKC inhibitor in vitro,14 suppresses phorbol myristate acetate (PMA)-induced fluid pinocytosis in human polymorphonuclear cells (PMNs) and blood lymphocytes.15,16 Phorbol ester 12-O-tetradecanoylphorbol 13-acetate (TPA), a potent activator of PKC, induces membrane ruffling and macropinocytosis in A431 cells.17 In the course of studying potential PKC involvement in fluid phase uptake by dendritic cells, we serendipitously discovered a pharmacological agent, rottlerin, that is a much more selective inhibitor of DC pinocytosis than the agents used currently. Rottlerin is a polycyclic aromatic compound produced by the rain forest tree Mallotus philippinensis. Rottlerin was reported to be a relatively selective inhibitor of PKC-delta18 but has been found subsequently to inhibit a handful of other kinases (see Discussion). The selectivity of rottlerin inhibition provided us with the unexpected opportunity to clarify some important issues regarding FPE.
Many other cell types have constitutive or inducible macropinocytosis, often viewed to have a nutritive function. Swanson and coworkers19 demonstrated dramatic rapidly inducible cytokine-induced FPE in murine bone marrow macrophages; they interpreted its function to be a nutritive one facilitating cell proliferation. Afterwards, constitutive as well as PMA-stimulated macropinocytosis by bone marrow macrophages has been ascribed a role in antigen presentation.20 We present evidence that efficient FPE, which is a hallmark of dendritic cells, is acquired by cytokine-stimulated monocytes early in the MDDC differentiation process and leads to enhanced antigen presentation. Using rottlerin as an informative tool, we demonstrate rottlerin-inhibitable FPE is a contributor to tetanus-toxoid (TT) specific T cell proliferative responses.
Materials and methods
Culture medium and reagents
The medium used throughout was RPMI-1640 supplemented with 2 mm l-glutamine, 1% non-essential amino acids, 1% pyruvate, 10 mm HEPES, 10% heat-inactivated fetal calf serum (FCS) and 100 µ/ml penicillin G sodium, 100 µg/ml streptomycin sulphate, 0·25 U/ml amphotericin B (all from Life Technologies, MD). Human recombinant granulocyte macrophage-colony stimulating factor (GM-CSF) and interleukin (IL)-4 was purchased from PeptroTech, Rocky Hill, NJ. Lucifer yellow CH (LY), fluorescein isothyocyanate (FITC)-dextran (Mr 70 000), FITC-transferrin and Alexa 488-phalloidin were from Molecular Probes (Eugene, OR). Phalloidin-FITC, amiloride hydrochloride:hydrate, 5-(N,N-dimethyl)-amiloride hydrochloride (DMA), cytochalasin D (CCD), carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP) and mannan from Saccharomyces cerevisiae were purchased from Sigma Chemical Co (St. Louis, MO). Bisindolylmaleimide I (BIM I), bisindolylmaleimide II (BIM II), Ro-31–8220, staurosporine, H-7, H-89, calphostin C and rottlerin were from Calbiochem (San Diego, CA). CD14 and CD−1 a monoclonal antibodies were purchased from Pharmingen (San Diego, CA).
Cell preparations
Elutriated human monocytes from healthy human volunteers were used to generate immature dendritic cells and cytokine-stimulated monocytes. CD14+ cells (∼99% pure) were cultured at 1 × 106 per ml in RPMI-10% FCS. For generation of short-term cytokine stimulated monocytes, cells were cultured for 1 or 24 hr with 50 ng/ml GM-CSF and 35 ng/ml IL-4. For generation of monocyte-derived dendritic cells, GM-CSF- and IL-4-containing cultures were continued for 6–8 days. Half the culture medium was changed with fresh medium containing 100 ng/ml GM-CSF and 70 ng/ml IL-4. The detached cells, the main population of CD1a+ cells after the culture period, were used as the source of immature dendritic cells. Peripheral blood T lymphocytes (PBT) were isolated from lymphocyte-enriched fractions prepared from healthy human volunteers by apheresis followed by sedimentation of cells through lymphocyte separation medium (ICN, Aurora, OH). PBT were washed three times with Dulbecco's Phosphate Buffered Saline (DPBS) and cryopreserved until use.
Immunoblot analysis of protein kinase C
PKC isoforms were examined by Western blot analysis using primary antibodies: monoclonal mouse anti-PKC-alpha, anti-PKC-beta, anti-PKC-delta, anti-PKC-theta and anti-PKC-eta (BD Transduction Laboratories, Lexington, KY) and polyclonal anti-PKC-zeta (Upstate Biotechnology, Inc., Lake Placid, NY).
Flow cytometric analysis of tracer uptake
Cell suspensions containing 3 × 105 cells in RPMI-10% FCS buffered with 25 mm HEPES were prewarmed for 10 min at 37° and pulsed with the tracer LY (1 mg/ml), FITC-dextran (0·5 mg/ml) and FITC-transferrin (200 µg/ml) at these concentrations unless indicated otherwise. After the indicated incubation period, uptake was stopped by washing the cells four times with cold phosphate buffered saline (PBS) containing 1% FCS and 0·01% NaN3 and were analysed on a FACScan (Becton Dickinson). In some experiments, cells were preincubated with the inhibitors as indicated in the figure legends. Data shown represent uptake in experimental conditions minus background uptake (cells pulsed at 4°).
Fluorescence microscopy
Day 7 dendritic cells were preincubated with rottlerin (2 µm or 10 µm) or buffer control for 30 min at 37°. LY was then added to the medium and uptake was allowed for 1 min. Thereafter cells were washed three times in PBS, fixed for 15 min with 3% paraformaldehyde (PFA) in PBS without calcium and magnesium at the same temperature. After PBS washing, cells were allowed to settle for 30 min at room temperature on the poly l-lysine-coated coverslip (pretreated with 100 µg/ml in PBS at 4° overnight), re-fixed with 4% PFA, mounted with fluoromount-G (Southern Biotechnology Associates, Inc., AL), and images were taken using a Zeiss epifluorescence axioplan2 microscope equipped with a 100× Plano-apo (N.A. 1·4) oil immersion lens and a Hamamatsu ORCA-ER CCD camera (Hamamatsu Photonics, Hamamatsu City, Japan).
For fluorescence imaging of actin distribution during adhesion, day 7 dendritic cells were allowed to adhere on the poly l-lysine-coated coverslip as above in the presence or absence of rottlerin. Following adherence, cells were fixed with 4% PFA for 30 min, washed three times with PBS and permeabilized with 0·2% Triton X-100 in PBS containing 1% bovine serum albumin (BSA) for 10 min at room temperature. Cells were washed three times with PBS, quenched with 0·1 m glycine for 15 min at room temperature, washed a two to three times with PBS and incubated for 1 hr in 2% BSA in PBS to block non-specific binding. They were washed two to three times in PBS and stained with Alexa 488-phaloidin (diluted 1 : 40 in 0·1% BSA/PBS) for 30 min at room temperature, washed with PBS followed by water, mounted with prolong antifade (Molecular Probes, Inc., OR) and viewed with a Zeiss fluorescence microscope as above.
Quantitation of filamentous actin
Day 7 dendritic cells were treated with media alone or rottlerin at 37°, washed three times with PBS and fixed for 15 min with 3% PFA in PBS without calcium and magnesium at the same temperature. After washing three times in PBS, cells were permeabilized with 0·2% Triton X-100 in PBS containing 1% BSA for 10 min and blocked for 30 min in 2% BSA containing PBS. Cells were then washed again with PBS and labelled with phalloidin-FITC conjugate for 30 min. Fluorescence was quantitated by flow cytometry.
Scanning electron microscopy
Day 7 dendritic cells were treated with media alone or 10 µm rottlerin in suspension, washed and then fixed by transferring to a 20-fold volume of fixative (3% glutaraldehyde, 0·1 m cacodylate, 7·5% sucrose, 0·05% CaCl2, pH 7·4). Cells were fixed for 1 hr, washed with PBS and allowed to adhere on poly l-lysine treated glass chips for 1 hr. Chips were transferred to and stored in fixative until subsequent processing. Fixed samples were then dehydrated in ethanol, critical-point dried with CO2 and sputter-coated with a 1 nm discontinuous layer of platinum. Images were taken using Hitachi S-900 field emission scanning electron microscope (Hitachi Scientific Instrument Ltd, Mountain View, CA).
T cell proliferative response to tetanus toxoid
Twenty-four-hr cytokine-stimulated or unstimulated monocytes were pulsed for 1 hr with antigen in the presence or absence of rottlerin at the indicated concentrations. Antigens used were TT protein (Wyeth-Ayerst Laboratories, Inc., Marietta, PA) or synthetic TT derived peptide p30 (TT 947–967; FNNFTVSFWLRVPKVSASHLE, provided by G. Corradin of University of Lausanne, Switzerland). After the antigen pulse monocytes were washed three times; 20 000 stimulator cells/well were then cocultured with 3 × 105 autologous responder cells for a total of 5 days and proliferation quantitated by [3H]thymidine incorporation.
Results
Rottlerin is a potent inhibitor of dendritic cell FPE
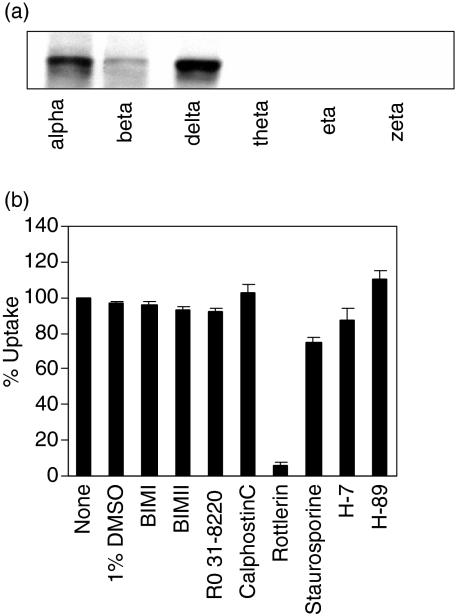
To analyse a potential role for PKC in FPE we first investigated the expression of PKC isoforms in monocyte-derived dendritic cells (MDDC) and the sensitivity of dendritic cell FPE to PKC inhibitors. Our results indicate that multiple PKC isoforms are present in dendritic cells; delta and alpha are particularly strongly detected in Western blot (Fig. 1a). However, PKC inhibitors (BIM I, BIM II, Ro-31–8220, calphostin C) did not influence FPE (Fig. 1b). Thus, PKC activity appears not to be critical for FPE. In contrast to the other PKC inhibitors, rottlerin inhibits more than 90% of FPE at the low concentration (10 µm) used typically for putative selective inhibition of PKC-delta activity. Because rottlerin has also been reported to inhibit a limited set of other kinases, including PKA,21 we explored whether a PKA inhibitor (H-89) or other broader spectrum kinase inhibitors (H-7 and staurosporine) would block FPE (Fig. 1b). None of these agents inhibited FPE. Therefore, inhibition by rottlerin is a special property of rottlerin not shared by a variety of other kinase inhibitors.
Figure 1.
Multiple protein kinase C (PKC) isoforms are expressed in monocyte derived dendritic cells (MDDC) but are not involved in fluid phase endocytosis (FPE). (a) Whole cell lysate from immature dendritic cells were analysed by Western blot using monoclonal anti-PKC α, β, δ, θ and η antibodies or polyclonal anti-PKC ζ antibodies. (b) MDDC were pretreated with the following inhibitors bisindolylmaleimide (BIMI) (100 nm), BIMII (100 nm), Ro 31–8220 (100 nm), calphostin C (1 µm), Rottlerin (10 µm), staurosporine (1 µm), H-7 (100 µm) and H-89 (10 µm) or 1% dimethylsulphoxide (DMSO) for 45 min. Lucifer yellow (LY) uptake was measured during the subsequent 45 min as described in Materials and methods. Dendritic cells incubated at 4° showed < 2% of the uptake at 37°. Values are expressed as a percentage of control (no inhibitors). Bars represent the mean ± SD (n = 3), pooling data from three separate experiments.
Characteristics of rottlerin-mediated inhibition
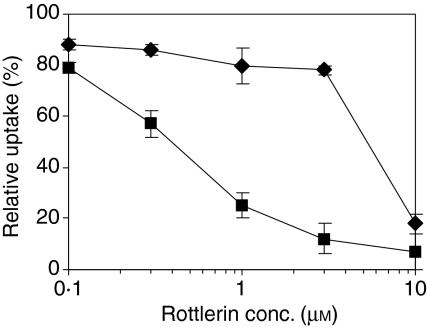
Inhibition of endocytosis of lucifer yellow (LY) by rottlerin is dose-dependent with half-maximal inhibition at 0·4 µm(Fig. 2). This concentration is about sevenfold lower than its reported half-maximal inhibition of PKC-delta.18 The specificity of rottlerin inhibition of FPE was assessed by comparisons with RME. We used transferrin uptake via the transferrin receptor as the model system to measure RME, because that process is often taken as the prototype of RME. The concentration of rottlerin required for half-maximal inhibition of RME was at least 10× higher than the concentration required for comparable inhibition of FPE (Fig. 2). The rottlerin concentration used in most subsequent experiments (2–3 µm) consistently gave more than 80% inhibition of FPE and less than 25% inhibition of RME.
Figure 2.
Rottlerin inhibits fluid phase endocytosis (FPE) much more than receptor-mediated endocytosis (RME). Monocyte derived dendritic cells (MDDCs) were pretreated with graded concentrations of rottlerin for 45 min and during the subsequent 45 min two kinds of uptake were measured: FPE measured using LY (squares) and RME measured using fluorescein isothiocyanate (FITC)-transferrin (diamonds). Values are expressed as a percentage of control (no inhibitors). Points represent the mean ± SD (n = 3), pooling data from three separate experiments. IC50 for FPE is 0·4 µm and for RME is 5 µm.
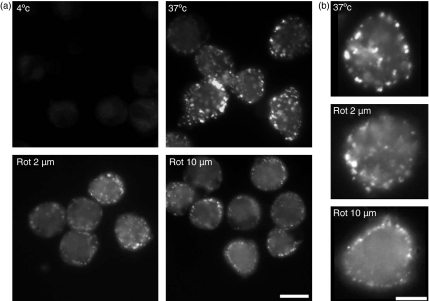
To confirm and extend the results of flow cytometric analysis, the effects of rottlerin on FPE were also examined by fluorescence microscopy (Fig. 3). As expected for highly macropinocytic cells, MDDC pulsed with LY for only 1 min show multiple LY-filled vesicles, many of which exceed 0·2 microns in diameter (Fig. 3a). The number of such highly fluorescent vesicles is marked reduced by treatment with 2 µm (or 10 µm) rottlerin. Both the number of macropinosomes and their fluorescence intensity is influenced by rottlerin (see also Fig. 3b, in which the brightness has been increased for images of the rottlerin-treated cells to facilitate inspection of morphology). At 2 µm rottlerin some macropinosomes can still form, although their fluorescence intensity is much lower. At 10 µm LY-containing vesicles are dim, like the 2 µm rottlerin-treated sample, and in addition are reduced in average size and restricted to the periphery of the MDDC. Fluorescence microscopy of RME using FITC-transferrin showed little or no inhibition of uptake at 2 µm rottlerin (data not shown), consistent with the results of flow cytometry. Thus, these images collected by fluorescence microscopy confirm that much of the LY uptake by MDDC is into macropinosomes and that reduced uptake observed by cytometry corresponds with decreased numbers of macropinosomes.
Figure 3.
Fluorescence microscopy confirms inhibition of macropinocytosis by rottlerin. Monocyte derived dendritic cells (MDDCs) were preincubated with rottlerin (2 µm or 10 µm) or buffer control for 30 min then examined by fluorescence microscopy after 1 min with Lucifer yellow (LY) at 37° except for the panel labelled 4° (which, like the panel labelled 37°, has no rottlerin). Scale bars indicate 10 microns. (a) Images acquired at equal amplification; (b) representative images in which amplification has been increased on the rottlerin-containing samples to facilitate image interpretation.
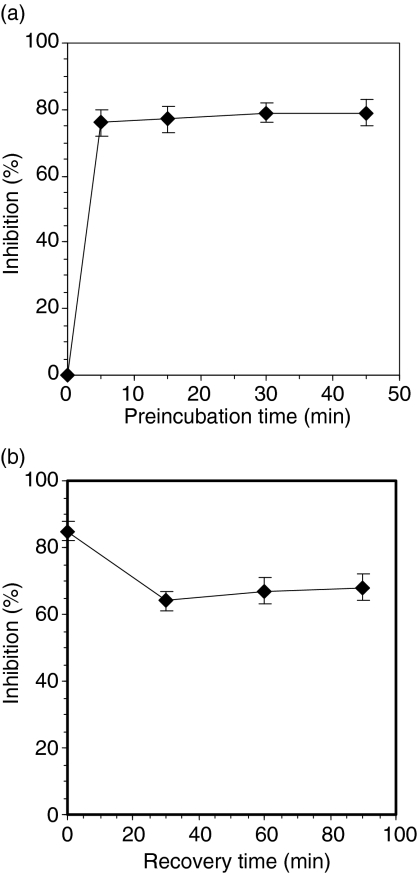
The kinetics of onset and duration of inhibition were analysed. Rottlerin inhibition occurred rapidly, reaching full effectiveness in 5 min (Fig. 4a); this is particularly remarkable given that a relatively low concentration of rottlerin was used (2 µm, just 5× above the half maximal inhibition). Inhibition continued after removal of rottlerin (Fig. 4b); little loss of effect was observed even 90 min after washout. One trivial explanation for the dramatic effect of rottlerin on macropinocytosis would be a toxic effect. We investigated various potential manifestations of toxicity to MDDC and have found none at the concentrations used. For example, trypan blue exclusion was not influenced following short-term exposure to 30 µm and that multiday exposure to 2 µm rottlerin was non-toxic, as it did not interfere with monocyte differentiation into MDDC (not shown).
Figure 4.
Rapid onset and prolonged duration of rottlerin inhibition. (a) Rapid onset. Monocyte derived dendritic cells (MDDC) were preincubated with 2 µm rottlerin at 37° for the indicated time, and their uptake of Lucifer yellow (LY) was measured during the subsequent 15 min. (b) Prolonged inhibition after washout. MDDC were preincubated in rottlerin for 45 min at 37°, washed and incubated in inhibitor-free medium for the time indicated. Uptake of LY during the subsequent 45 min at 37° was measured. Points represent the mean ± SD (n = 3), pooling data from three separate experiments.
Specificity of inhibition
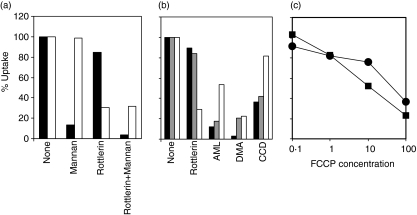
Further studies of the specificity of inhibition included analysis of a third tracer, FITC-dextran. Our analysis confirms the findings of others,1 that FITC-dextran is taken up predominantly by a mannose-receptor-dependent pathway because excess mannan inhibits almost 90% of its uptake (Fig. 5a). This dominant route of uptake is resistant to rottlerin (less than 20% inhibition). About 10% of the uptake of FITC-dextran is via a mannose-receptor independent pathway, resistant to inhibition by excess mannan. This pathway of FITC-dextran uptake resistant to mannan inhibition, considered to be FPE,1 is sensitive to rottlerin inhibition (> 95% inhibition, cf. mannan versus rottlerin + mannan). As expected, LY uptake is unaffected by mannan.
Figure 5.
Specificity of rottlerin for uptake via fluid phase endocytosis (FPE). (a) fluorescein isothiocyanate (FITC)-dextran is taken up predominantly by a mannan-inhibitable rottlerin-resistant pathway. Monocyte derived dendritic cells (MDDCs) were pretreated for 15 min with either 2 µm rottlerin or 3 mg/ml mannan or a combination of both, and their subsequent uptake of Lucifer yellow (LY) (open) or FITC-dextran (solid) was measured after 15 min. (b) Rottlerin is a better selective inhibitor of FPE than other commonly used pharmacological agents. MDDCs were preincubated with amiloride (3 mm), 5-(N,N-dimethyl)-amiloride hydrochloride (DMA) (500 µm) or cytochalasin D (CCD) (10 µg/ml) for 15 min and their subsequent uptake of LY (open), FITC-dextran (solid) or FITC-transferrin (grey) was measured. Values are expressed as percentage of control (no inhibitors). (c) Macropinocytosis is not selectively blocked by carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP). MDDCs were pre-exposed to graded concentrations of FCCP for 15 min at 37°; thereafter, LY (squares) or FITC-transferrin (circles) were added and uptake measured after 15 min.
Comparison of rottlerin with other inhibitors reported to inhibit FPE demonstrated that rottlerin is a much more selective FPE inhibitor than the others (Fig. 5b). Rottlerin shows strong inhibition of LY uptake (i.e. FPE), but not FITC-dextran or FITC-transferrin (i.e. RME). In contrast, amiloride, DMA and CCD do not preferentially inhibit LY uptake, but are generally more potent in inhibition of FITC-dextran or FITC-transferrin. Thus the ability of 2 µm rottlerin to preferentially inhibit MDDC macropinocytosis much more than RME is a unique property of this inhibitor. One important new finding about rottlerin is its capacity to uncouple mitochondrial oxidative phosphorylation.22 Because the critical consequence of this uncoupling is a decrease in the cellular ATP level, we tested the hypothesis that decreasing the cellular ATP level might selectively impair macropinocytosis. We tested whether treatment with the mitochondrial uncoupler, FCCP, could reproduce the selective inhibition of macropinocytosis observed with rottlerin. In contrast to that prediction, we find that informative concentrations of FCCP do not selectively inhibit FPE (Fig. 5c). These results suggest that rottlerin is not selectively inhibiting macropinocytosis by uncoupling mitochondrial oxidative phosphorylation.
Rottlerin modifies actin distribution
FPE by MDDC is mediated in large part by macropinocytosis, which involves actin-dependent ruffling at the cell surface, and closure of the ruffles to form large macropinosomes.23 We explored the possibility that rottlerin inhibition occurs by modifying filamentous actin or by blocking ruffle formation. We assessed F-actin by flow cytometric quantitation of FITC-phalloidin and found that overall levels of F-actin were not modified by treatment with rottlerin even at 10 µm(Fig. 6a). A higher-resolution view of surface topology was obtained by scanning electron microscopy (Fig. 6b). The prominent surface ruffles evident on MDDC are largely unaffected by rottlerin treatment for 5 min at 10 µm; although there is some suggestion of a coarser surface topology in the rottlerin-treated samples, the difference is not dramatic (see Discussion). We conclude that rottlerin inhibition is not mediated by a major inhibition of ruffles.
Figure 6.
Rottlerin does not decrease polymerized actin or change the surface topology of monocyte derived dendritic cells (MDDCs). (a) The amount of filamentous actin (F-actin) in MDDCs was determined by staining with fluorescein isothiocyanate (FITC)-phalloidin and determining fluorescence by flow cytometry as described in Materials and methods. Data shown are fluorescence histograms of MDDCs with and without treatment for 15 min with 10 µm rottlerin. (b) Scanning electron micrographs of MDDC treated for 5 min with buffer control or 10 µm rottlerin.
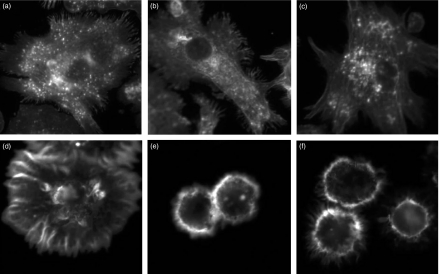
We considered the possibility that dynamic actin regulation might be altered by doses of rottlerin that did not cause an obvious change in surface morphology. We investigated this possibility by studying rottlerin affects on dynamic actin regulation during MDDC cell spreading. We observed that rottlerin profoundly alters MDDC spreading (Fig. 7), and that it does so at concentrations similar to those which affect macropinocytosis. In the absence of rottlerin, MDDC plated onto poly l-lysine-coated coverslips spread efficiently in 30 min and developed focal accumulations of actin consistent with focal contacts. Such spreading is altered minimally by low concentrations of rottlerin that do not inhibit FPE (0·1 µm and 0·3 µm; Fig. 7b,c). However, rottlerin dramatically blocks spreading at low concentrations (1 µm and 3 µm; Fig. 7d,e) that selectively blocked FPE. These studies reveal a dose-dependent effect of rottlerin on actin reorganization that correlates well with its inhibitory effect on FPE.
Figure 7.
Rottlerin impairs MDDC spreading. MDDCs were allowed to spread for 30 min at 37° on poly L-lysine-coated coverslips in the presence of graded concentrations of rottlerin (A: none; B: 0·1 µm; C: 0·3 µm; D: 1 µm; E: 3 µm; F: 10 µm); thereafter cells were fixed, permeabilized, stained with alexa 488-phalloidin and examined by fluorescence microscopy.
Rottlerin inhibits the efficient FPE which appears very early during dendritic cell differentiation
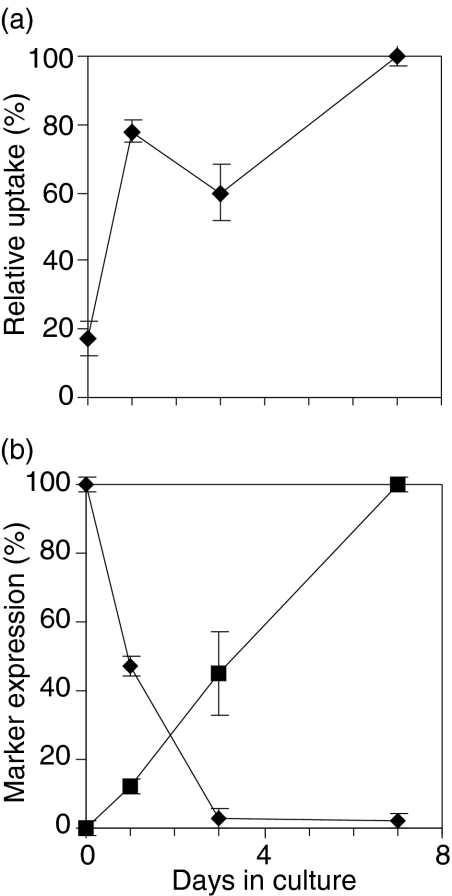
Efficient FPE is often viewed as a distinguishing characteristic of dendritic cells, but relatively little is known about the acquisition of this trait during dendritic cell differentiation. Therefore, we determined the time of appearance of efficient FPE during the generation of MDDC from human peripheral blood monocytes (Fig. 8a). By day 1 the cultured monocytes showed efficiency in FPE that is close to the efficiency of 7-day MDDC. In contrast, the phenotypic transition to CD1a-positive occurred more gradually during culture; maximal expression was not evident until day 7 (Fig. 8b). Similarly, conversion to CD14-negative phenotype required several days.
Figure 8.
Efficient FPE is observed early during monocyte differentiation into monocyte derived dendritic cells (MDDCs). Monocytes were cultured for 7 days in presence of granulocyte macrophage-colony stimulating factor (GM-CSF) and interleukin (IL)-4. (a) At intervals during culture, cells were harvested, and their 45 min uptake of Lucifer yellow (LY) measured by flow cytometry. (b) Expression of CD1a (squares) and CD14 (diamonds) was determined on parallel samples by flow cytometry. Results shown are the mean of three different experiments ± SD.
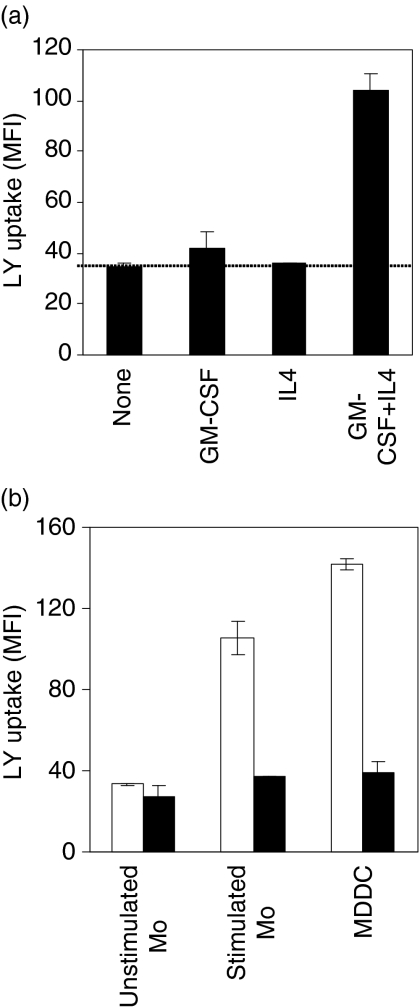
Additional analysis indicated that efficient FPE could be induced in monocytes within 1 hr (Fig. 9a). Specifically, 1-hr treatment with IL-4 and GM-CSF, the cytokines generally used to induce monocyte cell differentiation into dendritic cells, induced increased LY uptake. Neither of the cytokines alone is able to increase FPE over basal level; thus, GM-CSF and IL4 act synergistically to dramatically augment monocyte FPE within 1 hr.
Figure 9.
Characteristics of efficient fluid phase endocytosis (FPE) in monocytes. (a) Granulocyte macrophage-colony stimulating factor (GM-CSF) and interleukin (IL)-4 are both required to rapidly induce pinocytosis in monocytes. Lucifer yellow (LY) uptake was measured in fresh monocytes cultured for 1 hr in media alone or with GM-CSF and/or IL-4. The data show the fluorescence of the cells after 45 min and are the mean of three different experiments ± SD. (b) Rottlerin inhibits efficient FPE in cytokine-stimulated monocytes. The capacity of rottlerin to inhibit FPE was in monocytes precultured for 24 hr in media alone; monocyte precultured for 24 hr in the presence of GM-CSF + IL-4 and in monocyte derived dendritic cells (MDDCs) resulting from 7days' culture of monocytes in the presence of GM-CSF + IL-4. LY uptake was measured in the absence (filled bar) or presence (open bar) of 2 µm rottlerin. Points represent the mean ± SD (n = 3), pooling data from three separate experiments.
We investigated whether FPE in resting and cytokine-stimulated monocytes is equally susceptible to inhibition by rottlerin (Fig. 9b). Unlike the FPE of MDDC, the FPE of resting monocytes is resistant to inhibition by 2 µm rottlerin. A simple interpretation of these results is that two modes of FPE are operating concurrently in MDDC and stimulated monocytes: superimposed on the rottlerin-resistant basal mode seen in the resting monocytes is a new rottlerin-inhibitable mode of augmented FPE.
Rottlerin-inhibitable FPE contributes to antigen-presentation
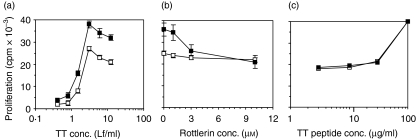
Although it is believed that fluid phase uptake of antigen can contribute to antigen presentation, it has not been directly proved by functional studies using inhibitors that selectively inhibit FPE but not RME. Because rottlerin is a more selective inhibitor of FPE than other agents studied previously, we explored the possibility that rottlerin inhibition studies could directly demonstrate a role for FPE in antigen presentation using cytokine-stimulated monocytes. Inhibition studies were undertaken in an in vitro model system of human T cell proliferative responses to tetanus toxoid (TT). To control carefully the conditions of antigen uptake, we used purified monocytes pulsed for 1 hr with TT. Monocytes cultured for 24 hr with IL-4 and GM-CSF induced consistently higher stimulation of T cells than monocytes cultured in the absence of cytokine (Fig. 10a). Analysis of the dose–response curve indicates a 1·5–2× increase in efficiency in presentation by the cytokine-stimulated monocytes. The proliferation induced by resting monocytes was minimally inhibited by rottlerin; uptake of tetanus toxoid by these resting monocytes probably occurs via RME, with some contribution from the rottlerin-resistant FPE (for example, seen in Fig. 8). In contrast, rottlerin treatment of the cytokine-stimulated monocytes reduces their functional capacity to induce T cell proliferation (Fig. 10b). Of particular note, (1) rottlerin inhibition reduces their stimulatory capacity back to the level of the monocytes not stimulated with cytokine; and (2) the dose–response characteristics of that reversal demonstrate that most of the inhibition is caused by 3 µm rottlerin, consistent with rottlerin's effect on augmented FPE. Thus the functional effects of rottlerin on antigen presentation have characteristics precisely concordant with rottlerin's effects on FPE. Inhibition by rottlerin is not due to toxicity; this is evident from its minimal inhibition using the unstimulated monocytes (Fig. 10a). In addition, rottlerin does not inhibit proliferation induced by cytokine-stimulated monocytes that present the relevant tetanus toxoid peptide, whose presentation does not require internalization (Fig. 10c). Thus, the inhibitory effect of rottlerin is not due to its toxic effect on T-cells or monocytes. On the contrary, the simplest interpretation is that rottlerin-mediated reduction of T cell proliferation results from its selective inhibition of certain types of FPE.
Figure 10.
Rottlerin inhibits increased efficiency of antigen presentation by cytokine-stimulated monocytes. (a) Dose–response analysis of proliferative response of autologous normal human T cells to tetanus-toxoid (TT) presented on two preparations of antigen-pulsed monocytes − either precultured for 24 hr in the presence (filled squares) or absence (open squares) of granulocyte macrophage-colony stimulating factor (GM-CSF) and interleukin (IL)-4. (b) The susceptibility of the proliferative response to rottlerin was compared for the cytokine-stimulated (filled squares) and unstimulated (open squares) monocytes pulsed with 3 Lf/ml TT protein. (c) Cytokine-stimulated monocytes were pulsed with graded concentrations of TT peptide p30 in the presence or absence of 3 µm rottlerin. Antigen-presenting cells were pulsed with TT protein or peptide for 1 hr, and their ability to induce proliferative response was measured on day 5 as described in Materials and methods. In all the cases, T cells alone or monocytes alone or in the presence of TT protein incorporated less than 400 cpm.
Discussion
Our discussion focuses on three conclusions from the foregoing studies: (1) rottlerin is a much more selective inhibitor of MDDC fluid phase uptake than the agents routinely used as well as being non-toxic, and acting quickly and irreversibly; (2) rottlerin inhibition clearly distinguishes highly efficient fluid phase uptake in MDDCs (rottlerin-inhibitable) from the inefficient fluid phase uptake (rottlerin-resistant) found in monocytes; and (3) rottlerin inhibitable efficient FPE found in cytokine-stimulated monocytes during MDDC generation contributes to antigen presentation.
Rottlerin inhibition of FPE
Analysis of FPE would be greatly facilitated by availability of selective inhibitors of FPE. However, the pharmacological agents now used widely as selective inhibitors of FPE can also inhibit RME. Inhibitors of the amiloride family (amiloride and DMA) are used most commonly as selective inhibitors of FPE but not RME. Such selectivity was first demonstrated for amiloride by West and coworkers24 in A431 cells. Following this, the specificity of amiloride for FPE has been assumed without further validation and has been used in a variety of other studies.1,20,25–27 However, the validity of this assumption is questioned by studies showing that amiloride or its analogues inhibits RME in renal tubule epithelial cells.28,29 Our results demonstrate that for MDDC, amiloride and DMA both inhibit RME (transferrin uptake); indeed, amiloride shows a pattern opposite than expected, preferentially inhibiting RME relative to FPE. CCD has also been used as an inhibitor of the macropinocytosis.1,19,30 However, our data demonstrate that in MDDC, CCD does not preferentially inhibited FPE. CCD is not a very efficient inhibitor of FPE (see Fig. 5b and others' data such as Sallusto et al.1); this may reflect the fact that some actin polymerization is resistant to CCD inhibition.31 Thus, no pharmacological agent is available which can be relied upon to inhibit FPE but not RME in diverse cell types.
Our studies identify rottlerin as a useful inhibitor of macropinocytosis in MDDC. We demonstrate that at low concentrations rottlerin is a selective inhibitor of FPE relative to RME. Specifically, its selectivity is much better than the agents outlined above (amiloride, DMA and CCD). Moreover, it has other properties which are desirable for an inhibitor. It is non-toxic, both in short-term assays and even in long-term culture of monocytes to derive dendritic cells. It has rapid onset, showing maximal inhibition within 5 min of addition to the cell culture. Its effects are irreversible, at least over 90 min.
Although our data demonstrate that rottlerin is the most selective of the known inhibitors for MDDC FPE, three aspects of rottlerin inhibition qualify its usefulness. First, as discussed in the next section, rottlerin inhibits some but not other fluid phase uptake. On one hand, this makes it useful for discriminating subtypes of FPE (see below), but on the other hand disqualifies it as a general-purpose inhibitor of fluid phase uptake. Secondly, like most inhibitors, its functional selectivity is restricted to a particular dose range. Its selectivity for FPE of MDDC is best between 1 and 3 µm. Thirdly, its mechanism of action is not yet understood. Although described initially as an inhibitor of PKCδ18 its use as a selective kinase inhibitor is problematic because (1) it inhibits multiple kinases;18,21,32 (2) some studies could not confirm rottlerin inhibition of particular kinases;18,21 (3) its mechanism of kinase inhibition appears to be complex;33 and (4) our studies with kinase inhibitors do not provide support for the concept that it is acting as a kinase inhibitor. Our studies also discount the likelihood it is inhibiting FPE by inhibiting mitochondrial oxidative phosphorylation.22 Our observation that it inhibits MDDC spreading and actin reorganization is consistent with its perturbing dynamic actin reorganization, but the exact molecular target remains unknown. One interesting possibility is that rottlerin perturbs recruitment of actin from podosomes which have been suggested recently to provide a reservoir of actin which can be mobilized for TLR-induced augmentation of pinocytosis in dendritic cells.34
Rottlerin distinguishes subtypes of FPE
Better understanding of endocytic processes requires: (a) discrimination of distinct kinds of endocytosis and (b) elucidation of the distinctive characteristics of each of those endocytic processes. The most widely understood distinction between endocytic processes is RME versus FPE. In this paper we address primarily FPE, most often using LY as the tracer. Within the category of FPE, there is accumulating evidence for heterogeneity. One notable heterogeneity relates to vesicle size (macropinocytosis versus micropinocytosis).35 Moreover, among macropinocytic processes there are differences (1) in intracellular destination for the cargo (cytosolic versus endosomal);1,20 (2) in particular signalling molecules involved; and (3) in dependence on gelsolin. For example, in fibroblasts, Rac activation leads to membrane ruffling and pinocytosis but in dendritic cells regulation of Rac activity does not appear to be the control point of pinocytosis.10,36 Similarly, in fibroblasts macropinocytosis depends on gelsolin37 but in dendritic cells it does not.38 Elucidation of such heterogeneity requires straightforward strategies to distinguish subtypes of endocytosis. We describe a new parameter which distinguishes subtypes of FPE, namely inhibition by low-dose rottlerin. The efficient FPE by cytokine-stimulated monocytes resembles dendritic cell FPE (and differs from unstimulated monocytes), in being sensitive to rottlerin inhibition.
Rottlerin inhibits augmented T cell proliferation
Our results show that the highly efficient FPE appears very early in the dendritic cell differentiation pathway when the cells resemble monocytes phenotypically (Figs 8 and 9). Our results also suggest that this enhanced fluid phase uptake in monocytes is associated with enhanced in vitro T cell responses (Fig. 10). We therefore hypothesize that blood monocytes migrating into sites of inflammation rapidly acquire efficient FPE and thereby enhance their capacity to function as APC because (1) monocytes can be recruited into inflammatory sites in skin39 and (2) GM-CSF and IL-4 are both found in skin at sites of inflammation, in part because of their release from mast cells present in skin.40–42
The radical idea that macropinocytosis provided a significant mode of antigen uptake for dendritic cells was proposed by Lanzavecchia and coworkers.1 Their data demonstrated that although FPE lacks a mechanism for surface enrichment, the extreme volume of fluid concentrated by this process in dendritic cells (close to its cell volume per hr) makes it a significant contributor to antigen uptake. Prior to that time, macropinocytosis by antigen-presenting cells such as bone marrow macrophages have been ascribed a nutritive role in keeping with considerations about macropinocytosis in non-immune cells.19 Lanzavecchia's findings provided the first evidence to argue for the immunological importance of macropinocytosis, namely efficient uptake; macropinocytosis delivers antigen efficiently to intracellular compartments understood to be involved in loading peptide onto MHC molecules. The hypothesis of the functional importance of macropinocytosis was supported by findings that increased macropinocysis was associated with increased efficiency of antigen presentation.1
Functional studies of selective inhibitors of macropinocytosis would provide the most direct evidence for its functional importance. We are aware of only three such studies using amiloride/dimethylamiloride, but testing only a single antigen, ovalbumin;20,43,44 it is notable that ovalbumin is an unusual very negatively charged, carbohydrate-modified molecule that is susceptible to uptake by RME.45,46 Here, we demonstrate that rottlerin inhibits antigen-specific proliferation stimulated by cytokine-stimulated monocytes. The rottlerin inhibition is partial and reduces stimulation only to the level observed with unstimulated monocytes, consistent with the presence of other modes of uptakes of tetanus toxoid. Our study with rottlerin is the first to demonstrate that FPE plays a role in antigen presentation of an antigen other than ovalbumin.
Acknowledgments
We thank Martin Brown, Amit Kumar, Paul Roche, Alessandra Mazzoni, Beth Hiltbold and Alberto Visintin for constructive advice and discussion; Giampietro Corradin and Wyeth-Ayerst Laboratories for provision of reagents. This research was supported by the Intramural Research Program of the NIH, National Cancer Institute.
Abbreviations
- FPE
fluid phase endocytosis
- RME
receptor-mediated endocytosis
- DC
dendritic cell
- MDDC
monocyte derived dendritic cells
- PKC
protein kinase C
- LY
Lucifer yellow
- DMA
5-(N,N-dimethyl)-amiloride hydrochloride
- CCD
cytochalasin D
- BIM
bisindolylmaleimide
- FCCP
carbonyl cyanide p-trifluoromethoxyphenylhydrazone
- TT
tetanus-toxoid
References
- 1.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thery C, Amigorena S. The cell biology of antigen presentation in dendritic cells. Curr Opin Immunol. 2001;13:45–51. doi: 10.1016/s0952-7915(00)00180-1. [DOI] [PubMed] [Google Scholar]
- 3.Norbury CC, Chambers BJ, Prescott AR, Ljunggren HG, Watts C. Constitutive macropinocytosis allows TAP-dependent major histocompatibility complex class I presentation of exogenous soluble antigen by bone marrow-derived dendritic cells. Eur J Immunol. 1997;27:280–8. doi: 10.1002/eji.1830270141. [DOI] [PubMed] [Google Scholar]
- 4.Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;106:263–6. doi: 10.1016/s0092-8674(01)00455-x. [DOI] [PubMed] [Google Scholar]
- 5.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–8. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 6.Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20:621–67. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 7.Ojcius DM, Bravo DA, Kanellopoulos JM, et al. Internalization of Chlamydia by dendritic cells and stimulation of Chlamydia-specific T cells. J Immunol. 1998;160:1297–303. [PubMed] [Google Scholar]
- 8.Wassenberg JJ, Dezfulian C, Nicchitta CV. Receptor mediated and fluid phase pathways for internalization of the ER Hsp90 chaperone GRP94 in murine macrophages. J Cell Sci. 1999;112:2167–75. doi: 10.1242/jcs.112.13.2167. [DOI] [PubMed] [Google Scholar]
- 9.von DA, Bailey E, Gibbs DM, Robinson JH. The route of bacterial uptake by macrophages influences the repertoire of epitopes presented to CD4 T cells. Eur J Immunol. 2002;32:3714–9. doi: 10.1002/1521-4141(200212)32:12<3714::AID-IMMU3714>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 10.West MA, Prescott AR, Eskelinen EL, Ridley AJ, Watts C. Rac is required for constitutive macropinocytosis by dendritic cells but does not control its downregulation. Curr Biol. 2000;10:839–48. doi: 10.1016/s0960-9822(00)00595-9. [DOI] [PubMed] [Google Scholar]
- 11.Garrett WS, Chen LM, Kroschewski R, et al. Developmental control of endocytosis in dendritic cells by Cdc42. Cell. 2000;102:325–34. doi: 10.1016/s0092-8674(00)00038-6. [DOI] [PubMed] [Google Scholar]
- 12.Yue L, Lu S, Garces J, Jin T, Li J. Protein kinase C-regulated dynamitin-macrophage-enriched myristoylated alanine-rice C kinase substrate interaction is involved in macrophage cell spreading. J Biol Chem. 2000;275:23948–56. doi: 10.1074/jbc.M001845200. [DOI] [PubMed] [Google Scholar]
- 13.Larsen EC, DiGennaro JA, Saito N, et al. Differential requirement for classic and novel PKC isoforms in respiratory burst and phagocytosis in RAW 264.7 cells. J Immunol. 2000;165:2809–17. doi: 10.4049/jimmunol.165.5.2809. [DOI] [PubMed] [Google Scholar]
- 14.Twomey B, Muid RE, Nixon JS, Sedgwick AD, Wilkinson SE, Dale MM. The effect of new potent selective inhibitors of protein kinase C on the neutrophil respiratory burst. Biochem Biophys Res Commun. 1990;171:1087–92. doi: 10.1016/0006-291x(90)90795-o. [DOI] [PubMed] [Google Scholar]
- 15.Keller HU, Niggli V. The PKC-inhibitor Ro 31–8220 selectively suppresses PMA- and diacylglycerol-induced fluid pinocytosis and actin polymerization in PMNs. Biochem Biophys Res Commun. 1993;194:1111–6. doi: 10.1006/bbrc.1993.1936. [DOI] [PubMed] [Google Scholar]
- 16.Trachsel S, Keller HU. Selective responses (actin polymerization, shape changes, locomotion, pinocytosis) to the PKC inhibitor Ro 31–8220 suggest that PKC discriminately regulates functions of human blood lymphocytes. J Leukoc Biol. 1995;57:587–91. doi: 10.1002/jlb.57.4.587. [DOI] [PubMed] [Google Scholar]
- 17.Grimmer SDB, Sandvig K. Membrane ruffling and macropinocytosis in A431 cells require cholesterol. J Cell Sci. 2002;115:2953–62. [Google Scholar]
- 18.Gschwendt M, Muller HJ, Kielbassa K, et al. Rottlerin, a novel protein kinase inhibitor. Biochem Biophys Res Commun. 1994;199:93–8. doi: 10.1006/bbrc.1994.1199. [DOI] [PubMed] [Google Scholar]
- 19.Racoosin EL, Swanson JA. Macrophage colony-stimulating factor (rM-CSF) stimulates pinocytosis in bone marrow-derived macrophages. J Exp Med. 1989;170:1635–48. doi: 10.1084/jem.170.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norbury CC, Hewlett LJ, Prescott AR, Shastri N, Watts C. Class I MHC presentation of exogenous soluble antigen via macropinocytosis in bone marrow macrophages. Immunity. 1995;3:783–91. doi: 10.1016/1074-7613(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 21.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soltoff SP. Rottlerin is a mitochondrial uncoupler that decreases cellular ATP levels and indirectly blocks protein kinase Cdelta tyrosine phosphorylation. J Biol Chem. 2001;276:37986–92. doi: 10.1074/jbc.M105073200. [DOI] [PubMed] [Google Scholar]
- 23.Ridley AJ. Membrane ruffling and signal transduction. Bioessays. 1994;16:321–7. doi: 10.1002/bies.950160506. [DOI] [PubMed] [Google Scholar]
- 24.West MA, Bretscher MS, Watts C. Distinct endocytotic pathways in epidermal growth factor-stimulated human carcinoma A431 cells. J Cell Biol. 1989;109:2731–9. doi: 10.1083/jcb.109.6.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dharmawardhane S, Sanders LC, Martin SS, Daniels RH, Bokoch GM. Localization of p21-activated kinase 1 (PAK1) to pinocytic vesicles and cortical actin structures in stimulated cells. J Cell Biol. 1997;138:1265–78. doi: 10.1083/jcb.138.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meier O, Boucke K, Hammer SV, et al. Adenovirus triggers macropinocytosis and endosomal leakage together with its clathrin-mediated uptake. J Cell Biol. 2002;158:1119–31. doi: 10.1083/jcb.200112067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basner-Tschakarjan E, Mirmohammadsadegh A, Baer A, Hengge UR. Uptake and trafficking of DNA in keratinocytes: evidence for DNA-binding proteins. Gene Ther. 2004;11:765–74. doi: 10.1038/sj.gt.3302221. [DOI] [PubMed] [Google Scholar]
- 28.Gekle M, Drumm K, Mildenberger S, Freudinger R, Gassner B, Silbernagl S. Inhibition of Na+-H+ exchange impairs receptor-mediated albumin endocytosis in renal proximal tubule-derived epithelial cells from opossum. J Physiol. 1999;520:709–21. doi: 10.1111/j.1469-7793.1999.00709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schelling JR, Linas SL. Angiotensin II-dependent proximal tubule sodium transport requires receptor-mediated endocytosis. Am J Physiol. 1994;266:C669–75. doi: 10.1152/ajpcell.1994.266.3.C669. [DOI] [PubMed] [Google Scholar]
- 30.Xu J, Ziemnicka D, Merz GS, Kotula L. Human spectrin Src homology 3 domain binding protein 1 regulates macropinocytosis in NIH 3T3 cells. J Cell Sci. 2000;113:3805–14. doi: 10.1242/jcs.113.21.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Percival JM, Thomas G, Cock TA, et al. Sorting of tropomyosin isoforms in synchronised NIH 3T3 fibroblasts: evidence for distinct microfilament populations. Cell Motil Cytoskeleton. 2000;47:189–208. doi: 10.1002/1097-0169(200011)47:3<189::AID-CM3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 32.Gschwendt M, Kittstein W, Marks F. Elongation factor-2 kinase: effective inhibition by the novel protein kinase inhibitor rottlerin and relative insensitivity towards staurosporine. FEBS Lett. 1994;338:85–8. doi: 10.1016/0014-5793(94)80121-5. [DOI] [PubMed] [Google Scholar]
- 33.McGovern SL, Shoichet BK. Kinase inhibitors: not just for kinases anymore. J Med Chem. 2003;46:1478–83. doi: 10.1021/jm020427b. [DOI] [PubMed] [Google Scholar]
- 34.West MA, Wallin RP, Matthews SP, et al. Enhanced dendritic cell antigen capture via toll-like receptor-induced actin remodeling. Science. 2004;305:1153–7. doi: 10.1126/science.1099153. [DOI] [PubMed] [Google Scholar]
- 35.Watts C, Marsh M. Endocytosis: what goes in and how? J Cell Sci. 1992;103:1–8. doi: 10.1242/jcs.103.1.1a. [DOI] [PubMed] [Google Scholar]
- 36.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–10. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 37.Azuma T, Witke W, Stossel TP, Hartwig JH, Kwiatkowski DJ. Gelsolin is a downstream effector of rac for fibroblast motility. EMBO J. 1998;17:1362–70. doi: 10.1093/emboj/17.5.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.West MA, Antoniou AN, Prescott AR, Azuma T, Kwiatkowski DJ, Watts C. Membrane ruffling, macropinocytosis and antigen presentation in the absence of gelsolin in murine dendritic cells. Eur J Immunol. 1999;29:3450–5. doi: 10.1002/(SICI)1521-4141(199911)29:11<3450::AID-IMMU3450>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 39.Gordon JR. Monocyte chemoattractant peptide-1 expression during cutaneous allergic reactions in mice is mast cell dependent and largely mediates the monocyte recruitment response. J Allergy Clin Immunol. 2000;106:110–6. doi: 10.1067/mai.2000.107036. [DOI] [PubMed] [Google Scholar]
- 40.Weiss DL, Brown MA. Regulation of IL-4 production in mast cells: a paradigm for cell-type-specific gene expression. Immunol Rev. 2001;179:35–47. doi: 10.1034/j.1600-065x.2001.790104.x. [DOI] [PubMed] [Google Scholar]
- 41.Wodnar-Filipowicz A, Heusser CH, Moroni C. Production of the haemopoietic growth factors GM-CSF and interleukin-3 by mast cells in response to IgE receptor-mediated activation. Nature. 1989;339:150–2. doi: 10.1038/339150a0. [DOI] [PubMed] [Google Scholar]
- 42.Sherman MA. The role of STAT6 in mast cell IL-4 production. Immunol Rev. 2001;179:48–56. doi: 10.1034/j.1600-065x.2001.790105.x. [DOI] [PubMed] [Google Scholar]
- 43.Brossart P, Bevan MJ. Presentation of exogenous protein antigens on major histocompatibility complex class I molecules by dendritic cells: pathway of presentation and regulation by cytokines. Blood. 1997;90:1594–9. [PMC free article] [PubMed] [Google Scholar]
- 44.Werling D, Hope JC, Chaplin P, Collins RA, Taylor G, Howard CJ. Involvement of caveolae in the uptake of respiratory syncytial virus antigen by dendritic cells. J Leukoc Biol. 1999;66:50–8. doi: 10.1002/jlb.66.1.50. [DOI] [PubMed] [Google Scholar]
- 45.Croguennec T, Nau F, Pezennec S, Brule G. Simple rapid procedure for preparation of large quantities of ovalbumin. J Agric Food Chem. 2000;48:4883–9. doi: 10.1021/jf991198d. [DOI] [PubMed] [Google Scholar]
- 46.Kjeken R, Mousavi SA, Brech A, Griffiths G, Berg T. Wortmannin-sensitive trafficking steps in the endocytic pathway in rat liver endothelial cells. Biochem J. 2001;357:497–503. doi: 10.1042/0264-6021:3570497. [DOI] [PMC free article] [PubMed] [Google Scholar]