Abstract
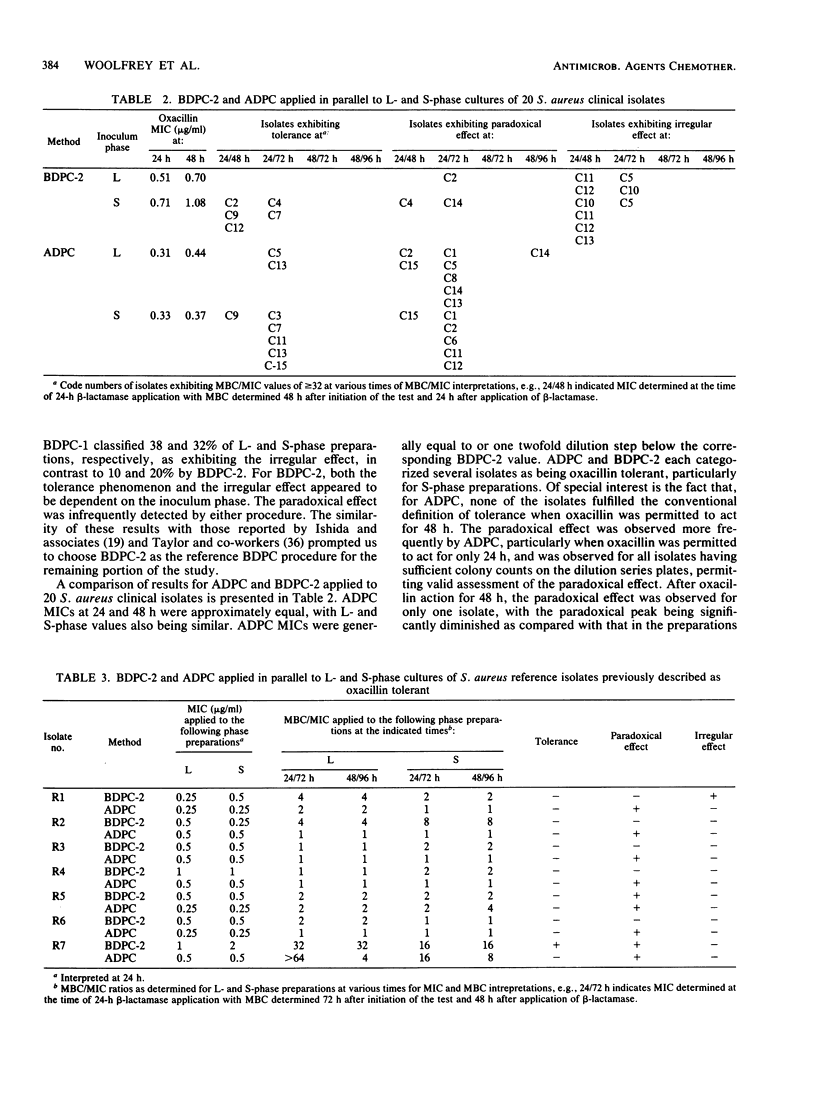
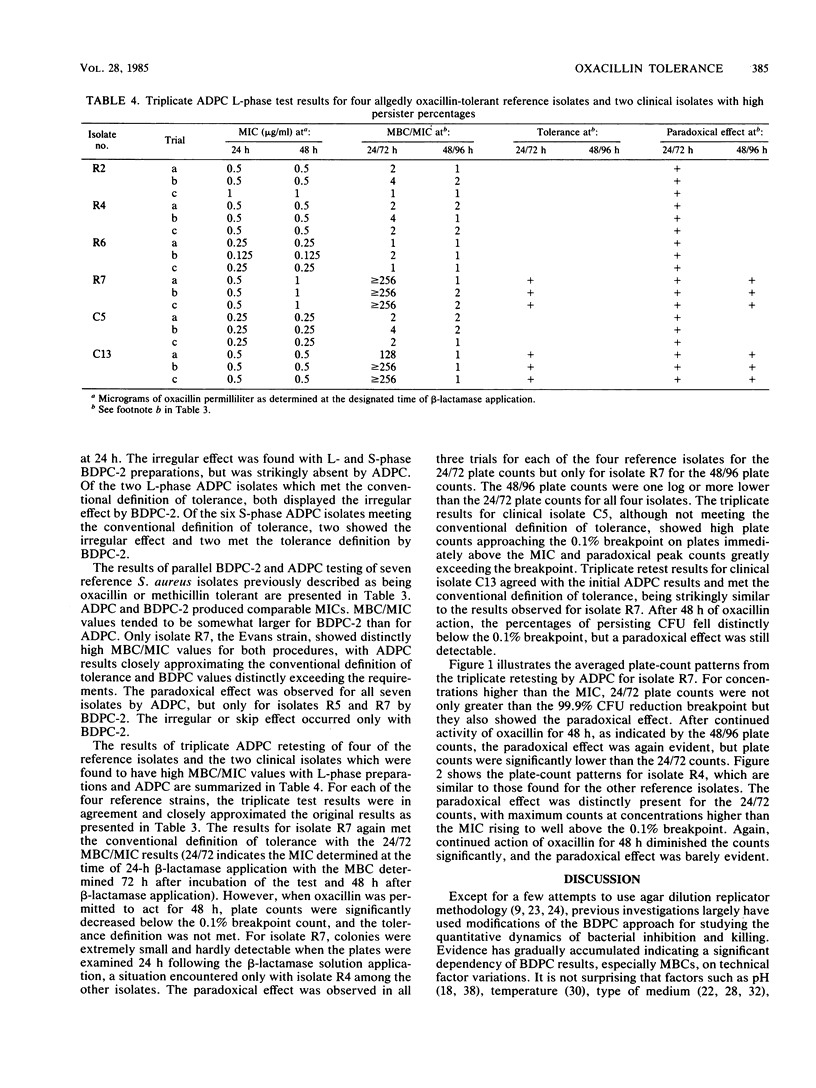
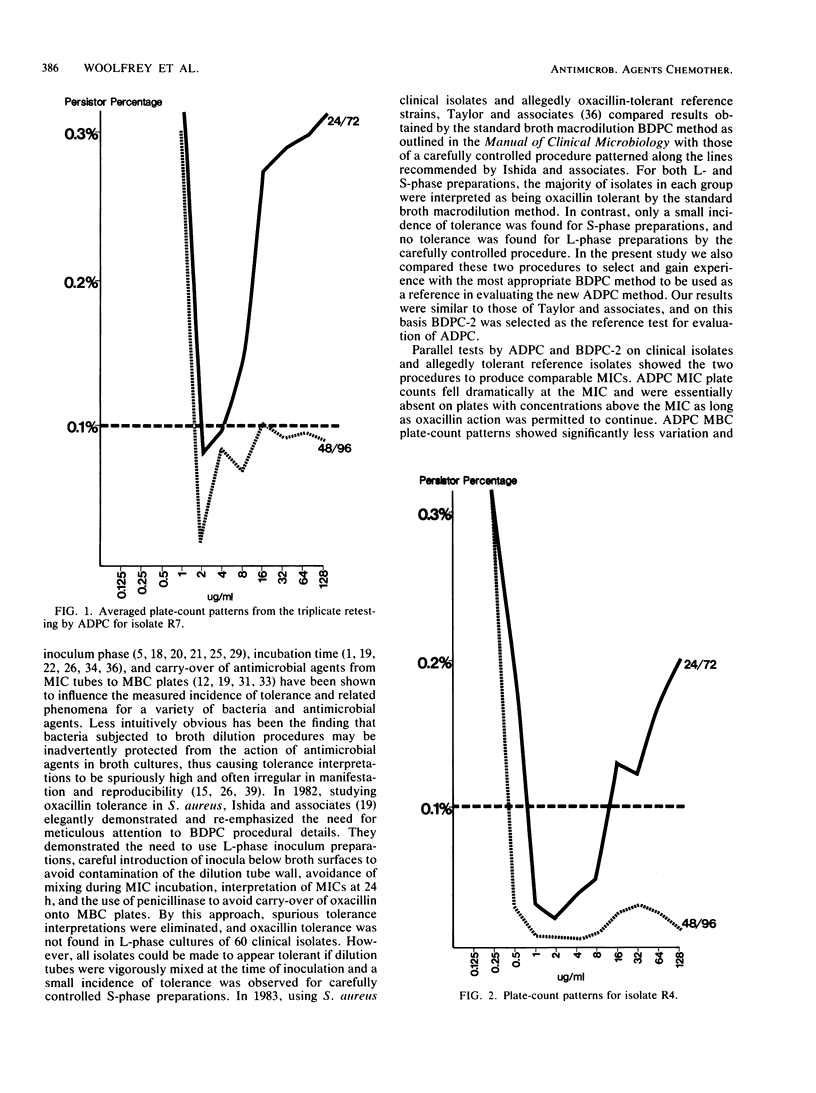
A novel agar dilution plate-count procedure for the quantitative measurement of bacterial inhibition and killing is described. For Staphylococcus aureus versus oxacillin, by the agar dilution plate-count procedure it was found that only 1 of 20 clinical isolates and 1 of 7 allegedly tolerant reference isolates met the conventional definition of tolerance. By using inocula of 10(5) CFU per plate, most isolates were demonstrated to have subpopulations of cells which, although inhibited, persisted for 24 h in concentrations significantly above their MICs. The persister percentages at 24 h appeared to be strain dependent, and all persisters exhibited the paradoxical effect. For each isolate, the persister percentage markedly decreased after action by oxacillin for 48 h, and the paradoxical effect was greatly diminished. Our findings suggest that tolerance is an artificial and arbitrary concept that does not adequately characterize the inhibition and killing dynamics associated with the persister phenomenon.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ampel N. M., Keating M. H., Moon-McDermott L., Peter G., Zinner S. H. Comparison of tube dilution and microtitre methods for the detection of antibiotic tolerance in strains of Staphylococcus aureus. J Antimicrob Chemother. 1984 May;13(5):417–421. doi: 10.1093/jac/13.5.417. [DOI] [PubMed] [Google Scholar]
- Best G. K., Koval A. V., Best N. H. Susceptibility of clinical isolates of Staphylococcus aureus to killing by oxacillin. Can J Microbiol. 1975 Nov;21(11):1692–1697. doi: 10.1139/m75-248. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Further observations on the zone phenomenon in the bactericidal action of penicillin. J Bacteriol. 1951 Nov;62(5):663–668. doi: 10.1128/jb.62.5.663-668.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes C. J., Stevens D. A., Groot obbink D. J., Ackerman V. P. A replicator method for the combined determination of minimum inhibitory concentration and minimum bactericidal concentration. J Antimicrob Chemother. 1985 Jan;15(1):53–60. doi: 10.1093/jac/15.1.53. [DOI] [PubMed] [Google Scholar]
- GUNNISON J. B., FRAHER M. A., JAWETZ E. PERSISTENCE OF STAPHYLOCOCCUS AUREUS IN PENICILLIN IN VITRO. J Gen Microbiol. 1964 May;35:335–349. doi: 10.1099/00221287-35-2-335. [DOI] [PubMed] [Google Scholar]
- Goessens W. H., Fontijne P., Michel M. F. Factors influencing detection of tolerance in Staphylococcus aureus. Antimicrob Agents Chemother. 1982 Sep;22(3):364–368. doi: 10.1128/aac.22.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goessens W. H., Fontijne P., Michel M. F. Responses of tolerant and nontolerant Staphylococcus aureus strains to methicillin treatment in an experimental infection in mice. Antimicrob Agents Chemother. 1984 Dec;26(6):829–832. doi: 10.1128/aac.26.6.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goessens W. H., Fontijne P., van Raffe M., Michel M. F. Tolerance percentage as a criterion for the detection of tolerant Staphylococcus aureus strains. Antimicrob Agents Chemother. 1984 May;25(5):575–578. doi: 10.1128/aac.25.5.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood D. Mucopeptide hydrolases and bacterial "persisters". Lancet. 1972 Sep 2;2(7775):465–466. doi: 10.1016/s0140-6736(72)91858-2. [DOI] [PubMed] [Google Scholar]
- Gwynn M. N., Webb T. L., Rolinson G. N. Regrowth of Pseudomonas aeruginosa and other bacteria after the bactericidal action of carbenicillin and other beta-lactam antibiotics. J Infect Dis. 1981 Sep;144(3):263–269. doi: 10.1093/infdis/144.3.263. [DOI] [PubMed] [Google Scholar]
- Hilty M. D., Venglarcik J. S., Best G. K. Oxacillin-tolerant staphylococcal bacteremia in children. J Pediatr. 1980 Jun;96(6):1035–1037. doi: 10.1016/s0022-3476(80)80633-0. [DOI] [PubMed] [Google Scholar]
- Horne D., Tomasz A. pH-dependent penicillin tolerance of group B streptococci. Antimicrob Agents Chemother. 1981 Jul;20(1):128–135. doi: 10.1128/aac.20.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida K., Guze P. A., Kalmanson G. M., Albrandt K., Guze L. B. Variables in demonstrating methicillin tolerance in Staphylococcus aureus strains. Antimicrob Agents Chemother. 1982 Apr;21(4):688–690. doi: 10.1128/aac.21.4.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. S., Anthony B. F. Importance of bacterial growth phase in determining minimal bactericidal concentrations of penicillin and methicillin. Antimicrob Agents Chemother. 1981 Jun;19(6):1075–1077. doi: 10.1128/aac.19.6.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. S., Anthony B. F. Penicillin tolerance in group B streptococci isolated from infected neonates. J Infect Dis. 1981 Nov;144(5):411–419. doi: 10.1093/infdis/144.5.411. [DOI] [PubMed] [Google Scholar]
- Kim K. S., Yoshimori R. N., Imagawa D. T., Anthony B. F. Importance of medium in demonstrating penicillin tolerance by group B streptococci. Antimicrob Agents Chemother. 1979 Aug;16(2):214–216. doi: 10.1128/aac.16.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda G. Studies on bactericidal activities of beta-lactam antibiotics on agar plates: the correlation with the antibacterial activities determined by the conventional methods. J Antibiot (Tokyo) 1976 Nov;29(11):1237–1240. doi: 10.7164/antibiotics.29.1237. [DOI] [PubMed] [Google Scholar]
- Masuda G., Tomioka S., Uchida H., Hasegawa M. Bacteriostatic and bactericidal activities of selected beta-lactam antibiotics studied on agar plates. Antimicrob Agents Chemother. 1977 Mar;11(3):376–382. doi: 10.1128/aac.11.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhall C. G., Apollo E. Effect of storage and changes in bacterial growth phase and antibiotic concentrations on antimicrobial tolerance in Staphylococcus aureus. Antimicrob Agents Chemother. 1980 Nov;18(5):784–788. doi: 10.1128/aac.18.5.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhall C. G., Medoff G., Marr J. J. Variation in the susceptibility of strains of Staphylococcus aureus to oxacillin, cephalothin, and gentamicin. Antimicrob Agents Chemother. 1976 Oct;10(4):707–712. doi: 10.1128/aac.10.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDERMOTT W. Microbial persistence. Yale J Biol Med. 1958 Feb;30(4):257–291. [PMC free article] [PubMed] [Google Scholar]
- Norden C. W., Keleti E. Antibiotic tolerance in strains of Staphylococcus aureus. J Antimicrob Chemother. 1981 Jun;7(6):599–605. doi: 10.1093/jac/7.6.599. [DOI] [PubMed] [Google Scholar]
- PARKER R. F. A paradoxical effect of penicillin. J Bacteriol. 1953 Jul;66(1):60–67. doi: 10.1128/jb.66.1.60-67.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. T., Edwards J. R., Wise E. M., Jr In vivo studies on the uptake and binding of beta-lactam antibiotics in relation to inhibition of wall synthesis and cell death. Ann N Y Acad Sci. 1974 May 10;235(0):300–309. doi: 10.1111/j.1749-6632.1974.tb43273.x. [DOI] [PubMed] [Google Scholar]
- Pearson R. D., Steigbigel R. T., Davis H. T., Chapman S. W. Method of reliable determination of minimal lethal antibiotic concentrations. Antimicrob Agents Chemother. 1980 Nov;18(5):699–708. doi: 10.1128/aac.18.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson L. R., Gerding D. N., Hall W. H., Schierl E. A. Medium-dependent variation in bactericidal activity of antibiotics against susceptible Staphylococcus aureus. Antimicrob Agents Chemother. 1978 Apr;13(4):665–668. doi: 10.1128/aac.13.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajashekaraiah K. R., Rice T., Rao V. S., Marsh D., Ramakrishna B., Kallick C. A. Clinical significance of tolerant strains of Staphylococcus aureus in patients with endocarditis. Ann Intern Med. 1980 Dec;93(6):796–801. doi: 10.7326/0003-4819-93-6-796. [DOI] [PubMed] [Google Scholar]
- Sabath L. D., Wheeler N., Laverdiere M., Blazevic D., Wilkinson B. J. A new type of penicillin resistance of Staphylococcus aureus. Lancet. 1977 Feb 26;1(8009):443–447. doi: 10.1016/s0140-6736(77)91941-9. [DOI] [PubMed] [Google Scholar]
- Shah P. M. Paradoxical effect of antibiotics. I. The 'Eagle effect'. J Antimicrob Chemother. 1982 Oct;10(4):259–260. doi: 10.1093/jac/10.4.259. [DOI] [PubMed] [Google Scholar]
- Shah P. M. Paradoxical effect of antibiotics. I. The 'Eagle effect'. J Antimicrob Chemother. 1982 Oct;10(4):259–260. doi: 10.1093/jac/10.4.259. [DOI] [PubMed] [Google Scholar]
- Taylor P. C., Schoenknecht F. D., Sherris J. C., Linner E. C. Determination of minimum bactericidal concentrations of oxacillin for Staphylococcus aureus: influence and significance of technical factors. Antimicrob Agents Chemother. 1983 Jan;23(1):142–150. doi: 10.1128/aac.23.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Albino A., Zanati E. Multiple antibiotic resistance in a bacterium with suppressed autolytic system. Nature. 1970 Jul 11;227(5254):138–140. doi: 10.1038/227138a0. [DOI] [PubMed] [Google Scholar]
- Venglarcik J. S., 3rd, Blair L. L., Dunkle L. M. pH-dependent oxacillin tolerance of Staphylococcus aureus. Antimicrob Agents Chemother. 1983 Feb;23(2):232–235. doi: 10.1128/aac.23.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinner S. H., Husson M., Klastersky J. Effect of mixing on rifampin bactericidal activity against staphylococci. Antimicrob Agents Chemother. 1981 Aug;20(2):267–269. doi: 10.1128/aac.20.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]



