Abstract
Period2 (Per2) is an essential component of the mammalian clock mechanism and robust circadian expression of Per2 is essential for the maintenance of circadian rhythms. Although recent studies have shown that the circadian E2 enhancer (a non-canonical E-box) accounts for most of the circadian transcriptional drive of mPer2, little is known about the other cis-elements of mPer2 oscillatory transcription. Here, we examined the contribution of E4BP4 to Per2 mRNA oscillation in the cell-autonomous clock. Knockdown experiments of E4BP4 in both Northern blots and real-time luciferase assays suggested that endogenous E4BP4 negatively regulates Per2 mRNA oscillation. Sequence analysis revealed two putative E4BP4-binding sites (termed A-site and B-site) on mammalian Per2 promoter regions. Luciferase assays with mutant constructs showed that a novel E4BP4-binding site (B-site) is responsible for E4BP4-mediated transcriptional repression of Per2. Furthermore, chromatin immunoprecipitation assays in vivo showed that the peak of E4BP4 binding to the B-site on the Per2 promoter almost matched the trough of Per2 mRNA expression. Importantly, real-time luciferase assays showed that the B-site in addition to the E2 enhancer is required for robust circadian expression of Per2 in the cell-autonomous clock. These findings indicated that E4BP4 is required for the negative regulation of mammalian circadian clocks.
INTRODUCTION
Physiological and behavioral circadian rhythms are features of organisms ranging from bacteria to humans and are driven by an endogenous clock that consists of transcriptional/translational feedback loops of clock genes (1–4). The first clock mutants were isolated by a forward genetics approach using eclosion rhythms as a phenotype to clock components in Drosophila (5). These mutant flies exhibited similar defects in locomotor activity rhythms and the corresponding molecular defects were later identified in the period (per) gene (6,7). Since then, several additional clock genes, including timeless, clock, cycle, doubletime and recently vrille, have been identified in Drosophila (8–12). Orthologs of most Drosophila circadian clock genes have been identified in mammals, highlighting general conservation of the clock mechanism.
Three mammalian homologues (Per1, Per2 and Per3) of the Drosophila circadian clock gene per have been identified (13–19). Gene targeting studies have demonstrated that mPer2Brdm1 mutant mice display a short-circadian period followed by a loss of circadian rhythmicity in constant darkness (20). In contrast, a deletion of mPer1 only shortens the period length and mPer3 knockout mice have an essentially normal clock (21,22), indicating that Per2 plays a prominent role among the three mammalian Per genes. Moreover, constitutively overexpressed mPer2 mRNA rapidly damps cellular rhythm (23), indicating that robust circadian expression of Per2 is essential for the maintenance of circadian rhythms.
Recent studies have shown that the circadian E2 enhancer (a non-canonical E-box) accounts for most of the circadian transcriptional drive of the mPer2 gene by CLOCK:BMAL1 (24,25), but little is known about the other cis-elements of mPer2 oscillatory transcription.
The bZIP transcription factor E4BP4 (also called NFIL3) is a mammalian homologue of vrille (vri) that functions as a key negative component of the Drosophila circadian clock (12,26,27). E4BP4 probably plays an important role in the phase-delaying process of chickens as a light-dependent suppressor of cPer2 (28). Although E4BP4 is believed to be involved in the mammalian circadian clock (25,29,30), direct evidence has yet to support a requirement for E4BP4-mediated regulation of these clocks.
Here, we show that E4BP4 functions as a repressor of Per2 transcription through a novel E4BP4-binding site in the promoter. We also show that E4BP4 binding is required for robust circadian expression of Per2 in the cell-autonomous clock.
MATERIALS AND METHODS
Cell culture
Mouse fibroblast NIH3T3 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and a mixture of penicillin and streptomycin at 37°C under a humidified 5% CO2 atmosphere.
Small interfering RNA (siRNA)
We designed E4BP4 siRNA for knockdown experiments using BLOCK-iT™ RNAi Designer (https://rnaidesigner.invitrogen.com/rnaiexpress/), and BLOCK-iT Fluorescent Oligo (Invitrogen) served as a control. These oligonucleotides were introduced into NIH3T3 cells at a final concentration of 10 nM using X-treamGENE (Roche Diagnostics) or Lipofectamine 2000 (Invitrogen) according to the suppliers' protocols.
Western blotting
NIH3T3 cells were transfected with the expression vectors, Myc-tagged E4BP4 or Myc-tagged HLF after siRNA manipulation. After a 24 h incubation, proteins were separated on 10% SDS–PAGE gels (31) and transferred to nitrocellulose membranes (Bio-Rad). After blocking nonspecific binding with 3% dry milk in PBS, proteins were probed with anti-Myc monoclonal antibody (clone 9E10; Roche Diagnostics) and then incubated with a horseradish peroxidase-conjugated anti-mouse IgG (Upstate). Immunoreactive proteins were visualized using ECL (Amersham Biosciences) according to the manufacturer's instructions. The same membrane was reprobed with anti-actin antibody (clone C4, CHEMICOM).
Northern blotting
Total RNA was prepared using ISOGEN (Nippon Gene) and then poly(A)+ RNA was purified using a GenElute mRNA Miniprep Kit (Sigma-Aldrich). Northern blotting proceeded as described (32). Probes labeled with 32P were generated from cDNA fragments of Per2 (bases 1123–1830; GenBank accession no. AF036893), E4BP4 (bases 61–770; GenBank accession no. U83148), Bmal1 (bases 231–910; GenBank accession no. AF015953) and β-actin. The relative expression level of each gene to that of β-actin was calculated using Image Gauge (FUJIFILM).
Real-time luciferase assay
Fragments of DNA containing the Per2 promoter region and its derivatives were cloned into pGL3-dLuc that contains a rapid degradation domain modified from mouse ornithine decarboxylase at the carboxy-terminal end of firefly luciferase (33). After transfecting reporter plasmids using PolyFect (Qiagen), NIH3T3 cells were stimulated with 100 nM dexamethasone for 2 h and then incubated with DMEM containing 0.1 mM luciferin (Promega), 25 mM HEPES (pH 7.2) and 10% FBS. Bioluminescence was measured and integrated for 1 min at intervals of 10 min using Kronos AB-2500 (ATTO).
Transient luciferase assay
The Per2 promoter region containing E4BP4-binding sites and its derivatives were cloned into pGL3-Basic vector (Promega). The constructs were co-transfected with phRG-TK (Promega) as an internal control into NIH3T3 cells. Luciferase activities were measured using the Dual-Luciferase Reporter Assay System (Promega) and a Luminometer Model TD-20/20 (Turner Designs). The transcriptional activities were normalized relative to Renilla luciferase activities.
Gel shift assay
Gel shifts were examined as described (34). Briefly, nuclear extracts were purified from NIH3T3 cells using CelLytic NuCLEAR EXTRACTION KIT (Sigma). Recombinant-E4BP4 was purchased from ABNOVA. The probes (A-site, from −251 to −92; B-site, from +112 to +332 of mPer2) were amplified using the following primer sets: A-site, 5′-GGAAGTGGACGCGCCTACTCG-3′ (forward) and 5′-CGGAACCTGAGAGCTACGCTC-3′ (reverse); B-site, 5′-TTGACGCGGCGAAGCGGTGAGTG-3′ (forward) and 5′-GGGACGCAGTGTGAACCTGG-3′ (reverse). Nucleotide sequences of the 16-bp oligonucleotide probes for the A- and B-sites were 5′-CGTCTTATGTAAAGAG-3′ and 5′-CGTCTTACGTAACCGG-3′, respectively. These probes were end-labeled with [γ-32P]ATP using T4 polynucleotide kinase (New England BioLabs). The DNA probes were suspended in 10 μl of 16 mM HEPES (pH 7.5), 150 mM KCl, 16% (v/v) glycerol, 1.6 mM MgCl2, 0.8 mM dithiothreitol, 0.4 mM PMSF, 1 mM EDTA, 0.8 mg/ml BSA, 0.06 mg/ml poly(dI–dC) and 0.01% NP-40 in the presence of or absence of competitor oligonucleotides and incubated with the nuclear extracts or E4BP4 protein. The anti-E4BP4 antibody was also added for super-shift assays. The samples were resolved by electrophoresis on 4% polyacrylamide gels in 40 mM Tris–acetate, 1 mM EDTA and 5% glycerol at 110 V for 2 h.
Chromatin immunoprecipitation (ChIP) assay
Assays of ChIP proceeded as described (34). Briefly, NIH3T3 cells were cross-linked with 1% formaldehyde for 15 min at room temperature and then washed twice with ice-cold PBS. The cells were shattered with lysis buffer (25 mM Tris-HCl (pH 8.0), 140 mM NaCl, 1% Triton X-100, 0.1% SDS, 3 mM EDTA and 1 mM PMSF) on ice for 30 min. Sonication to shear DNA into 100–300 bp fragments was followed by centrifugation and supernatants containing soluble chromatin were collected. The chromatin fraction was incubated with anti-E4BP4 antibody (E-16, Santa Cruz Biotechnology) overnight at 4°C, followed by salmon sperm DNA/protein G agarose (Upstate). Chromatin immunocomplexes were washed three times, once with wash buffer 1 (20 mM Tris-HCl (pH 8.0), 150 mM NaCl, 0.1% SDS, 1% Triton X-100 and 2 mM EDTA), once with wash buffer 2 (20 mM Tris-HCl (pH 8.0), 500 mM NaCl, 0.1% SDS, 1% Triton X-100 and 2 mM EDTA) and once with wash buffer 3 (10 mM Tris-HCl (pH 8.0), 0.25 M LiCl, 1% Nonidet P-40, 1% deoxycholate and 1 mM EDTA). All washes proceeded at 4°C for 5 min. The samples were then washed twice with TE buffer. The immunocomplexes were removed with 1% SDS and 0.1 M NaHCO3 and then heated overnight at 65°C to reverse the crosslinks. The crosslinks of DNA input samples were similarly reversed. Sample DNA was purified and then putative E4BP4 target regions (A-site, from −251 to −92; B-site, from +112 to +332 of mPer2) were amplified by PCR using the following primer sets: A- site, 5′-GGAAGTGGACGCGCCTACTCG-3′ (forward) and 5′-CGGAACCTGAGAGCTACGCTC-3′ (reverse); B-site, 5′-TTGACGCGGCGAAGCGGTGAGTG-3′ (forward) and 5′-GGGACGCAGTGTGAACCTGG-3′ (reverse).
RESULTS
E4BP4 down-regulates Per2 transcription
E4BP4 is a mammalian homologue of vrille (vri) that functions as a key negative component of the Drosophila circadian clock (12,26,27). However, whether or not E4BP4 is required for mammalian circadian clocks remains unclear. We initially examined the effect of E4BP4 upon Per2 transcription. To determine whether E4BP4 regulates Per2 gene expression, we performed knockdown experiments using small interfering RNA (siRNA) for E4BP4 (E4BP4 siRNA). The induction of E4BP4 siRNA into NIH3T3 cells resulted in a significant decrease in the protein level of exogenously expressed Myc-tagged E4BP4, whereas the level of Myc-tagged Hepatic Leukemia Factor (HLF) was not affected (Figure 1A, lane 2), suggesting that the siRNA specifically knocked down E4BP4. We then examined the mRNA levels of E4BP4 and Per2 by Northern blotting. After introducing E4BP4 siRNA into NIH3T3 cells, the mRNA level of endogenous E4BP4 decreased to ∼60% of that in non-transfected (NT) cells (Figure 1B, lane 5). On the other hand, that of Per2 increased to ∼130% of that in NT cells (Figure 1B, lane 6). The levels of neither E4BP4 mRNA (Figure 1B, lane 3) nor Per2 mRNA (Figure 1B, lane 4) were affected by induction with scrambled siRNA (Control siRNA). These results suggest that E4BP4 suppresses Per2 transcription.
Figure 1.
E4BP4 is a negative regulator of Per2 transcription. (A) RNA interference with E4BP4. NIH3T3 cells were transfected with scrambled siRNA (Control siRNA) or specific siRNA for E4BP4 (E4BP4 siRNA). Myc-tagged E4BP4 was co-transfected with Myc-tagged HLF (as a negative control) 24 h later. At 48 h after siRNA transfection, the E4BP4 protein level was determined by Western blotting using anti-Myc antibody. The same membrane was stripped and reprobed with anti-actin antibody. (B) Knockdown of E4BP4 increased Per2 transcription. NIH3T3 cells transfected with siRNAs (Control siRNA or E4BP4 siRNA) or non-transfected (NT) were analyzed for E4BP4 and Per2 mRNA by Northern blotting. Poly(A)+ RNA was purified from total RNA of two independent experiments. Expression levels were normalized to those of β-actin. Values are relative to that of NT cells.
The circadian clock is cell-autonomous (35,36). Circadian oscillators are located not only in the suprachiasmatic nucleus (SCN) of the brain, which is the central circadian pacemaker in mammals, but also in most peripheral tissues (19,37,38) and even in established cell lines (39). To examine the role of E4BP4 on the circadian expression of Per2 in the cell-autonomous clock, we performed real-time luciferase assays (33,40) in NIH3T3 cells using the reporter plasmid containing the mPer2 (−798 to +331) promoter to drive destabilized luciferase (mPer2-dLuc). The transcriptional start site (TSS) is indicated as +1 (24). After the introduction of E4BP4 siRNA, the cells were transfected with mPer2-dLuc and circadian gene expression was induced with 100 nM dexamethasone (41). We measured bioluminescence in the presence of luciferin and integrated signals for 1 min at intervals of 10 min. As reported (24,25,30), the transcriptional fluctuation from mPer2-dLuc showed robust circadian oscillation. The induction of E4BP4 siRNA caused a remarkable overall 1.86-fold increase in the transcriptional activity of mPer2-dLuc compared with Control siRNA (Figure 2A). Conversely, exogenously expressed E4BP4 resulted in a gradual reduction in the circadian expression of Per2 (Figure 2B). These results suggested that E4BP4 functions as a negative regulator of Per2 oscillation in the cell-autonomous clock.
Figure 2.
E4BP4 negatively regulates Per2 oscillation in cell-autonomous clock. (A) Effect of E4BP4-knockdown by siRNA. B, Effect of E4BP4-overexpression. Promoter region of mPer2 (−798 to +331 relative to the cap site) was examined in real-time reporter gene assays. After introduction of siRNAs (A) or E4BP4-expression vector (B), bioluminescence was measured and integrated for 1 min at intervals of 10 min. The results are representative of three independent experiments.
Two putative E4BP4-binding sites on the Per2 promoter region
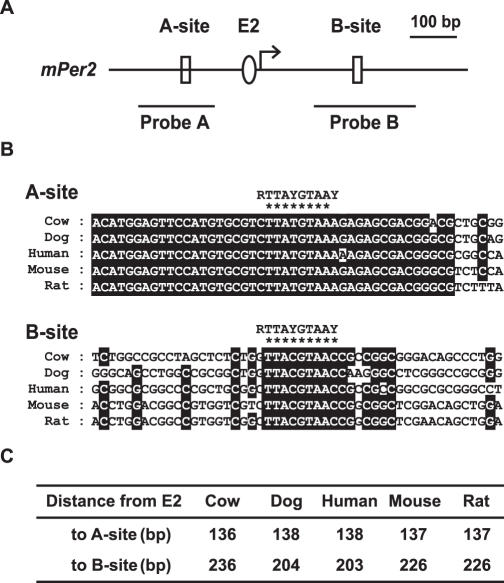
Although many putative E4BP4-binding sites are located in clock and clock-related genes (30), whether the E4BP4-mediated negative regulation of Per2 is direct or indirect remains unknown. To examine whether E4BP4 directly represses the transcriptional activity of Per2, we searched the mouse Per2 promoter region and genomic gene sequences for the E4BP4-binding site, RT(G/T)AYGTAAY (where R is a purine and Y is a pyrimidine) (42). Sequence analysis revealed two putative E4BP4-binding sites (termed A-site at −151 and B-site at +197) around the TSS (Figure 3A). The nucleotide sequences of the A- and B-sites matched 8/10 and 9/10 bp of the consensus E4BP4-binding sequence, respectively. Further analysis revealed that the nucleotide sequence around the A-site is highly conserved among mammalian Per2 promoter regions (cow, dog, human, mouse and rat) (Figure 3B, upper panel). On the other hand, a B-site is located at a diverse region of intron 1 (Figure 3B, lower panel). Interestingly, the location of both sites with respect to the E2 enhancer was highly conserved beyond species (Figure 3C). These data suggest that the A- and B-sites are functionally important for the E4BP4-mediated negative regulation of Per2.
Figure 3.
Two putative E4BP4-binding sites on mammalian Per2 promoter regions. (A) Maps of mPer2 promoter region. Arrow and oval indicate transcription start site (TSS) and E2 enhancer (position at –20 relative to cap site) (24), respectively. Two putative E4BP4-binding sites are shown as open boxes. Positions of probes used for gel shift analysis are indicated as horizontal bars. (B) Sequence alignment of region around E4BP4-binding sites. Conserved sequences are shown as white characters on black. Consensus E4BP4-binding sequences are shown above and identical bases are indicated by asterisks. (C) Distance between putative E4BP4-binding sites and E2.
E4BP4 directly represses the transcriptional activity of Per2 through the B-site
To understand the functional importance of the A- and B-sites for E4BP4-mediated transcriptional repression of Per2, we performed luciferase assays with mutant constructs of the mPer2 (−798 to +331) promoter (Figure 4A) to prevent E4BP4 binding (Supplementary Figure S1). The mutated sequences of the A- and B-sites were 5′-CCAGTGTAAA-3′ and 5′-CCAGCGTAAC-3′, respectively (43). E4BP4-mediated transcriptional repression of these mutant constructs was examined, and normalized expression level was calculated relative to the luciferase activity in the absence of E4BP4. Consistent with the observations in Figure 2B, exogenously expressed E4BP4 repressed the transcriptional activity of the wild type of mPer2(−798 to +331) promoter (Wild, % of repression by E4BP4 was 41.0%). A mutation of the A-site (A mut; 47.2%) resulted in the same transcriptional repression as with the Wild type. However, mutation of the B-site (B mut; 9.67%) and of both the A- and B-sites (A/B mut; 11.7%) recovered from the repression (Figure 4B). Similar results were obtained using deletion constructs of E4BP4-binding sites (data not shown). As the PAR transcription factors (DBP, HLF and TEF) are known to bind to the identical nucleotide sequence as E4BP4 in vitro (29,30), we also examined the effect of DBP, HLF and TEF on the mPer2 promoter activity using same mutant and deletion constructs. However, neither the A- nor B-site was responsible for the transcriptional activation of mPer2 by these PAR transcription factors (Supplementary Figure S2). These results suggest that the B-site is functionally important for the E4BP4-mediated transcriptional repression of Per2.
Figure 4.
B-site is responsible for E4BP4-mediated transcriptional repression of Per2. (A) Schematic representation of mutant constructs of mPer2 promoter. Arrow indicates TSS. Open boxes, putative E4BP4-binding sites. Each or both of the putative E4BP4-binding sites were mutated (A-site, 5′-CTTATGTAAA-3′ to 5′-CCAGTGTAAA-3′; B-site, 5′-CTTACGTAAC-3′ to 5′-CCAGCGTAAC-3′). (B) Analysis of E4BP4-binding sites on Per2 promoter. Transcriptional assay was performed with indicated mutant constructs. E4BP4 expression plasmid is present (+) or absent (−). Normalized expression level was calculated relative to luciferase activity in absence of E4BP4. Values are means ± SEM of three replicates from a single assay. (*) Significant difference between presence versus absence of E4BP4 (P < 0.001). Results are representative of two independent experiments.
To clarify the importance of the B-site for E4BP4-mediated transcriptional repression of the Per2 promoter through the DNA-binding activity of E4BP4, we performed gel shift assays using nuclear extracts from the NIH3T3 cells expressing Myc-tagged E4BP4 and the probes shown in Figure 3A. We used end-labeled DNA fragments of ∼200 bp containing either the A- or the B-site probes (Figure 3A). We observed shifted bands for both probes and the bands disappeared in the presence of an unlabeled competitor containing the consensus E4BP4-binding sequence, indicating that the protein-DNA complexes were specific for the E4BP4-binding site (Figure 5A, asterisk in probe A and double asterisk in probe B). However, the supersifted band with an anti-E4BP4 antibody was observed in only probe B (Figure 5A, arrowhead). This was also confirmed using an anti-Myc antibody (data not shown). These results suggest that E4BP4 binds to the B-site and forms a DNA-protein complex. We questioned why only the probe B (double asterisk) showed a supershifted band. Figure 3B shows that the nucleotide sequences of A- and B-sites were not identical. Therefore, we examined the affinity of E4BP4 for the A- and B-sites using gel shift assays, with recombinant-E4BP4 protein and 16-bp core probes for each site. Figure 5B and C shows that the B-site had higher affinity than the A-site for E4BP4. Further analysis determined that the Kds for binding of the recombinat-E4BP4 to the A- and B-sites were 2.58 and 0.579 nM, respectively (Figure 5D). These results indicated that E4BP4 preferentially binds to the B-site rather than to the A-site in vitro.
Figure 5.
E4BP4 preferentially binds to B-site in vitro. (A) Gel shift analysis using nuclear extracts from NIH3T3 cells transfected with the E4BP4-expression vector. Positions of probes A and B are shown in Figure 3A and competing nucleotide sequence is 5′-TCGAGAAAAAATTATGTAACGGTC-3′. * and **, band specifically bound to E4BP4-binding site; arrowhead, supershifted band. The band specifically bound to E4BP4-binding site in probe B (**) not but in probe A (*) was supershifted with an anti-E4BP4 antibody. (B) Gel shift analysis using recombinant-E4BP4. Oligonucleotide probes (A-site, 5′-CGTCTTATGTAAAGAG-3′; B-site, 5′-CGTCTTACGTAACCGG-3′) were incubated with increasing amounts of recombinant-E4BP4 (0, 0.4, 2, 10 and 50 ng). Arrowhead, band bound to E4BP4. (C) Quantitation of E4BP4-oligonucleotide probe complex. (D) Determination of Kds for binding of recombinant-E4BP4 to A- and B-sites. Recombinant-E4BP4 (10 ng) was incubated with increasing amounts of radiolabeled 16-bp core probes for A- and B-sites. After gel electrophoresis and autoradiography, radioactive bands corresponding to the bound and free forms were quantified. Concentration of bound probe was plotted against total input probe to show saturation curves. Kd values were determined from these data on Scatchard plots. Slope of best-fit line is equal to −1/Kd.
We then performed ChIP assays in NIH3T3 cells expressing Myc-tagged E4BP4 to determine the situation in vivo. Consistent with the observations in Figure 5 in vitro, the ChIP assays also suggested that E4BP4 binds to the B-site much more than to the A-site on the Per2 promoter in vivo (data not shown). Taken together, these results indicated that E4BP4 directly represses Per2 transcription via the B-site on the promoter.
Binding of E4BP4 correlates with negative regulation of Per2
The circadian expression of E4BP4 is similar to that of the anti-phase to Per2 oscillations in both the SCN and the liver (29,33). We therefore postulated that E4BP4 plays an important role in Per2 oscillation in the cell-autonomous core clock. To test this hypothesis, we analyzed the temporal expression profile of E4BP4 mRNA in NIH3T3 cells after stimulation with 100 nM dexamethasone (44). Northern blots showed rhythmic E4BP4 mRNA expression with a peak at 36 h and a trough at 24 h, an anti-phase to Per2 oscillation and a similar phase to Bmal1 oscillation (Figure 6A). We then examined the temporal binding of endogenous E4BP4 on the Per2 promoter by ChIP analysis. The ChIP assay showed that the peak of endogenous E4BP4 binding to the Per2 promoter almost matched the trough of Per2 mRNA expression (Figure 6B; see 18 and 36 h). The correlation between the binding activity of E4BP4 and the transcriptional repression of Per2 suggests that E4BP4 plays an important role as a negative regulator for the circadian expression of Per2 in the cell-autonomous core clock.
Figure 6.
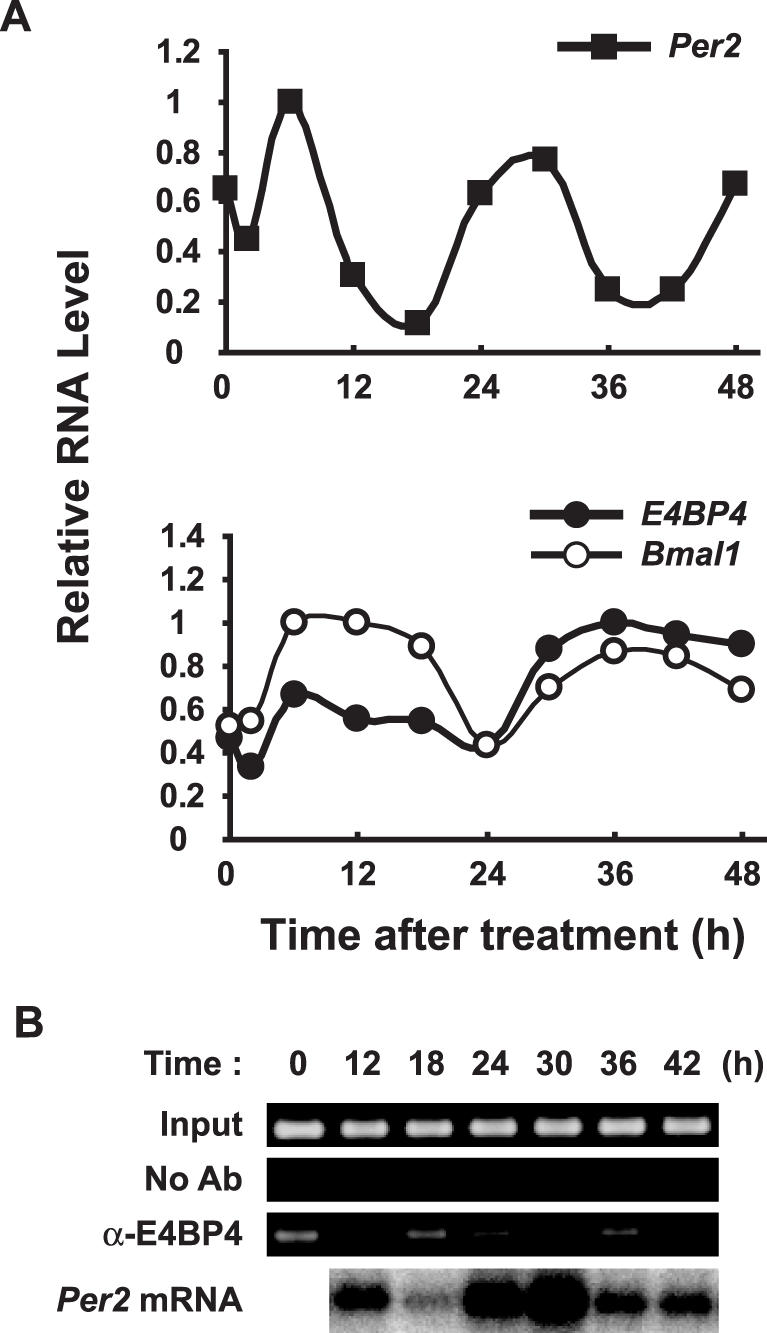
Binding of E4BP4 correlates with negative Per2 regulation. (A) E4BP4 transcript shows anti-phase to Per2 oscillation. NIH3T3 cells were stimulated with 100 nM dexamethasone, and mRNA was analyzed by northern blotting. Levels of RNA were normalized to β-actin expression and peak values of individual curves were set to 1. (B) Oscillatory binding of E4BP4 to Per2 promoter in vivo. NIH3T3 cells were stimulated with dexamethasone and ChIP assays of B-sites were applied. Bottom panel, Per2 mRNA oscillation. No Ab, without antibody; α-E4BP4, with anti-E4BP4 antibody.
B-site is responsible for the generation of high amplitude in Per2 oscillation
To clarify the role of E4BP4 binding in Per2 mRNA oscillation in the circadian clock, we performed real-time reporter assays using mutants of the A- and B-sites. Circadian oscillation of the construct with the wild type of mPer2 promoter (Wild-dLuc) was obvious (Figure 7A). Superimposing the oscillation profile of the mutant construct of the A-site (A mut-dLuc) on that of the wild type, confirmed that both oscillation profiles were similar, indicating that the A-site is not prerequisite for the circadian expression of Per2 (Figure 7B). On the other hand, the amplitude of oscillation by mutants of the B-site (B mut-dLuc) and of both the A- and B-sites (A/B mut-dLuc) was lower than that of Wild-dLuc (Figure 7C and D). These findings suggest that the B-site is responsible for generating the high amplitude of Per2 oscillation.
Figure 7.
(A–H) B-site drives the circadian expression of Per2. NIH3T3 cells were transfected with indicated mutant constructs, incubated with dexamethasone and then bioluminescence was measured. For accurate comparison, light gray dots show bioluminescence from Wild-dLuc. Peak values of individual curves were set to 1. Results are representative of three independent experiments that generated similar results. Wild, wild type mPer2 promoter; A mut, mutated A-site; B mut, mutated B-site; E2 mut, mutated E2 enhancer (5′-GCTAGT-3′).
Binding of E4BP4 to the B-site is required for circadian expression of Per2
The E2 enhancer is required for Per2 oscillation, which is mediated by CLOCK:BMAL1 and it is located 226 bp upstream of the B-site (24,25). To understand the relationship between the E2 enhancer and the B-site for Per2 oscillation, we performed real-time reporter assays using mutants of the E2 enhancer that lack transcriptional activation by CLOCK:BMAL1 (Supplementary Figure S3) (24). Surprisingly, the mutant of the E2 enhancer (E2 mut-dLuc), which contains both of the intact E4BP4 sites, retained the ability to potently drive the circadian oscillation of Per2 (Figure 7E), whereas the circadian rhythmicity for the mutant of the E2 enhancer and the B-site (E2/B mut-dLuc) as well as the mutant of the E2 enhancer and the A/B-sites (E2/A/B mut-dLuc) was lost (Figure 7G and H). The mutant of both the E2 enhancer and the A-site (E2/A mut-dLuc) retained a clear oscillatory profile but it was subtly changed as compared with E2 mut-dLuc (Figure 7F). These results strengthened the notion that not only the E2 enhancer, but also the B-site for E4BP4 binding is critical for the circadian expression of Per2 mRNA in the cell-autonomous core clock.
DISCUSSION
E4BP4 is a mammalian homologue of vrille (vri) that functions as a key negative component of the Drosophila circadian clock (12,26,27). E4BP4 in chickens probably plays an important role in the phase-delaying process as a light-dependent suppressor of cPer2 (28). E4BP4 is rhythmically expressed in mammals with an anti-phase to Period1 (Per1) oscillation in the liver and the SCN and exogenously expressed E4BP4 directly represses the Per1 promoter activity (29). These results indicated that E4BP4 functions as a key negative component of mammalian circadian clocks such as in Drosophila. However, no direct evidence has supported this notion until now. This study is the first to demonstrate that endogenous E4BP4 negatively regulates Per2 transcription in mammals (Figure 1B), which is consistent with findings that E4BP4 represses the transcription of several genes (42,43,45). This was also confirmed in mPer2 oscillatory transcription (Figure 2A). Figure 2 shows that the modulation of E4BP4 expression remarkably affected the amplitude, but not the period during mPer2 oscillation. An mPer2 mutant displays a short-circadian period followed by a loss of circadian rhythmicity in constant darkness (20), and our data also indicated a subtle elongation and shortening of the period induced by down- and up-regulated E4BP4 expression, respectively (Figure 2). The effect upon the period of oscillation might depend on the expression level and a distinguishing change in E4BP4 expression might be required. These results show that E4BP4 is involved in the circadian expression of Per2, which is one of the essential components of mammalian circadian clocks.
We identified two putative E4BP4-binding sites on the Per2 promoter region (Figure 3). Ueda et al. described the A-site as an E4BP4-binding site that can oscillate SV40 promoter activity in a similar phase to Per2 in established cell lines (30). Because the nucleotide sequence around the A-site is highly conserved, the A-site has simply been thought to play an important role. In this study, we showed that the novel E4BP4-binding site, the B-site, is required for the robust circadian expression of Per2 (Figures 4 and 7). Furthermore, the importance of B-sites for E4BP4-mediated transcriptional repression of Per2 is confirmed by the DNA-binding activity of E4BP4 in vitro and in vivo (Figure 5). Different nucleotides at the center of the consensus sequence, that is, ‘T’ and ‘C’ on the A- and B-sites, respectively, might explain the preference for the B-site.
Yoo et al. have recently identified a circadian enhancer (E2) with a non-canonical 5′-CACGTT-3′ E-box located 20 bp upstream of the mPer2 transcription start site and demonstrated that a 210 bp promoter region with the E2 enhancer but without the B-site, is sufficient for Per2 oscillation (24). Here, we performed real-time luciferase assays in NIH3T3 cells using the mPer2 (−798 to +331) promoter containing the E2 enhancer as well as the novel E4BP4-binding site (B-site). Our findings showed that the novel cis-element for E4BP4 binding is required for robust circadian expression of Per2 in the cell-autonomous core clock as well as the E2 enhancer, indicating that E4BP4 is a key negative regulator of the mammalian circadian clock.
The Drosophila circadian oscillator consists of interlocked period/timeless and dClock transcriptional/translational feedback loops (46–49). Within these loops, VRI negatively regulates period expression, which is activated by the dCLOCK:CYCLE complex (CYCLE is also known as dBMAL1), through the repression of dClock promoter activity (12,26,27). We showed that E4BP4, which is a mammalian homologue of vri, functions as a repressor of Per2 transcription through the novel E4BP4-binding site (B-site) and that E4BP4 must bind to the B-site for the robust circadian expression of Per2 in the cell-autonomous clock. These findings demonstrate the importance of negative regulation by the direct binding of E4BP4 as well as of positive regulation by CLOCK:BMAL1 in the mammalian circadian clock. Taken together, VRI/E4BP4 seems to function as a negative factor of Period oscillation in the circadian clock of Drosophila and mammals.
Missense mutations in clock genes have recently been linked to familial advanced (50,51) and delayed (52) sleep phase syndromes, an abnormality in the circadian timing system that affects the timing of sleep. Here we show the importance of negative regulation by E4BP4 for the circadian expression of Per2. As with these clock components, the identification of a nucleotide polymorphism in E4BP4 should bring new insight into sleep disorders.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
Acknowledgments
The authors thank Dr Y. Nakajima for providing the plasmid containing mPer2 promoter. We also thank Ms C. Iitaka, Dr K. Ohsaki and Mr Y. Hara for helpful discussion. This project was supported by operational subsidies from AIST (METI) and from the University of Tsukuba, and the Ministry of Education, Culture, Sports, Science and Technology. Funding to pay the Open Access publication charges for this article was provided by AIST (METI).
Conflict of interest statement. None declared.
REFERENCES
- 1.Dunlap J.C. Molecular bases for circadian clocks. Cell. 1999;96:271–290. doi: 10.1016/s0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]
- 2.Kako K., Ishida N. The role of transcription factors in circadian gene expression. Neurosci. Res. 1998;31:257–264. doi: 10.1016/s0168-0102(98)00054-6. [DOI] [PubMed] [Google Scholar]
- 3.Reppert S.M., Weaver D.R. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 4.Panda S., Hogenesch J.B., Kay S.A. Circadian rhythms from flies to human. Nature. 2002;417:329–335. doi: 10.1038/417329a. [DOI] [PubMed] [Google Scholar]
- 5.Konopka R.J., Benzer S. Clock mutants of Drosophila melanogaster. Proc. Natl Acad. Sci. USA. 1971;68:2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bargiello T.A., Jackson F.R., Young M.W. Restoration of circadian behavioural rhythms by gene transfer in Drosophila. Nature. 1984;312:752–754. doi: 10.1038/312752a0. [DOI] [PubMed] [Google Scholar]
- 7.Reddy P., Zehring W.A., Wheeler D.A., Pirrotta V., Hadfield C., Hall J.C., Rosbash M. Molecular analysis of the period locus in Drosophila melanogaster and identification of a transcript involved in biological rhythms. Cell. 1984;38:701–710. doi: 10.1016/0092-8674(84)90265-4. [DOI] [PubMed] [Google Scholar]
- 8.Myers M.P., Wager-Smith K., Wesley C.S., Young M.W., Sehgal A. Positional cloning and sequence analysis of the Drosophila clock gene, timeless. Science. 1995;270:805–808. doi: 10.1126/science.270.5237.805. [DOI] [PubMed] [Google Scholar]
- 9.Allada R., White N.E., So W.V., Hall J.C., Rosbash M. A mutant Drosophila homolog of mammalian Clock disrupts circadian rhythms and transcription of period and timeless. Cell. 1998;93:791–804. doi: 10.1016/s0092-8674(00)81440-3. [DOI] [PubMed] [Google Scholar]
- 10.Rutila J.E., Suri V., Le M., So W.V., Rosbash M., Hall J.C. CYCLE is a second bHLH-PAS clock protein essential for circadian rhythmicity and transcription of Drosophila period and timeless. Cell. 1998;93:805–814. doi: 10.1016/s0092-8674(00)81441-5. [DOI] [PubMed] [Google Scholar]
- 11.Price J.L., Blau J., Rothenfluh A., Abodeely M., Kloss B., Young M.W. double-time is a novel Drosophila clock gene that regulates PERIOD protein accumulation. Cell. 1998;94:83–95. doi: 10.1016/s0092-8674(00)81224-6. [DOI] [PubMed] [Google Scholar]
- 12.Blau J., Young M.W. Cycling vrille expression is required for a functional Drosophila clock. Cell. 1999;99:661–671. doi: 10.1016/s0092-8674(00)81554-8. [DOI] [PubMed] [Google Scholar]
- 13.Tei H., Okamura H., Shigeyoshi Y., Fukuhara C., Ozawa R., Hirose M., Sakaki Y. Circadian oscillation of a mammalian homologue of the Drosophila period gene. Nature. 1997;389:512–516. doi: 10.1038/39086. [DOI] [PubMed] [Google Scholar]
- 14.Sun Z.S., Albrecht U., Zhuchenko O., Bailey J., Eichele G., Lee C.C. RIGUI, a putative mammalian ortholog of the Drosophila period gene. Cell. 1997;90:1003–1011. doi: 10.1016/s0092-8674(00)80366-9. [DOI] [PubMed] [Google Scholar]
- 15.Shearman L.P., Zylka M.J., Weaver D.R., Kolakowski L.F., Jr, Reppert S.M. Two period homologs: circadian expression and photic regulation in the suprachiasmatic nuclei. Neuron. 1997;19:1261–1269. doi: 10.1016/s0896-6273(00)80417-1. [DOI] [PubMed] [Google Scholar]
- 16.Albrecht U., Sun Z.S., Eichele G., Lee C.C. A differential response of two putative mammalian circadian regulators, mper1 and mper2, to light. Cell. 1997;91:1055–1064. doi: 10.1016/s0092-8674(00)80495-x. [DOI] [PubMed] [Google Scholar]
- 17.Takumi T., Matsubara C., Shigeyoshi Y., Taguchi K., Yagita K., Maebayashi Y., Sakakida Y., Okumura K., Takashima N., Okamura H. A new mammalian period gene predominantly expressed in the suprachiasmatic nucleus. Genes Cells. 1998;3:167–176. doi: 10.1046/j.1365-2443.1998.00178.x. [DOI] [PubMed] [Google Scholar]
- 18.Takumi T., Taguchi K., Miyake S., Sakakida Y., Takashima N., Matsubara C., Maebayashi Y., Okumura K., Takekida S., Yamamoto S., et al. A light-independent oscillatory gene mPer3 in mouse SCN and OVLT. EMBO J. 1998;17:4753–4759. doi: 10.1093/emboj/17.16.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakamoto K., Nagase T., Fukui H., Horikawa K., Okada T., Tanaka H., Sato K., Miyake Y., Ohara O., Kako K., et al. Multitissue circadian expression of rat period homolog (rPer2) mRNA is governed by the mammalian circadian clock, the suprachiasmatic nucleus in the brain. J. Biol. Chem. 1998;273:27039–27042. doi: 10.1074/jbc.273.42.27039. [DOI] [PubMed] [Google Scholar]
- 20.Zheng B., Larkin D.W., Albrecht U., Sun Z.S., Sage M., Eichele G., Lee C.C., Bradley A. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature. 1999;400:169–173. doi: 10.1038/22118. [DOI] [PubMed] [Google Scholar]
- 21.Bae K., Jin X., Maywood E.S., Hastings M.H., Reppert S.M., Weaver D.R. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron. 2001;30:525–536. doi: 10.1016/s0896-6273(01)00302-6. [DOI] [PubMed] [Google Scholar]
- 22.Zheng B., Albrecht U., Kaasik K., Sage M., Lu W., Vaishnav S., Li Q., Sun Z.S., Eichele G., Bradley A., et al. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell. 2001;105:683–694. doi: 10.1016/s0092-8674(01)00380-4. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto Y., Yagita K., Okamura H. Role of cyclic mPer2 expression in the mammalian cellular clock. Mol. Cell. Biol. 2005;25:1912–1921. doi: 10.1128/MCB.25.5.1912-1921.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoo S.H., Ko C.H., Lowrey P.L., Buhr E.D., Song E.J., Chang S., Yoo O.J., Yamazaki S., Lee C., Takahashi J.S. A noncanonical E-box enhancer drives mouse Period2 circadian oscillations in vivo. Proc. Natl Acad. Sci. USA. 2005;102:2608–2613. doi: 10.1073/pnas.0409763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akashi M., Ichise T., Mamine T., Takumi T. Molecular mechanism of cell-autonomous circadian gene expression of Period2, a crucial regulator of the mammalian circadian clock. Mol. Biol. Cell. 2006;17:555–565. doi: 10.1091/mbc.E05-05-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cyran S.A., Buchsbaum A.M., Reddy K.L., Lin M.C., Glossop N.R., Hardin P.E., Young M.W., Storti R.V., Blau J. vrille, Pdp1, and dClock form a second feedback loop in the Drosophila circadian clock. Cell. 2003;112:329–341. doi: 10.1016/s0092-8674(03)00074-6. [DOI] [PubMed] [Google Scholar]
- 27.Glossop N.R., Houl J.H., Zheng H., Ng F.S., Dudek S.M., Hardin P.E. VRILLE feeds back to control circadian transcription of Clock in the Drosophila circadian oscillator. Neuron. 2003;37:249–261. doi: 10.1016/s0896-6273(03)00002-3. [DOI] [PubMed] [Google Scholar]
- 28.Doi M., Nakajima Y., Okano T., Fukada Y. Light-induced phase-delay of the chicken pineal circadian clock is associated with the induction of cE4bp4, a potential transcriptional repressor of cPer2 gene. Proc. Natl Acad. Sci. USA. 2001;98:8089–8094. doi: 10.1073/pnas.141090998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitsui S., Yamaguchi S., Matsuo T., Ishida Y., Okamura H. Antagonistic role of E4BP4 and PAR proteins in the circadian oscillatory mechanism. Genes Dev. 2001;15:995–1006. doi: 10.1101/gad.873501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ueda H.R., Hayashi S., Chen W., Sano M., Machida M., Shigeyoshi Y., Iino M., Hashimoto S. System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nature Genet. 2005;37:187–192. doi: 10.1038/ng1504. [DOI] [PubMed] [Google Scholar]
- 31.Tojo M., Matsuzaki K., Minami T., Honda Y., Yasuda H., Chiba T., Saya H., Fujii-Kuriyama Y., Nakao M. The aryl hydrocarbon receptor nuclear transporter is modulated by the SUMO-1 conjugation system. J. Biol. Chem. 2002;277:46576–46585. doi: 10.1074/jbc.M205987200. [DOI] [PubMed] [Google Scholar]
- 32.Oishi K., Amagai N., Shirai H., Kadota K., Ohkura N., Ishida N. Genome-wide expression analysis reveals 100 adrenal gland-dependent circadian genes in the mouse liver. DNA Res. 2005;12:191–202. doi: 10.1093/dnares/dsi003. [DOI] [PubMed] [Google Scholar]
- 33.Ueda H.R., Chen W., Adachi A., Wakamatsu H., Hayashi S., Takasugi T., Nagano M., Nakahama K., Suzuki Y., Sugano S., et al. A transcription factor response element for gene expression during circadian night. Nature. 2002;418:534–539. doi: 10.1038/nature00906. [DOI] [PubMed] [Google Scholar]
- 34.Onishi Y., Kiyama R. Interaction of NF-E2 in the human β-globin locus control region before chromatin remodeling. J. Biol. Chem. 2003;278:8163–8171. doi: 10.1074/jbc.M209612200. [DOI] [PubMed] [Google Scholar]
- 35.Welsh D.K., Logothetis D.E., Meister M., Reppert S.M. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- 36.Nagoshi E., Saini C., Bauer C., Laroche T., Naef F., Schibler U. Circadian gene expression in individual fibroblasts: cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell. 2004;119:693–705. doi: 10.1016/j.cell.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 37.Yamazaki S., Numano R., Abe M., Hida A., Takahashi R., Ueda M., Block G.D., Sakaki Y., Menaker M., Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 38.Yoo S.H., Yamazaki S., Lowrey P.L., Shimomura K., Ko C.H., Buhr E.D., Siepka S.M., Hong H.K., Oh W.J., Yoo O.J., et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc. Natl Acad. Sci. USA. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balsalobre A., Damiola F., Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- 40.Brandes C., Plautz J.D., Stanewsky R., Jamison C.F., Straume M., Wood K.V., Kay S.A., Hall J.C. Novel features of drosophila period transcription revealed by real-time luciferase reporting. Neuron. 1996;16:687–692. doi: 10.1016/s0896-6273(00)80088-4. [DOI] [PubMed] [Google Scholar]
- 41.Balsalobre A., Marcacci L., Schibler U. Multiple signaling pathways elicit circadian gene expression in cultured Rat-1 fibroblasts. Curr. Biol. 2000;10:1291–1294. doi: 10.1016/s0960-9822(00)00758-2. [DOI] [PubMed] [Google Scholar]
- 42.Cowell I.G., Skinner A., Hurst H.C. Transcriptional repression by a novel member of the bZIP family of transcription factors. Mol. Cell Biol. 1992;12:3070–3077. doi: 10.1128/mcb.12.7.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hough C., Cuthbert C.D., Notley C., Brown C., Hegadorn C., Berber E., Lillicrap D. Cell type-specific regulation of von Willebrand factor expression by the E4BP4 transcriptional repressor. Blood. 2005;105:1531–1539. doi: 10.1182/blood-2002-10-3093. [DOI] [PubMed] [Google Scholar]
- 44.Balsalobre A., Brown S.A., Marcacci L., Tronche F., Kellendonk C., Reichardt H.M., Schutz G., Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 45.Lai C.K., Ting L.P. Transcriptional repression of human hepatitis B virus genes by a bZIP family member, E4BP4. J. Virol. 1999;73:3197–3209. doi: 10.1128/jvi.73.4.3197-3209.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allada R., Emery P., Takahashi J.S., Rosbash M. Stopping time: the genetics of fly and mouse circadian clocks. Annu. Rev. Neurosci. 2001;24:1091–1119. doi: 10.1146/annurev.neuro.24.1.1091. [DOI] [PubMed] [Google Scholar]
- 47.Hall J.C. Systems approaches to biological rhythms in Drosophila. Methods Enzymol. 2005;393:61–185. doi: 10.1016/S0076-6879(05)93004-8. [DOI] [PubMed] [Google Scholar]
- 48.Stanewsky R. Genetic analysis of the circadian system in Drosophila melanogaster and mammals. J. Neurobiol. 2003;54:111–147. doi: 10.1002/neu.10164. [DOI] [PubMed] [Google Scholar]
- 49.Young M.W., Kay S.A. Time zones: a comparative genetics of circadian clocks. Nature Rev. Genet. 2001;2:702–715. doi: 10.1038/35088576. [DOI] [PubMed] [Google Scholar]
- 50.Toh K.L., Jones C.R., He Y., Eide E.J., Hinz W.A., Virshup D.M., Ptacek L.J., Fu Y.H. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001;291:1040–1043. doi: 10.1126/science.1057499. [DOI] [PubMed] [Google Scholar]
- 51.Xu Y., Padiath Q.S., Shapiro R.E., Jones C.R., Wu S.C., Saigoh N., Saigoh K., Ptacek L.J., Fu Y.H. Functional consequences of a CKIδ mutation causing familial advanced sleep phase syndrome. Nature. 2005;434:640–644. doi: 10.1038/nature03453. [DOI] [PubMed] [Google Scholar]
- 52.Ebisawa T., Uchiyama M., Kajimura N., Mishima K., Kamei Y., Katoh M., Watanabe T., Sekimoto M., Shibui K., Kim K., et al. Association of structural polymorphisms in the human period3 gene with delayed sleep phase syndrome. EMBO Rep. 2001;2:342–346. doi: 10.1093/embo-reports/kve070. [DOI] [PMC free article] [PubMed] [Google Scholar]