Abstract
The development of successful vaccination strategies for eliciting cytotoxic T lymphocytes (CTLs) will be facilitated by the definition of strategies for subdividing CTLs into functionally distinct subpopulations. We assessed whether surface expression of a number of cell-surface proteins could be used to define functionally distinct subpopulations of memory CTLs in mice immunized with a recombinant vaccinia virus expressing human immunodeficiency virus (HIV)-1 envelope (Env). We found changes in cell-surface expression of CD11a, CD44, CD45RB, CD49d, CD54 and CD62L on Env-specific CD8+ T cells that appeared to differentiate them from other CD8+ T cells within 1 week to 1 month following immunization. Further, we saw an up-regulation of CD62L surface expression on Env-specific CD8+ memory T cells several months after immunization. However, CD62L expression did not correlate with differences in the abilities of CTLs to proliferate or produce interferon gamma (IFN-γ) and tumour necrosis factor alpha (TNF-α) in vitro in response to Env peptide stimulation. Moreover, the expression of CD62L did not allow differentiation of CTLs into subpopulations with distinct expansion kinetics in vivo after adoptive transfer into naïve mice and subsequent boosting of these mice with a recombinant adenovirus expressing HIV-1 Env. Therefore, the definition of memory CD8+ T-cell subpopulations on the basis of CD62L expression in mice does not allow the delineation of functionally distinct CTL subpopulations.
Keywords: CD8+ T lymphocyte, cell surface molecule, memory, mouse, vaccination
Introduction
A number of persistent or chronic pathogens, including Mycobacterium tuberculosis, human immunodeficiency virus (HIV) and hepatitis C virus, are contained by cellular immune responses, and efforts to create effective vaccines to protect against these organisms have therefore focused on the generation of cytotoxic T lymphocytes (CTLs).1 The goal of these vaccines is to induce long-term memory cell populations that will rapidly expand upon exposure to the replicating pathogen.2 The vaccine strategies being explored for protection against these organisms include plasmid DNA and live attenuated or replication-deficient recombinant viral vectors, as they induce potent, high-frequency CD8+ T-cell responses.3
Distinct populations of memory CD8+ T cells have been defined based on phenotypic, homing and functional characteristics.4,5 Effector memory T cells (TEMs) migrate to peripheral tissues and sites of inflammation. Upon re-encountering a specific antigen, these cells rapidly mediate effector functions, including perforin-associated cytolytic activity and cytokine secretion. In this manner, TEMs provide immediate protection against infection or reactivation of micro-organisms. Central memory T cells (TCMs) express lymph node homing receptors that lead to cellular retention in secondary lymphoid organs. Although they can differentiate with time into cytolytic and interferon (IFN)-γ-producing cells, they do not rapidly mediate effector functions following antigen exposure.4–7 TCMs have a greater capacity to persist in vivo as well as a higher proliferative capacity than TEMs. Thus, it is presumed that TCMs have the greatest potential for conferring protective immunity against pathogens, as they will rapidly expand on exposure to a pathogen and differentiate into effector cells that will populate peripheral sites.1,8
The development of vaccine strategies for inducing effective cellular immune responses would be greatly facilitated by the definition of cell surface proteins whose selective expression would allow the differentiation of antigen-specific memory CD8+ T cells into TEMs and TCMs. An ideal immunization protocol may induce both subsets of memory cells: TCMs that proliferate in secondary lymphoid tissue to expand the effector lymphocyte population, and TEMs that can immediately fight invading pathogens at the site of infection.1 However, as TCMs are purported to have a greater proliferative capacity than TEMs, priming immunizations may be most effective if they expand the largest possible population of TCMs. Moreover, the most effective timing for delivery of boost immunizations should be at the time of maximal TCM expansion.3 These issues would be clarified by an ability to monitor the development of subsets of antigen-specific memory CD8+ T cells in vivo.
The present study was initiated to explore the utility of selected monoclonal antibodies for defining functionally distinct subpopulations of vaccine-elicited CTLs. In fact, we found that the expression of cell surface molecules that had been associated with memory CTLs was not useful for differentiating between functionally distinct subpopulations of vaccine-induced CTLs.
Materials and methods
Mice
Female Balb/c mice (6–12 weeks of age) were obtained from Charles River Laboratories (Cambridge, MA) or Taconic (Germantown, NY). All animal studies were performed in accordance with the Harvard University Handbook for the Care and Use of Laboratory Animals.
Viruses and immunizations
Recombinant vaccinia virus expressing HIV-1 B10 gp160 (rVac-Env) was generously provided by Dennis Panicali, Therion Biologics Corporation (Cambridge, MA). Mice were immunized intraperitoneally (i.p.) with 2 × 107 plaque-forming units (PFU) of rVac-Env. Recombinant adenovirus expressing HIV-1 envelope (Env) (rAdeno-Env) was generously provided by Dr Gary Nabel (Vaccine Research Center, National Institutes of Health, Bethesda, MD). Mice were immunized intramuscularly (i.m.) in the quadriceps with 106.5 particles of rAdeno-Env.
H-2Dd/p18 tetramer construction
Tetrameric H-2Dd major histocompatibility complex (MHC) class I with the p18 peptide RGPGRAFVTI from the V3 loop of HIV-1 Env9 (H-2Dd/p18 tetramer) was produced as previously described10,11 using streptavidin coupled to phycoerythrin (PE) (DAKO Corporation, Glostrup, Denmark).
Surface staining of blood and splenocyte samples for phenotypic analyses
Peripheral blood mononuclear cells (PBMCs) from 100 µl of heparinized blood were prepared as previously described, with red blood cells (RBCs) lysed in a solution of NH4Cl-Tris.11,12 Isolated splenocytes or PBMCs were washed in phosphate-buffered saline (PBS) containing 2% fetal calf serum (FCS) and stained with 0·1–0·2 µg H-2Dd/p18 tetramer-PE along with anti-CD62L-fluorescein isothiocyanate (FITC) and anti-CD8α-PerCP (clones MEL-14 and 53-6·7, respectively; BD Pharmingen, San Diego, CA) or anti-CD8α-allophycocyanin (APC) (clone CT-CD8a; Caltag Laboratories, Burlingame, CA). Additional phenotypic analyses were performed by staining splenic samples with H-2Dd/p18 tetramer-PE and anti-CD8α-PerCP along with one of the following antibodies conjugated to FITC: anti-CD11a, anti-CD25, anti-CD44, anti-CD45RB, anti-CD49d, anti-CD54 or anti-CD95 (clones M17/4, 7D4, IM7, 16 A, R1-2, 3E2 and Jo2, respectively; BD Pharmingen). Samples were fixed in 1·5% paraformaldehyde and analysed on a FACSCalibur using CellQuest software (BD Immunocytometry, San Jose, CA).
Splenocyte sorting into CD62L+ and CD62L– T cells
In vitro assays
At 1, 2 and 8 months after rVac-Env immunization, splenocytes from two mice were isolated and pooled. T cells were negatively selected using the Pan T Cell Isolation Kit and an AutoMACS separator according to the manufacturer's instructions (Miltenyi Biotec GmbH, Gladbach, Germany). A subsample of the recovered T cells was removed for use in assays, and the remainder were incubated with anti-CD62L beads (Miltenyi) and sorted again via AutoMACS into CD62L+ and CD62L– T-cell fractions. Each fraction, as well as unsorted cells, was stained with H-2Dd/p18 tetramer-PE, anti-CD62L-FITC, anti-CD3ε-PerCP (clone 145-2C11; BD Pharmingen) and anti-CD8α-APC to monitor sorting efficiency as well as the proportion of H-2Dd/p18 tetramer+ CD8+ cells in each fraction. The T-cell fractions for each experiment were 92–98% pure. Between 41 and 57% of the CD62L+ subpopulations were comprised of T cells staining positively for CD62L, while between 87 and 94% of CD62L– subpopulations were comprised of T cells staining negatively for CD62L surface expression. The apparently large proportion of CD62L− cells in the analysed CD62L+ subpopulation was a result of the blocking of the anti-CD62L-FITC staining antibody by the previously bound anti-CD62L sorting beads, and therefore does not reflect the true purity of the CD62L+ subpopulation (data not shown).
In vivo assays
Splenocytes from 13 to 16 rVac-Env-immunized mice (> 4 months postimmunization) were pooled and sorted into CD62L+ CD8+ T and CD62L− CD8+ T-cell fractions. This was accomplished by first incubating splenocytes with a combination of anti-CD4-, anti-CD19- and anti-CD11b-conjugated paramagnetic beads (Miltenyi) according to the manufacturer's instructions. Cells were sorted by AutoMACS, and the negative fraction was incubated with anti-CD62L-conjugated beads for separation into CD62L+ and CD62L– memory subpopulations. To determine the sorting efficiency and percentage of H-2Dd/p18 tetramer+ CD8+ cells in each lymphocyte subpopulation, subsamples of each fraction were stained with H-2Dd/p18 tetramer-PE, anti-CD3ε-PerCP, anti-CD8α-APC, and anti-CD62L-FITC, anti-CD19-FITC or anti-CD11b-FITC (clones 1D3 and M1/70, respectively; BD Pharmingen), as well as with anti-CD4-PE or anti-CD4-APC (clone CT-CD4; Caltag) in combination with anti-CD3ε and anti-CD8α antibodies. The T-cell fractions for each experiment were 41–71% pure. Generally <1% of isolated subpopulations were CD4+ T cells. Approximately 50% of CD8+ T cells in CD62L+ subpopulations stained positively for CD62L surface expression, while >95% of CD8+ T cells in CD62L– subpopulations stained negatively for CD62L surface expression.
In vitro proliferation
Following sorting, unfractionated T cells, CD62L+ T cells and CD62L– T cells were labelled with 0·8 µm CFSE (Molecular Probes, Eugene, OR) and then rested overnight at 4 × 106 cells/ml in media containing 5% FCS to avoid confounding effects resulting from the stimulation of the CD62L+ population during the process of sorting. The following day, 3 × 105 cells of each of the three T-cell subpopulations were placed into 96-well plates with or without 100 ng/ml p18 peptide. On the second day of culture, 5 U/ml recombinant rat interleukin (IL)-2 (Sigma-Aldrich Company, St Louis, MO) was added to all cells. Cells were harvested on days 0–8 of culture and stained with H-2Dd/p18 tetramer-PE, anti-CD3ε-PerCP and anti-CD8α-APC for flow cytometric analysis of CFSE dilution.
Intracellular cytokine stimulation and staining
Following an overnight rest, unfractionated T cells, CD62L+ T cells and CD62L– T cells were stained with H-2Dd/p18 tetramer-PE and then washed. In parallel, aliquots of as many as 2 × 106 of each cell population were exposed to no peptide or 1 µg/ml p18 peptide and 2 µg/ml each of anti-CD28 and anti-CD49d (clones 37·51 and R1-2, respectively; BD Pharmingen). As controls, aliquots of cells were also exposed to 10 µg/ml phorbol 12-myristate 13-acetate (PMA) and 50 µg/ml ionomycin (Sigma). All samples were treated with Golgi Plug (containing brefeldin A; BD Pharmingen) and then incubated for 6 hr at 37°. Following incubation, samples were stained with H-2Dd/p18 tetramer-PE, anti-CD62L-FITC and anti-CD8α-PerCP, and then permeabilized by incubation with Cytofix/Cytoperm (BD Pharmingen) and stained with anti-IFN-γ- or anti-tumour necrosis factor (TNF)-α-APC (clones XMG1·2 and MP6-XT22, respectively; BD Pharmingen) for flow cytometric analysis.
In vivo proliferation
Splenocytes from 13 to 16 mice immunized with rVac-Env were pooled and then sorted into CD62L+ CD8+ T-cell and CD62L– CD8+ T-cell fractions as described above. Two separate experiments were performed, one at 5 months and one at 8 months post-rVac-Env immunization. Cells were labelled with 8–10 µm CFSE at 37° for 30–60 min and then rested overnight at 4 × 106 cells/ml in media containing 5% mouse serum (Jackson ImmunoResearch Laboratories, West Grove, PA) to avoid confounding effects resulting from the stimulation of the CD62L+ population during the process of sorting. The following day, sorted cells were transferred into naïve Balb/c mice by tail vein injection. The total number of cells transferred was ≤50 × 106 donor cells per recipient mouse and contained 2–8 × 105 H-2Dd/p18 tetramer+ CD8+ cells. In a given experiment, each mouse received an equivalent number of H-2Dd/p18 tetramer+ CD8+ T cells. Recipients of these Env-specific memory CD8+ T cells were boosted immediately following adoptive cell transfer with 106.5 particles of rAdeno-Env. Following transfer and boosting, peripheral blood samples were obtained from recipient mice and the proliferation of the infused donor cell populations was determined by evaluating CFSE dilution and staining with H-2Dd/p18 tetramer-PE, anti-CD3ε-PerCP and anti-CD8α-APC by flow cytometric analysis. Control experiments were performed similarly using donor splenocytes from naïve mice (data not shown).
Results
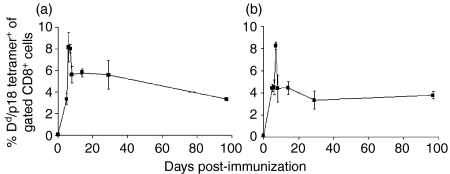
We first established a mouse model to explore phenotypic strategies for defining HIV-1-vaccine-elicited memory CTLs. A single i.p. inoculation of Balb/c mice with 2 × 107 PFU of recombinant vaccinia-HIV-1 BH10 gp160 elicited a high-frequency and durable HIV-1 Env-specific CTL response (Fig. 1). The magnitude of the CTL response in these mice was determined by monitoring the well-defined H-2Dd-restricted p18 epitope-specific CD8+ CTL response in PBMCs using tetramer staining and flow cytometric analysis (Fig. 1a). This epitope-specific CTL response was maximal in PBMCs on day 6 following immunization, with 8·2 ± 1·4% of CD8+ T cells binding the H-2Dd/p18 tetramer. This CTL population decayed rapidly thereafter, reaching a plateau 2 days later of 5·6 ± 0·8% of CD8+ PBMCs. Importantly, H-2Dd/p18 tetramer-binding CD8+ T cells were readily detected at least as late as 8 months following a single inoculation of the recombinant vaccinia-HIV-1 Env construct (4·5 ± 0·5% of CD8+ PBMCs; data not shown). Moreover, these circulating H-2Dd/p18 tetramer+ cells represent a systemic antigen-specific cell population, as tetramer-binding CD8+ cell populations of comparable magnitude were detected in the spleens of these same mice (Fig. 1b).
Figure 1.
Kinetics of p18-specific CD8+ cell responses following immunization with recombinant vaccinia virus expressing human immunodeficiency virus (HIV)-1 B10 gp160 (rVac-Env). Dd/p18 tetramer+ CD8+ cells as a percentage of total CD8+ cells were monitored in (a) peripheral blood mononuclear cells (PBMCs) and (b) spleen cells of Balb/c mice inoculated intraperitoneally (i.p.) with 2 × 107 plaque-forming units (PFU) of rVac-Env. Plotted values represent the median ± standard error for n = 5–10 (a) or n = 4 (b) mice.
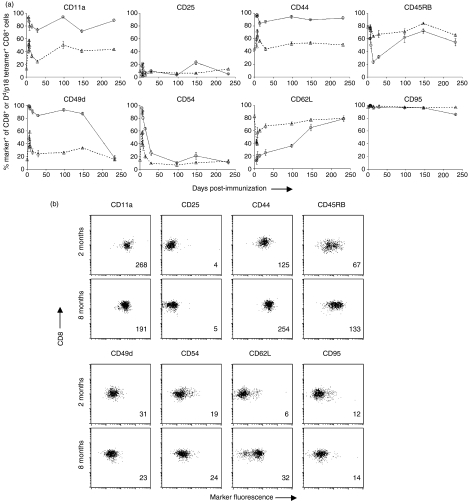
In view of the impressive durability of the HIV-1 Env epitope-specific CD8+ T-cell responses elicited by rVac-Env immunization, we sought to determine whether we could detect phenotypic and functional heterogeneity in these CTLs. Our aim was to determine whether phenotypic markers could be established that define the memory subset of CTLs in these vaccinated mice. To this end, naïve mice and mice inoculated with rVac-Env 5, 6, 7, 8 and 14 days previously, as well as 1, 3, 5 and 8 months previously, were killed and splenocytes were evaluated by monoclonal antibody staining and flow cytometric analysis. CD8+ T cells and H-2Dd/p18 tetramer+ CD8+ T cells were evaluated for expression over time of the following molecules: CD11a, CD25, CD44, CD45RB, CD49d, CD54, CD62L and CD95 (Fig. 2). These phenotypic analyses demonstrated that all p18-specific CD8+ T cells expressed the activation-associated surface adhesion molecules CD11a (integrin αL) and CD44 (hyaluronate receptor) virtually as soon as the CTLs were detected, and maintained expression of these molecules throughout the duration of the study. CD11a and CD44 were detected on only approximately half of all CD8+ T cells of these mice. In contrast to this, expression of both CD25 (IL-2Rα) and CD95 (Fas) on H-2Dd/p18 tetramer+ CD8+ T cells remained indistinguishable from their expression by the entire CD8+ T-cell population throughout the period of evaluation. CD54 [intercellular adhesion molecule type 1 (ICAM-1)] was expressed on almost all H-2Dd/p18 tetramer+ CD8+ T cells early following recombinant vaccinia inoculation and fell to levels seen on all CD8+ T cells (10–20% CD54+) by 1 month following vaccination. While only a small percentage of H-2Dd/p18 tetramer+ CD8+ T cells expressed the cell surface protein CD45RB (protein tyrosine phosphatase) and CD62L (l-selectin) early after vaccination (∼ 20%), the expression of these molecules was indistinguishable from that on all CD8+ T cells by 5 months following vaccination. H-2Dd/p18 tetramer+ CD8+ T cells shifted from >80% CD49d (integrin α4) expression positive until 5 months following immunization to a percentage indistinguishable from that of all CD8+ T cells (∼ 20% CD49d+) by approximately 8 months following vaccination.
Figure 2.
Activation/maturation-associated surface molecule expression on CD8+ T cells and Dd/p18 tetramer+ CD8+ cells following immunization with recombinant vaccinia virus expressing human immunodeficiency virus (HIV)-1 B10 gp160 (rVac-Env). Shown are the percentages of total CD8+ cells and Dd/p18 tetramer+ CD8+ cells (a) or dot plots and mean fluorescence intensity (MFI) of total CD8+ cells (b) expressing the indicated surface proteins in spleens of Balb/c mice inoculated intraperitoneally (i.p.) with 2 × 107 plaque-forming units (PFU) of rVac-Env. In (a), plotted values represent the median ± standard error for n = 4 mice. Circles, Dd/p18 tetramer+; triangles, CD8+. In (b), a representative dot plot is shown from the day 29 (2 month) and day 232 (8 month) post-infection (p.i.) time-points; the MFI of these CD8+ cell populations is indicated in the lower right-hand corner of each plot.
In view of the dynamic changes in CD62L expression by the vaccine-elicited H-2Dd/p18 tetramer+ CD8+ T cells (Fig. 2) and the previously reported expression of this molecule on memory T cells,6,13–16 we sought to determine whether the expression of CD62L could be used to differentiate between effector and memory CTLs. We therefore isolated CD62L+ and CD62L– subsets of H-2Dd/p18 tetramer+ CD8+ T cells at various times postimmunization and evaluated their functional properties using both in vitro and in vivo assays. Because stimulation of CD62L+ T cells results in the rapid loss of surface CD62L expression,17–20 we fractionated CD62L+ and CD62L– T-cell subsets prior to performing assays. Moreover, as it is also possible that the procedures used to isolate these lymphocyte subsets might expose CD62L+ T cells to a costimulatory signal that might alter the responsiveness of the cells, we rested the lymphocyte subsets in culture overnight following fractionation before subjecting them to functional evaluation.
In vitro proliferative capacity
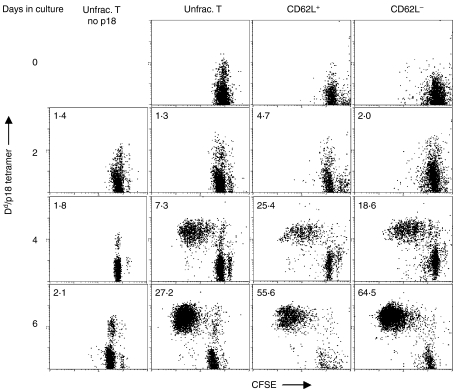
Splenocytes were obtained from mice 1, 2 or 8 months following rVac-Env immunization and were evaluated as unfractionated, CD62L+ or CD62L– T cells. These lymphocyte populations were stimulated by p18 peptide and their in vitro proliferation was assessed (Figs 3 and 4). The proliferation of H-2Dd/p18 tetramer+ CD8+ T cells obtained from mice 2 months post-rVac-Env immunization was first evaluated by flow cytometric assessment of CFSE-labelled cells (Fig. 3). In the absence of p18 peptide stimulation, CD8+ T cells in culture did not divide. Following exposure to p18 peptide, the unfractionated, CD62L+ and CD62L– T cells demonstrated a comparable dilution of CFSE after 2, 4 and 6 days in culture with p18, indicating comparable in vitro proliferation. In addition, the fold-increase in the total H-2Dd/p18 tetramer+ CD8+ T-cell numbers after 2, 4 and 6 days in culture with p18 (normalized to day 0 lymphocyte numbers) noted in Fig. 3 indicates comparable proliferation of CD62L+ and CD62L– T cells. Splenocytes obtained from mice at 1 and 8 months following rVac-Env immunization were similarly evaluated; the unfractionated, CD62L+ and CD62L– T cells demonstrated a comparable dilution of CFSE (data not shown).
Figure 3.
In vitro p18 peptide stimulation of CD62L+ and CD62L– Dd/p18 tetramer+ CD8+ T-cell division. Splenocytes from two Balb/c mice immunized with 2 × 107 plaque-forming units (PFU) of recombinant vaccinia virus expressing human immunodeficiency virus (HIV)-1 B10 gp160 (rVac-Env) were harvested at 2 months postinoculation. Populations of unfractionated (unfrac.) T cells, CD62L+ T cells and CD62L– T cells (3 × 105 cells/well in 96-well plates) were labelled with 0·8 µm CFSE and cultured with 100 ng/ml p18 peptide and 5 U/ml rat interleukin (IL)-2 added on day 2 of culture. Loss of CFSE fluorescence by splenocytes after 2, 4, and 6 days of culture indicates division of Dd/p18 tetramer+ CD8+ T cells. The fold-increase in total Dd/p18 tetramer+ CD8+ T-cell number in cultures (normalized to day 0 lymphocyte numbers) is indicated in the upper left corner of each dot plot.
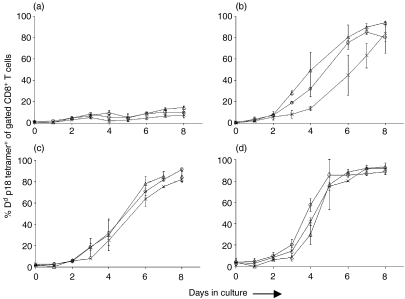
Figure 4.
In vitro p18 peptide stimulation of CD62L+ and CD62L– Dd/p18 tetramer+ CD8+ T-cell division. Splenocytes from two Balb/c mice immunized with 2 × 107 plaque-forming units (PFU) of recombinant vaccinia virus expressing human immunodeficiency virus (HIV)-1 B10 gp160 (rVac-Env) were harvested at the indicated times postinoculation. Populations of unfractionated T cells (crosses), CD62L+ T cells (circles) and CD62L– T cells (triangles) (3 × 105 cells/well in 96-well plates) were cultured with 100 ng/ml p18 peptide and 5 U/ml rat interleukin (IL)-2 added on day 2 of culture. (a) No peptide; (b) 1 month post-infection (p.i.); (c) 2 months p.i.; (d) 8 months p.i. The kinetics of expansion of Dd/p18 tetramer+ CD8+ T cells is shown over 8 days in culture. Plotted values represent the median ± standard error of two separately performed experiments.
Further, we evaluated the magnitude and kinetics of expansion of the p18-specific CTL subpopulations following in vitro exposure to p18 peptide (Fig. 4). An expansion of H-2Dd/p18 tetramer+ CD8+ T cells was evident by 3 days after initiating the cultures of p18- and IL-2-stimulated splenocytes, and continued until day 8 of culture, by which time >80% of CD8+ T cells in all cultures were p18-specific. In the presence of p18 peptide stimulation but in the absence of IL-2, cultured H-2Dd/p18 tetramer+ CD8+ T cells divided much more slowly and generally did not exceed 40% of CD8+ T cells (data not shown). The kinetics of the expansion of H-2Dd/p18 tetramer+ CD8+ T cells in the IL-2- and p18 peptide-exposed cultures were similar in unfractionated, CD62L+ and CD62L– CD8+ T cells at all evaluated time-points. Moreover, this same finding was noted in splenocytes obtained from mice 1, 2 and 8 months following vaccination. Therefore, CD62L expression did not differentiate between subpopulations of CTLs with different proliferative capacities.
IFN-γ and TNF-α production
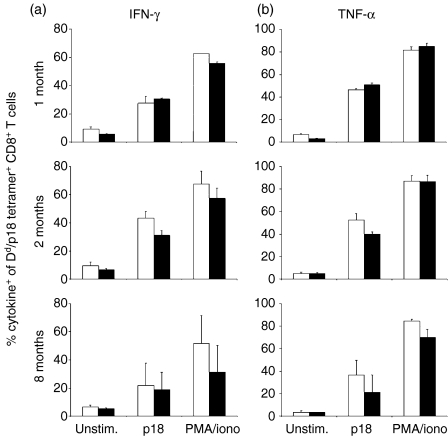
We then evaluated the cytokine production profile of these subpopulations of vaccine-elicited CTLs. CD62L+ and CD62L– CD8+ splenic T cells were isolated from mice immunized with rVac-Env 1, 2 or 8 months previously. These cell populations were stimulated in vitro for 6 hr with p18 peptide and assessed by intracellular cytokine staining for production of IFN-γ(Fig. 5a) and TNF-α (Fig. 5b). Modest differences in p18-stimulated cytokine production by these cell subpopulations were apparent at these various time-points. A similar evaluation of IL-2 production by the CD62L+ and CD62L– CD8+ splenic T-cell subpopulations was not performed as we observed that fewer than 15% of p18-specific CD8+ T cells produced IL-2 following a 10-hr stimulation with p18 peptide (data not shown). Cytokine production by p18-stimulated lymphocyte subpopulations was maximal in splenocytes obtained 2 months following vaccination, with 30–45% of H-2Dd/p18 tetramer+ CD8+ T cells producing IFN-γ and 40–55% producing TNF-α. At 8 months following vaccination, H-2Dd/p18 tetramer+ CD8+ T cells produced lower levels of both cytokines following p18 peptide stimulation, with 20% producing IFN-γ and 20–35% producing TNF-α. Importantly, no significant differences were noted in the production of these cytokines between the CD62L+ and CD62L– H-2Dd/p18 tetramer+ CD8+ lymphocytes. Similar results were obtained in parallel studies performed following a 3-hr rather than a 6-hr in vitro exposure of the isolated T-cell subpopulations to p18 peptide (data not shown). Therefore, CD62L expression did not allow the differentiation of distinct subpopulations of CTLs with different cytokine production profiles.
Figure 5.
In vitro p18 peptide stimulation of interferon (IFN)-γ and tumour necrosis factor (TNF)-α production by CD62L+ and CD62L– Dd/p18 tetramer+ CD8+ T cells. Splenocytes from two Balb/c mice immunized with 2 × 107 plaque-forming units (PFU) of recombinant vaccinia virus expressing human immunodeficiency virus (HIV)-1 B10 gp160 (rVac-Env) were harvested at the indicated times post-infection (p.i.). Populations of up to 2 × 106, CD62L+ T cells (open bars) and CD62L– T cells (filled bars) were incubated with no peptide (Unstim.) or 1 µg/ml p18 peptide and 2 µg/ml each of anti-CD28 and anti-CD49d antibodies (p18). Control cells were incubated with 10 µg/ml phorbol 12-myristate 13-acetate (PMA) and 50 µg/ml ionomycin (PMA/iono). All samples were treated with Golgi Plug and incubated for 6 hr at 37° before flow cytometric analysis for production of IFN-γ (a) or TNF-α (b) Plotted values represent the mean ± standard error of two separately performed experiments.
In vivo proliferative capacity
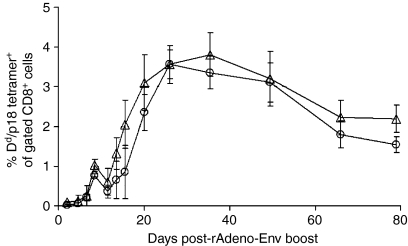
While these in vitro analyses of proliferative function and cytokine production did not suggest that CD62L expression provided a useful means of separating vaccine-elicited CD8+ T lymphocytes into functionally distinct subpopulations, we evaluated vaccine-generated CD62L+ and CD62L– CTLs for their ability to expand in vivo following a secondary exposure to antigen. Mice were immunized with rVac-Env and splenocytes were obtained 5 or 8 months later. These Env peptide-primed splenocytes were incubated with anti-CD19-, anti-CD11b- and anti-CD4-conjugated magnetic beads. CD4+ T cells were removed from the immune splenocytes to avoid the confounding functional effect of an unequal distribution of p18-specific CD4+ T cells between CD62L+ and CD62L– CD8+ T-cell subpopulations (data not shown). The isolated CD8+ T cells were then incubated with anti-CD62L-conjugated magnetic beads to generate cell subpopulations for adoptive transfer. Figure 6 shows the combined results of two experiments in which rVac-Env vaccine-induced memory CD62L+ or CD62L– H-2Dd/p18 tetramer+ CD8+ T cells were adoptively transferred in equal numbers into naïve Balb/c mice. These recipient mice were then immediately immunized with 106.5 particles of rAdeno-Env. We had previously determined that 106.5 particles of rAdeno-Env would induce the expansion of pre-existing H-2Dd/p18 tetramer+ CD8+ T-cell populations but would not elicit a measurable H-2Dd/p18 tetramer+ CD8+ T-cell response in the peripheral blood of naïve mice (data not shown). No differences in the magnitude or kinetics of the responses of CD62L+ or CD62L– H-2Dd/p18 tetramer+ CD8+ T cells were seen in the recipient mice following rAdeno-Env immunization. Both CD62L+ and CD62L– memory H-2Dd/p18 tetramer+ CD8+ T-cell subpopulations began expanding in PBMCs 1 week following immunization, and reached plateau levels of 3–4% of CD8+ T cells 4–5 weeks later. Thus, CD62L expression did not provide a useful phenotypic marker for fractionating functionally distinct rVac-Env-elicited CD8+ memory CTL subpopulations.
Figure 6.
In vivo expansion of CD62L+ (circles) and CD62L– (triangles) Dd/p18 tetramer+ CD8+ T cells following antigen stimulation. A total of 2–8 × 105 CD62L+ or CD62L– Dd/p18 tetramer+ CD8+ T cells were harvested from spleens of 13–16 Balb/c mice at 5 or 8 months postinoculation with 2 × 107 plaque-forming units (PFU) of recombinant vaccinia virus expressing human immunodeficiency virus (HIV)-1 B10 gp160 (rVac-Env), sorted into CD62L+ and CD62L– CD8+ T-cell populations, and adoptively transferred into naïve Balb/c mice. Recipient mice were boosted with 106.5 particles of rAdeno-Env intramuscularly (i.m.) at the time of adoptive transfer. Plotted values represent the median ± standard error of two separately performed experiments with n = 5–7 (CD62L+) or n = 4–6 (CD62L–) recipient mice.
Discussion
These studies were initiated in order to define a phenotypic profile of recombinant poxvirus-elicited memory CTLs to facilitate the process of devising strategies to expand CTLs maximally through vaccination. A prospective evaluation of recombinant vaccinia-HIV-1 Env-induced CTLs demonstrated a dramatic change in CD62L expression in the weeks following immunization, whereas the expression of CD11a, CD44 and CD49d on recombinant vaccinia-HIV-1 Env-induced CTLs was relatively stable. In fact, up-regulation of CD62L on memory T cells following antigen exposure has been observed in several model murine systems, including skin allografts and infection with Listeria monocytogenes, influenza virus, and Sendai virus.15,16,21–23 However, the kinetics of the switch of antigen-specific CD8+ T cells from CD62L– to CD62L+ varies widely. For example, by 5 weeks following Listeria infection and by 2 months following skin allografting, more than 60% of antigen-specific memory T cells in mice express CD62L. However, the expression of CD62L on virus-specific T cells did not occur until approximately 5 months following Sendai virus infection, and until 1 year or more following influenza virus infection.15,16,21–23 The kinetics of CD62L expression on the H-2Dd/p18 tetramer+ CD8+ T lymphocytes in the present study were thus consistent with previous findings.
The significance and impact of CD62L surface expression on memory T cells are not clear. CD62L plays an important role in lymphocyte rolling along endothelial surfaces during trafficking to lymphoid tissues. Cells lacking surface CD62L are confined mainly to non-lymphoid tissues.24,25 CD62L can also function as a signalling receptor by virtue of its association with tyrosine kinases. Its ligation in the process of lymphocyte trafficking and homing leads to increased tyrosine phosphorylation of several cellular proteins, and results in increased MAPK activity and the translocation of NFAT to the nucleus.26,27 Both the trafficking and the signalling functions of CD62L could explain an association of CD62L with memory T cells.
Several groups have proposed that memory T cells do not immediately display all of their hallmark functional traits early in their development, but instead gradually acquire memory cell characteristics.4,8,28 Thus a gradual re-expression of CD62L on memory T cells after recombinant vaccinia immunization (Fig. 2) might represent a useful surrogate marker for the acquisition of characteristic memory cell functions. We reasoned that such functions, in particular the ability to proliferate rapidly in response to antigenic stimulation, might be harnessed to improve our ability to generate memory CTLs by inoculation with recombinant vaccinia vectors. We therefore tested CD62L+ and CD62L– CD8+ memory T cells at 1, 2 and 8 months post-recombinant vaccinia immunization to determine whether there was indeed a kinetic of functional acquisition that correlated with CD62L surface expression. We analysed the capacity of CD62L+ and CD62L– CD8+ memory T-cell subpopulations to proliferate in culture (Figs 3 and 4) and to produce cytokine (Fig. 5) in response to specific p18 peptide stimulation. In the future, similar analyses comparing the functional capacities of CTLs with differential surface expression of the IL-7Rα chain (CD127) may prove useful, as recent evidence has suggested that it is the IL-7Rαhi cells that differentiate into long-lived memory cells.29,30
Based on the results of previous studies, we expected CD62L– T cells to produce cytokines more efficiently than CD62L+ T cells following restimulation with the p18 peptide, while CD62L+ T cells would re-enter the cell cycle more quickly than CD62L– T cells.6,13,23,31–35 We also expected that the functional capacities of memory CD8+ T cells would evolve with the passage of time postinoculation.8,28,36,37 However, we found the rVac-Env-induced CD62L+ and CD62L– H-2Dd/18 tetramer+ CD8+ T-cell subpopulations were functionally indistinguishable. These cell subpopulations demonstrated comparable dilution of CFSE labelling, expansion in culture, and production of cytokines following p18 stimulation at all evaluated time-points following immunization.
We also assessed the abilities of CD62L+ and CD62L– H-2Dd/p18 tetramer+ CD8+ T-cell subpopulations to expand in vivo following adoptive transfer into naïve mice and a repeated antigen exposure by boosting the recipient mice with a recombinant adenovirus vector expressing HIV-1 Env. We removed CD4+ cells from the T-cell subpopulations for these in vivo experiments to avoid the complication of transferring unequal numbers of Env-specific CD4+ T cells along with the CD62L+ and CD62L– H-2Dd/p18+ CD8+ T-cell subpopulations. The absence of Env-specific CD4+ cells in the transferred cell subpopulations should not have hindered the p18-driven secondary expansion of H-2Dd/p18 tetramer+ CD8+ T cells, as the absence of CD4+ cells during recall responses has minimal effects on the ability of previously generated memory CD8+ cells to respond.38–40 Nevertheless, we saw no reproducible differences in the in vivo proliferative abilities of CD62L+ and CD62L– H-2Dd/p18 tetramer+ CD8+ T cells (Fig. 6), with indistinguishable kinetics of expansion of these cells following stimulation with the p18 antigen.
However, a number of technical limitations may have biased the outcome of these adoptive transfer experiments. Because these studies were not performed using transgenic mice, we were limited to transferring 8 × 105 p18-specific CD8+ T cells into recipient mice. We also could not rule out the possibility that the H-2Dd/p18 tetramer+ CD8+ T-cell responses generated in the recipient mice were the result of a primary immune response against the p18 epitope. Arguing against that possibility, however, was the observation that 106.5 particles of recombinant adenovirus is a quantity of virus that expands p18-primed but not naïve CD8+ T cells and, further, does so with the characteristic kinetics of a secondary immune response (data not shown).
The findings of the present study in mice are not in accord with the described paradigm of functionally distinct populations of human memory TEMs and TCMs associated with distinct surface expression of CCR7 and CD62L.4,6,13,31–34,41 However, the results of the present studies are consistent with experiments in lymphocytic chonomeningitis virus (LCMV)- or Listeria-infected mice demonstrating that both CCR7+ CD62L+ (TCM) and CCR7– CD62L– (TEM) memory CD8+ T cells can rapidly kill in vivo, proliferate in vitro, and produce the cytokines IFN-γ and TNF-α in response to specific antigenic stimulation.8,15,42–45 Further, a third subpopulation of memory CD8+ T cells has recently been proposed: intermediate memory T cells (TIMs), with a mixed CD62L– and CCR7+ phenotype.46 Because equal numbers of antigen-specific TEMs and TIMs resided in the spleens of LCMV-infected mice,46 it is possible that a TIM subpopulation of cells may have influenced the results of the present studies. Recent attempts to define antigen-specific CD8+ TCM and TEM subpopulations in humans have proved increasingly complex, with the expression of as many as five separate cell-surface proteins (CD27, CD28, CD45RA, CCR5 and CCR7) being evaluated.47 Finally, as cells expressing the two lymph node homing receptors CD62L and CCR7 have been seen in non-lymphoid organs, expression of these molecules may not accurately predict the functional capacities of cells found in either lymphoid or non-lymphoid compartments.46,48–51 The present study suggests that CD62L expression alone is not sufficient to define distinct functional subpopulations of memory CD8+ T cell subsets in mice following rVac-Env immunization.
Acknowledgments
The authors wish to thank Robert A. Seder for helpful discussions, and Kristin R. Beaudry and Karen L. Hershberger for technical assistance. This work was supported by National Institutes of Health Grant AI-20729 (to N.L.L.).
References
- 1.Esser MT, Marchese RD, Kierstead LS, Tussey LG, Wang F, Chirmule N, Washabaugh MW. Memory T cells and vaccines. Vaccine. 2003;21:419–30. doi: 10.1016/s0264-410x(02)00407-3. [DOI] [PubMed] [Google Scholar]
- 2.Veiga-Fernandes H, Walter U, Bourgeois C, McLean A, Rocha B. Response of naive and memory CD8+ T cells to antigen stimulation in vivo. Nat Immunol. 2000;1:47–53. doi: 10.1038/76907. [DOI] [PubMed] [Google Scholar]
- 3.Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol. 2003;4:835–42. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- 4.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 5.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–63. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 6.Tussey L, Speller S, Gallimore A, Vessey R. Functionally distinct CD8+ memory T cell subsets in persistent EBV infection are differentiated by migratory receptor expression. Eur J Immunol. 2000;30:1823–9. doi: 10.1002/1521-4141(200007)30:7<1823::AID-IMMU1823>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 7.Berenzon D, Schwenk RJ, Letellier L, Guebre-Xabier M, Williams J, Krzych U. Protracted protection to Plasmodium berghei malaria is linked to functionally and phenotypically heterogeneous liver memory CD8+ T cells. J Immunol. 2003;171:2024–34. doi: 10.4049/jimmunol.171.4.2024. [DOI] [PubMed] [Google Scholar]
- 8.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–34. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 9.Shirai M, Kurokohchi K, Pendleton CD, Arichi T, Boyd LF, Takahashi H, Margulies DH, Berzofsky JA. Reciprocal cytotoxic T lymphocyte cross-reactivity interactions between two major epitopes within HIV-1 gp160. J Immunol. 1996;157:4399–411. [PubMed] [Google Scholar]
- 10.Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–6. doi: 10.1126/science.274.5284.94. [DOI] [PubMed] [Google Scholar]
- 11.Kuroda MJ, Schmitz JE, Barouch DH, et al. Analysis of Gag-specific cytotoxic T lymphocytes in simian immunodeficiency virus-infected rhesus monkeys by cell staining with a tetrameric major histocompatibility complex class I-peptide complex. J Exp Med. 1998;187:1373–81. doi: 10.1084/jem.187.9.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santra S, Barouch DH, Jackson SS, Kuroda MJ, Schmitz JE, Lifton MA, Sharpe AH, Letvin NL. Functional equivalency of B7-1 and B7-2 for costimulating plasmid DNA vaccine-elicited CTL responses. J Immunol. 2000;165:6791–5. doi: 10.4049/jimmunol.165.12.6791. [DOI] [PubMed] [Google Scholar]
- 13.Ahmadzadeh M, Hussain SF, Farber DL. Heterogeneity of the memory CD4 T cell response: persisting effectors and resting memory T cells. J Immunol. 2001;166:926–35. doi: 10.4049/jimmunol.166.2.926. [DOI] [PubMed] [Google Scholar]
- 14.Farber DL, Ahmadzadeh M. Dissecting the complexity of the memory T cell response. Immunol Res. 2002;25:247–59. doi: 10.1385/IR:25:3:247. [DOI] [PubMed] [Google Scholar]
- 15.Li XY, Matsuzaki G, Yoshikai Y, Muramori K, Nomoto K. T cells expressing both 1-selectin and CD44 molecules increase in number in peritoneal exudate cells and in vitro-stimulated spleen cells from mice immunized intraperitoneally with Listeria monocytogenes. Immunology. 1993;78:28–34. [PMC free article] [PubMed] [Google Scholar]
- 16.Mobley JL, Rigby SM, Dailey MO. Regulation of adhesion molecule expression by CD8 T cells in vivo. II. Expression of 1-selectin (CD62L) by memory cytolytic T cells responding to minor histocompatibility antigens. J Immunol. 1994;153:5443–552. [PubMed] [Google Scholar]
- 17.Kahn J, Ingraham RH, Shirley F, Migaki GI, Kishimoto TK. Membrane proximal cleavage of 1-selectin: identification of the cleavage site and a 6-kD transmembrane peptide fragment of 1-selectin. J Cell Biol. 1994;125:461–70. doi: 10.1083/jcb.125.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kishimoto TK, Kahn J, Migaki G, Mainolfi E, Shirley F, Ingraham R, Rothlein R. Regulation of 1-selectin expression by membrane proximal proteolysis. Agents Actions Suppl. 1995;47:121–34. doi: 10.1007/978-3-0348-7343-7_11. [DOI] [PubMed] [Google Scholar]
- 19.Kishimoto TK, Warnock RA, Jutila MA, Butcher EC, Lane C, Anderson DC, Smith CW. Antibodies against human neutrophil LECAM-1 (LAM-1/Leu-8/DREG-56 antigen) and endothelial cell ELAM-1 inhibit a common CD18-independent adhesion pathway in vitro. Blood. 1991;78:805–11. [PubMed] [Google Scholar]
- 20.Palecanda A, Walcheck B, Bishop DK, Jutila MA. Rapid activation-independent shedding of leukocyte 1-selectin induced by cross-linking of the surface antigen. Eur J Immunol. 1992;22:1279–86. doi: 10.1002/eji.1830220524. [DOI] [PubMed] [Google Scholar]
- 21.Busch DH, Pamer EG. T lymphocyte dynamics during Listeria monocytogenes infection. Immunol Lett. 1999;65:93–8. doi: 10.1016/s0165-2478(98)00130-8. [DOI] [PubMed] [Google Scholar]
- 22.Tripp RA, Hou S, Doherty PC. Temporal loss of the activated 1-selectin-low phenotype for virus-specific CD8+ memory T cells. J Immunol. 1995;154:5870–5. [PubMed] [Google Scholar]
- 23.Usherwood EJ, Hogan RJ, Crowther G, Surman SL, Hogg TL, Altman JD, Woodland DL. Functionally heterogeneous CD8(+) T-cell memory is induced by Sendai virus infection of mice. J Virol. 1999;73:7278–86. doi: 10.1128/jvi.73.9.7278-7286.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giblin PA, Hwang ST, Katsumoto TR, Rosen SD. Ligation of 1-selectin on T lymphocytes activates beta1 integrins and promotes adhesion to fibronectin. J Immunol. 1997;159:3498–507. [PubMed] [Google Scholar]
- 25.Picker LJ. Control of lymphocyte homing. Curr Opin Immunol. 1994;6:394–406. doi: 10.1016/0952-7915(94)90118-x. [DOI] [PubMed] [Google Scholar]
- 26.Waddell TK, Fialkow L, Chan CK, Kishimoto TK, Downey GP. Signaling functions of 1-selectin. Enhancement of tyrosine phosphorylation and activation of MAP kinase. J Biol Chem. 1995;270:15403–11. doi: 10.1074/jbc.270.25.15403. [DOI] [PubMed] [Google Scholar]
- 27.Brenner BC, Kadel S, Grigorovich S, Linderkamp O. Mechanisms of 1-selectin-induced activation of the nuclear factor of activated T lymphocytes (NFAT) Biochem Biophys Res Commun. 2002;291:237–44. doi: 10.1006/bbrc.2002.6451. [DOI] [PubMed] [Google Scholar]
- 28.Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837–51. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- 29.Huster KM, Busch V, Schiemann M, Linkemann K, Kerksiek KM, Wagner H, Busch DH. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc Natl Acad Sci USA. 2004;101:5610–5. doi: 10.1073/pnas.0308054101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–8. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 31.Hengel RL, Thaker V, Pavlick MV, et al. Cutting edge: 1-selectin (CD62L) expression distinguishes small resting memory CD4+ T cells that preferentially respond to recall antigen. J Immunol. 2003;170:28–32. doi: 10.4049/jimmunol.170.1.28. [DOI] [PubMed] [Google Scholar]
- 32.Kanegane H, Kasahara Y, Niida Y, Yachie A, Sughii S, Takatsu K, Taniguchi N, Miyawaki T. Expression of 1-selectin (CD62L) discriminates Th1- and Th2-like cytokine-producing memory CD4+ T cells. Immunology. 1996;87:186–90. doi: 10.1046/j.1365-2567.1996.446530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oehen S, Brduscha-Riem K. Differentiation of naive CTL to effector and memory CTL: correlation of effector function with phenotype and cell division. J Immunol. 1998;161:5338–46. [PubMed] [Google Scholar]
- 34.Ohgama J, Katoh M, Hirano M, et al. Functional studies on MEL-14+ and MEL-14– T cells in peripheral lymphoid tissues. Immunobiology. 1994;190:225–42. doi: 10.1016/S0171-2985(11)80271-8. [DOI] [PubMed] [Google Scholar]
- 35.Roman E, Miller E, Harmsen A, Wiley J, Von Andrian UH, Huston G, Swain SL. CD4 effector T cell subsets in the response to influenza: heterogeneity, migration, and function. J Exp Med. 2002;196:957–68. doi: 10.1084/jem.20021052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masopust D, Kaech SM, Wherry EJ, Ahmed R. The role of programming in memory T-cell development. Curr Opin Immunol. 2004;16:217–25. doi: 10.1016/j.coi.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Rufer N, Zippelius A, Batard P, et al. Ex vivo characterization of human CD8+ T subsets with distinct replicative history and partial effector functions. Blood. 2003;102:1779–87. doi: 10.1182/blood-2003-02-0420. [DOI] [PubMed] [Google Scholar]
- 38.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–9. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 39.Sun JC, Bevan MJ. Cutting edge: long-lived CD8 memory and protective immunity in the absence of CD40 expression on CD8 T cells. J Immunol. 2004;172:3385–9. doi: 10.4049/jimmunol.172.6.3385. [DOI] [PubMed] [Google Scholar]
- 40.Marzo AL, Vezys V, Klonowski KD, Lee SJ, Muralimohan G, Moore M, Tough DF, Lefrancois L. Fully functional memory CD8 T cells in the absence of CD4 T cells. J Immunol. 2004;173:969–75. doi: 10.4049/jimmunol.173.2.969. [DOI] [PubMed] [Google Scholar]
- 41.Wolint P, Betts MR, Koup RA, Oxenius A. Immediate cytotoxicity but not degranulation distinguishes effector and memory subsets of CD8+ T cells. J Exp Med. 2004;199:925–36. doi: 10.1084/jem.20031799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barber DL, Wherry EJ, Ahmed R. Cutting edge: rapid in vivo killing by memory CD8 T cells. J Immunol. 2003;171:27–31. doi: 10.4049/jimmunol.171.1.27. [DOI] [PubMed] [Google Scholar]
- 43.Unsoeld H, Krautwald S, Voehringer D, Kunzendorf U, Pircher H. Cutting edge: CCR7+ and CCR7– memory T cells do not differ in immediate effector cell function. J Immunol. 2002;169:638–41. doi: 10.4049/jimmunol.169.2.638. [DOI] [PubMed] [Google Scholar]
- 44.Ellefsen K, Harari A, Champagne P, Bart PA, Sekaly RP, Pantaleo G. Distribution and functional analysis of memory antiviral CD8 T cell responses in HIV-1 and cytomegalovirus infections. Eur J Immunol. 2002;32:3756–64. doi: 10.1002/1521-4141(200212)32:12<3756::AID-IMMU3756>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 45.Ravkov EV, Myrick CM, Altman JD. Immediate early effector functions of virus-specific CD8+CCR7+ memory cells in humans defined by HLA and CC chemokine ligand 19 tetramers. J Immunol. 2003;170:2461–8. doi: 10.4049/jimmunol.170.5.2461. [DOI] [PubMed] [Google Scholar]
- 46.Unsoeld H, Pircher H. Complex memory T-cell phenotypes revealed by coexpression of CD62L and CCR7. J Virol. 2005;79:4510–3. doi: 10.1128/JVI.79.7.4510-4513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tomiyama H, Matsuda T, Takiguchi M. Differentiation of human CD8(+) T cells from a memory to memory/effector phenotype. J Immunol. 2002;168:5538–50. doi: 10.4049/jimmunol.168.11.5538. [DOI] [PubMed] [Google Scholar]
- 48.Lo JC, Chin RK, Lee Y, et al. Differential regulation of CCL21 in lymphoid/nonlymphoid tissues for effectively attracting T cells to peripheral tissues. J Clin Invest. 2003;112:1495–505. doi: 10.1172/JCI19188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keramidaris E, Merson TD, Steeber DA, Tedder TF, Tang ML. 1-selectin and intercellular adhesion molecule 1 mediate lymphocyte migration to the inflamed airway/lung during an allergic inflammatory response in an animal model of asthma. J Allergy Clin Immunol. 2001;107:734–8. doi: 10.1067/mai.2001.114050. [DOI] [PubMed] [Google Scholar]
- 50.Junt T, Scandella E, Forster R, Krebs P, Krautwald S, Lipp M, Hengartner H, Ludewig B. Impact of CCR7 on priming and distribution of antiviral effector and memory CTL. J Immunol. 2004;173:6684–93. doi: 10.4049/jimmunol.173.11.6684. [DOI] [PubMed] [Google Scholar]
- 51.Baumhueter S, Dybdal N, Kyle C, Lasky LA. Global vascular expression of murine CD34, a sialomucin-like endothelial ligand for 1-selectin. Blood. 1994;84:2554–65. [PubMed] [Google Scholar]