Abstract
Ceftriaxone, a new third-generation cephalosporin, appears to be promising for the therapy of acute bacterial meningitis. The 90% MBCs of ceftriaxone against 54 recent cerebrospinal fluid isolates of Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae were less than or equal to 0.06 to 0.25 micrograms/ml. We examined the efficacy and safety of ceftriaxone therapy of meningitis in Bahia, Brazil. The study was conducted in two phases; in phase A, ceftriaxone was coadministered with ampicillin. The mean cerebrospinal fluid concentrations of ceftriaxone 24 h after an intravenous dose of 80 mg/kg were 4.2 and 2.3 micrograms/ml on days 4 to 6 and 10 to 12 of therapy, respectively. These concentrations were 8- to more than 100-fold greater than the 90% MBCs against the relevant pathogens. In phase B, ceftriaxone (administered once daily at a dose of 80 mg/kg after an initial dose of 100 mg/kg) was compared with conventional dosages of ampicillin and chloramphenicol in a prospective randomized trial of 36 children and adults with meningitis. The groups were comparable based on clinical, laboratory, and etiological parameters. Ceftriaxone given once daily produced results equivalent to those obtained with ampicillin plus chloramphenicol, as judged by cure rate, case fatality ratio, resolution with sequelae, type and severity of sequelae, time to sterility of cerebrospinal fluid, and potentially drug-related adverse effects. The cerebrospinal fluid bactericidal titers obtained 16 to 24 h after ceftriaxone dosing were usually 1:512 to greater than 1:2,048 even late in the treatment course, compared with values of 1:8 to 1:32 in patients receiving ampicillin plus chloramphenicol. Ceftriaxone clearly deserves further evaluation for the therapy of meningitis; the optimal dose, dosing frequency (every 12 h or every 24 h), and duration of therapy remain to be determined.
Full text
PDF


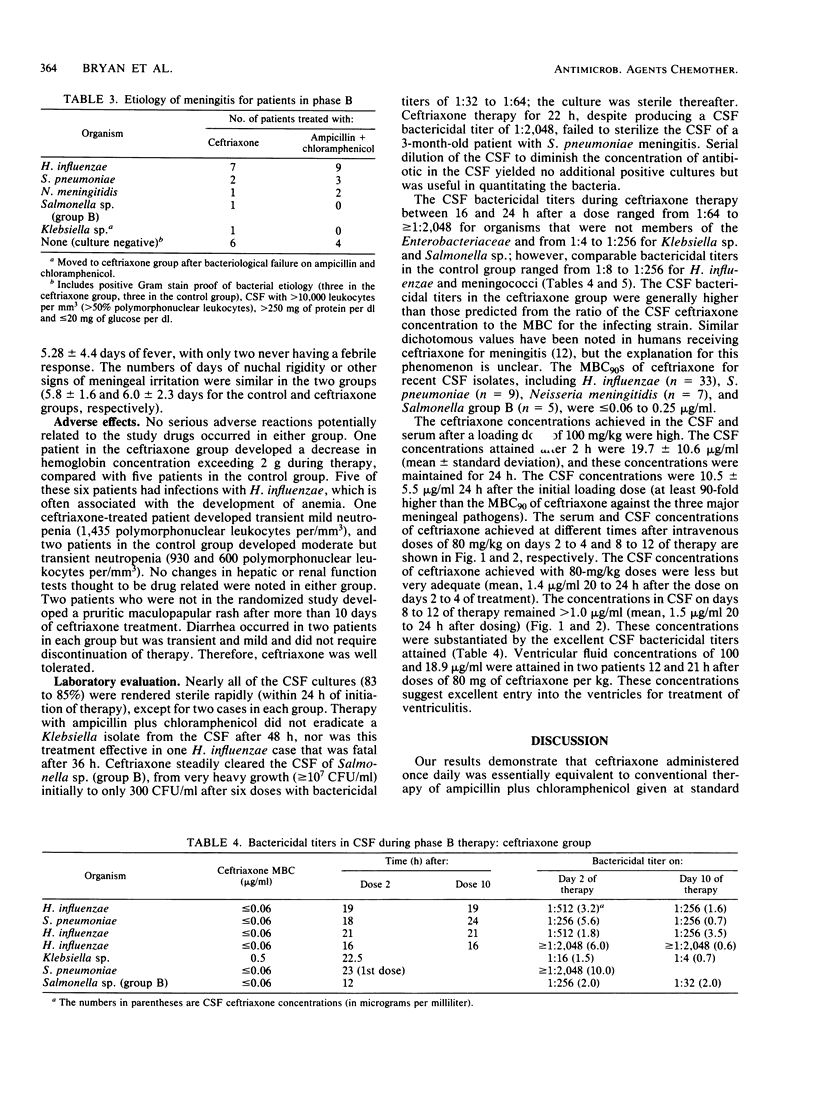
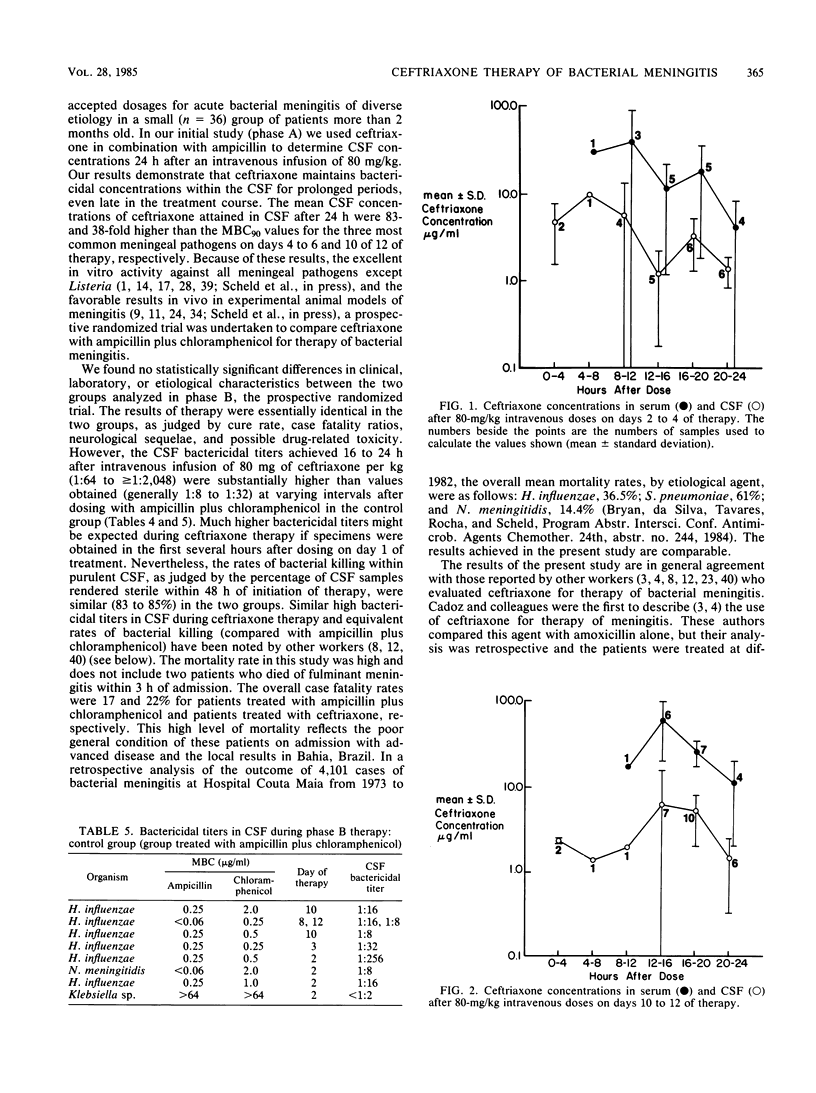



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angehrn P., Probst P. J., Reiner R., Then R. L. Ro 13-9904, a long-acting broad-spectrum cephalosporin: in vitro and in vivo studies. Antimicrob Agents Chemother. 1980 Dec;18(6):913–921. doi: 10.1128/aac.18.6.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belohradsky B. H., Bruch K., Geiss D., Kafetzis D., Marget W., Peters G. Intravenous cefotaxime in children with bacterial meningitis. Lancet. 1980 Jan 12;1(8159):61–63. doi: 10.1016/s0140-6736(80)90491-2. [DOI] [PubMed] [Google Scholar]
- Cadoz M., Denis F., Félix H., Diop Mar I. Treatment of purulent meningitis with a new cephalosporin-Rocephin (Ro 13-9904). Clinical, bacteriological and pharmacological observations in 24 cases. Chemotherapy. 1981;27 (Suppl 1):57–61. doi: 10.1159/000238030. [DOI] [PubMed] [Google Scholar]
- Cadoz M., Denis F., Guerma T., Prince-David M., Diop Mar I. Comparison bactériologique, pharmacologique et clinique de l'amoxycilline et du ceftriaxone dans 300 méningites purulentes. Pathol Biol (Paris) 1982 Jun;30(6 Pt 2):522–525. [PubMed] [Google Scholar]
- Chadwick E. G., Yogev R., Shulman S. T., Weinfeld R. E., Patel I. H. Single-dose ceftriaxone pharmacokinetics in pediatric patients with central nervous system infections. J Pediatr. 1983 Jan;102(1):134–137. doi: 10.1016/s0022-3476(83)80311-4. [DOI] [PubMed] [Google Scholar]
- Cherubin C. E., Corrado M. L., Nair S. R., Gombert M. E., Landesman S., Humbert G. Treatment of gram-negative bacillary meningitis: role of the new cephalosporin antibiotics. Rev Infect Dis. 1982 Sep-Oct;4 (Suppl):S453–S464. doi: 10.1093/clinids/4.supplement_2.s453. [DOI] [PubMed] [Google Scholar]
- Cherubin C. E., Marr J. S., Sierra M. F., Becker S. Listeria and gram-negative bacillary meningitis in New York City, 1972-1979. Frequent causes of meningitis in adults. Am J Med. 1981 Aug;71(2):199–209. doi: 10.1016/0002-9343(81)90106-6. [DOI] [PubMed] [Google Scholar]
- Congeni B. L. Comparison of ceftriaxone and traditional therapy of bacterial meningitis. Antimicrob Agents Chemother. 1984 Jan;25(1):40–44. doi: 10.1128/aac.25.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor E., Melick C., Yogev R. In-vitro and in-vivo efficacy of ceftriaxone, moxalactam, and chloramphenicol against Haemophilus influenzae type b. J Antimicrob Chemother. 1982 Dec;10(6):517–525. doi: 10.1093/jac/10.6.517. [DOI] [PubMed] [Google Scholar]
- Decazes J. M., Ernst J. D., Sande M. A. Correlation of in vitro time-kill curves and kinetics of bacterial killing in cerebrospinal fluid during ceftriaxone therapy of experimental Escherichia coli meningitis. Antimicrob Agents Chemother. 1983 Oct;24(4):463–467. doi: 10.1128/aac.24.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio M., McCracken G. H., Jr, Nelson J. D., Chrane D., Shelton S. Pharmacokinetics and cerebrospinal fluid bactericidal activity of ceftriaxone in the treatment of pediatric patients with bacterial meningitis. Antimicrob Agents Chemother. 1982 Oct;22(4):622–627. doi: 10.1128/aac.22.4.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaplane D., Yogev R., Shulman S. T. Ceftriaxone therapy of group B streptococcal bacteraemia and meningitis in infant rats. J Antimicrob Chemother. 1983 Jan;11(1):69–73. doi: 10.1093/jac/11.1.69. [DOI] [PubMed] [Google Scholar]
- Fass R. J. Comparative in vitro activities of third-generation cephalosporins. Arch Intern Med. 1983 Sep;143(9):1743–1745. [PubMed] [Google Scholar]
- Freedman J. M., Hoffman S. H., Scheld W. M., Lynch M. A., da Silva H. R., Rocha H., Sande M. A. Moxalactam for the treatment of bacterial meningitis in children. J Infect Dis. 1983 Nov;148(5):886–891. doi: 10.1093/infdis/148.5.886. [DOI] [PubMed] [Google Scholar]
- Gustafson T. L., Kelley R. A., Hutcheson R. H., Jr, Schaffner W., Sell S. H. Statewide survey of the antimicrobial susceptibilities of Haemophilus influenzae producing invasive disease in Tennessee. Pediatr Infect Dis. 1983 Mar-Apr;2(2):119–122. doi: 10.1097/00006454-198303000-00010. [DOI] [PubMed] [Google Scholar]
- Hall M. J., Westmacott D., Wong-Kai-In P. Comparative in-vitro activity and mode of action of ceftriaxone (Ro 13-9904), a new highly potent cephalosporin. J Antimicrob Chemother. 1981 Sep;8(3):193–203. doi: 10.1093/jac/8.3.193. [DOI] [PubMed] [Google Scholar]
- Istre G. R., Humphreys J. T., Albrecht K. D., Thornsberry C., Swenson J. M., Hopkins R. S. Chloramphenicol and penicillin resistance in pneumococci isolated from blood and cerebrospinal fluid: a prevalence study in metropolitan Denver. J Clin Microbiol. 1983 Mar;17(3):472–475. doi: 10.1128/jcm.17.3.472-475.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landesman S. H., Corrado M. L., Shah P. M., Armengaud M., Barza M., Cherubin C. E. Past and current roles for cephalosporin antibiotics in treatment of meningitis. Emphasis on use in gram-negative bacillary meningitis. Am J Med. 1981 Oct;71(4):693–703. doi: 10.1016/0002-9343(81)90240-0. [DOI] [PubMed] [Google Scholar]
- Latif R., Dajani A. S. Ceftriaxone diffusion into cerebrospinal fluid of children with meningitis. Antimicrob Agents Chemother. 1983 Jan;23(1):46–48. doi: 10.1128/aac.23.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchou B., Tran Van Tho, Armengaud M. Diffusion of ceftriaxone (Ro 13-9004/001) in the cerebrospinal fluid. Comparison with other beta-lactam antibiotics in dogs with healthy meninges and in dogs with experimental meningitis. Chemotherapy. 1981;27 (Suppl 1):37–41. doi: 10.1159/000238027. [DOI] [PubMed] [Google Scholar]
- Martin E. Once-daily administration of ceftriaxone in the treatment of meningitis and other serious infections in children. Eur J Clin Microbiol. 1983 Oct;2(5):509–515. doi: 10.1007/BF02013918. [DOI] [PubMed] [Google Scholar]
- McCracken G. H., Jr Management of bacterial meningitis in infants and children. Current status and future prospects. Am J Med. 1984 May 15;76(5A):215–223. doi: 10.1016/0002-9343(84)90267-5. [DOI] [PubMed] [Google Scholar]
- McCracken G. H., Jr, Nelson J. D., Grimm L. Pharmacokinetics and bacteriological efficacy of cefoperazone, ceftriaxone, and moxalactam in experimental Streptococcus pneumoniae and Haemophilus influenzae meningitis. Antimicrob Agents Chemother. 1982 Feb;21(2):262–267. doi: 10.1128/aac.21.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken G. H., Jr, Schaad U. B. The pharmacologic basis for moxalactam therapy for gram-negative enteric bacillary meningitis of infancy. Rev Infect Dis. 1982 Nov-Dec;4 (Suppl):S603–S605. doi: 10.1093/clinids/4.supplement_3.s603. [DOI] [PubMed] [Google Scholar]
- McCracken G. H., Jr, Siegel J. D., Threlkeld N., Thomas M. Ceftriaxone pharmacokinetics in newborn infants. Antimicrob Agents Chemother. 1983 Feb;23(2):341–343. doi: 10.1128/aac.23.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel J., Dickman D., Greenberg Z., Bergner-Rabinowitz S. Serotype distribution of penicillin-resistant pneumococci and their susceptibilities to seven antimicrobial agents. Antimicrob Agents Chemother. 1983 Mar;23(3):397–401. doi: 10.1128/aac.23.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C. The new beta-lactamase-stable cephalosporins. Ann Intern Med. 1982 Sep;97(3):408–419. doi: 10.7326/0003-4819-97-3-408. [DOI] [PubMed] [Google Scholar]
- Pakter R. L., Russell T. R., Mielke C. H., West D. Coagulopathy associated with the use of moxalactam. JAMA. 1982 Sep 3;248(9):1100–1100. [PubMed] [Google Scholar]
- Pollock A. A., Tee P. E., Patel I. H., Spicehandler J., Simberkoff M. S., Rahal J. J., Jr Pharmacokinetic characteristics of intravenous ceftriaxone in normal adults. Antimicrob Agents Chemother. 1982 Nov;22(5):816–823. doi: 10.1128/aac.22.5.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. C., Swenson C. D., Owens L. M., Smith A. L. Characterization of chloramphenicol-resistant Haemophilus influenzae. Antimicrob Agents Chemother. 1980 Oct;18(4):610–615. doi: 10.1128/aac.18.4.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sande M. A. Antibiotic therapy of bacterial meningitis: lessons we've learned. Am J Med. 1981 Oct;71(4):507–510. doi: 10.1016/0002-9343(81)90191-1. [DOI] [PubMed] [Google Scholar]
- Schaad U. B., Stoeckel K. Single-dose pharmacokinetics of ceftriaxone in infants and young children. Antimicrob Agents Chemother. 1982 Feb;21(2):248–253. doi: 10.1128/aac.21.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheld W. M., Sande M. A. Bactericidal versus bacteriostatic antibiotic therapy of experimental pneumococcal meningitis in rabbits. J Clin Invest. 1983 Mar;71(3):411–419. doi: 10.1172/JCI110785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheld W. M. The potential uses of ceftriaxone. Eur J Clin Microbiol. 1983 Oct;2(5):485–488. doi: 10.1007/BF02013913. [DOI] [PubMed] [Google Scholar]
- Seddon M., Wise R., Gillett A. P., Livingston R. Pharmacokinetics of Ro 13-9904, a broad-spectrum cephalosporin. Antimicrob Agents Chemother. 1980 Aug;18(2):240–242. doi: 10.1128/aac.18.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton S., Nelson J. D., McCracken G. H., Jr In vitro susceptibility of gram-negative bacilli from pediatric patients to moxalactam, cefotaxime, Ro 13-9904, and other cephalosporins. Antimicrob Agents Chemother. 1980 Sep;18(3):476–479. doi: 10.1128/aac.18.3.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele R. W., Bradsher R. W. Comparison of ceftriaxone with standard therapy for bacterial meningitis. J Pediatr. 1983 Jul;103(1):138–141. doi: 10.1016/s0022-3476(83)80801-4. [DOI] [PubMed] [Google Scholar]
- Steele R. W., Eyre L. B., Bradsher R. W., Weinfeld R. E., Patel I. H., Spicehandler J. Pharmacokinetics of ceftriaxone in pediatric patients with meningitis. Antimicrob Agents Chemother. 1983 Feb;23(2):191–194. doi: 10.1128/aac.23.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel K., McNamara P. J., Brandt R., Plozza-Nottebrock H., Ziegler W. H. Effects of concentration-dependent plasma protein binding on ceftriaxone kinetics. Clin Pharmacol Ther. 1981 May;29(5):650–657. doi: 10.1038/clpt.1981.90. [DOI] [PubMed] [Google Scholar]
- Uchiyama N., Greene G. R., Kitts D. B., Thrupp L. D. Meningitis due to Haemophilus influenzae type b resistant to ampicillin and chloramphenicol. J Pediatr. 1980 Sep;97(3):421–424. doi: 10.1016/s0022-3476(80)80193-4. [DOI] [PubMed] [Google Scholar]
- Ward J. I., Tsai T. F., Filice G. A., Fraser D. W. Prevalence of ampicillin- and chloramphenicol-resistant strains of Haemophilus influenzae causing meningitis and bacteremia: national survey of hospital laboratories. J Infect Dis. 1978 Sep;138(3):421–424. doi: 10.1093/infdis/138.3.421. [DOI] [PubMed] [Google Scholar]
- West S. E., Goodkin R., Kaplan A. M. Neonatal salmonella meningitis complicated by cerebral abscesses. West J Med. 1977 Aug;127(2):142–145. [PMC free article] [PubMed] [Google Scholar]
- del Rio M. A., Chrane D., Shelton S., McCracken G. H., Jr, Nelson J. D. Ceftriaxone versus ampicillin and chloramphenicol for treatment of bacterial meningitis in children. Lancet. 1983 Jun 4;1(8336):1241–1244. doi: 10.1016/s0140-6736(83)92696-x. [DOI] [PubMed] [Google Scholar]



