Abstract
Neurons that utilize melanin-concentrating hormone (MCH) and others that employ hypocretin as neurotransmitter are located in the hypothalamus and project diffusely throughout the CNS, including areas that participate in the generation and maintenance of the states of sleep and wakefulness. In the present report, immunohistochemical methods were employed to examine the distribution of MCHergic and hypocretinergic neurons. In order to test the hypothesis that the MCHergic system is capable of influencing specific behavioral states, we studied Fos immunoreactivity in MCH-containing neurons during 1) quiet wakefulness, 2) active wakefulness with motor activity, 3) active wakefulness without motor activity, 4) quiet sleep, and, 5) active sleep induced by carbachol (AS-carbachol). We determined that MCHergic neuronal somata in the cat are intermingled with hypocretinergic neurons in the dorsal and lateral hypothalamus, principally in the tuberal and tuberomammillary regions; however, hypocretinergic neurons extended more in the anterior-posterior axis than MCHergic neurons. Axosomatic and axodendritic contacts were common between these neurons. In contrast to hypocretinergic neurons, which are known to be active during motor activity and AS-carbachol, Fos immunoreactivity was not observed in MCH-containing neurons in conjunction with any of the preceding behavioral conditions. Non-MCHergic, non-hypocretinergic neurons that expressed c-fos during active wakefulness with motor activity were intermingled with MCH and hypocretin-containing neurons, suggesting that these neurons are related to some aspect of motor function. Further studies are required to elucidate the functional sequela of the interactions between MCHergic and hypocretinergic neurons and the phenotype of the other neurons that were active during motor activity.
Keywords: Melanin-concentrating hormone, MCH, hypocretin, orexin, sleep, REM
1. Introduction
The melanin-concentrating hormone (MCH) was initially characterized as a circulating factor mediating color change in teleost fish (Kawauchi, et al., 1983). Thereafter, MCH was identified in the rat and was found to be fully conserved in all mammals analyzed so far, including humans (Forray, 2003, Shi, 2004). MCH is generated via a proteolytic cleavage of a precursor that generates two additional peptides, neuropeptide E-I and neuropeptide G-E. The biological function of MCH is mediated by two G-protein coupled receptors known as MCH1R and MCHR2, although MCHR2 is only expressed in carnivores and primates (Forray, 2003, Shi, 2004). The conservation of this peptide across species suggests that MCH is involved in critical physiological processes. In this regard, there is evidence that MCH plays a key role in the central regulation of feeding and energy homeostasis. In genetically modified obese mice there is an up-regulation of MCH mRNA, and MCH produces a dose-dependent increase in food intake when administered intraventricularly in rats (Qu, et al., 1996, Rossi, et al., 1997, Rovere, et al., 1996). This stimulatory effect on food intake has been confirmed in transgenic mice in which the overexpression of MCH causes obesity, whereas mice lacking MCH are hypophagic and lean (Ludwig, et al., 2001, Shimada, et al., 1998). In addition, there are data indicating that the pharmacological blockade of MCH receptors may be used in a therapeutic manner to treat obesity, depression and anxiety, suggesting that the MCHergic system participates in other functions (Borowsky, et al., 2002).
Neurons that utilize MCH as a neurotransmitter have been described in the lateral hypothalamus and zona incerta of the rat (Bittencourt, et al., 1992, Skofitsch, et al., 1985). These neurons project throughout the central nervous system with dense projections to areas associated with the regulation of sleep and wakefulness (Bittencourt, et al., 1992, Jones, 2005, Skofitsch, et al., 1985); high densities of MCH receptors are also present in these areas (Kilduff and De Lecea, 2001). Based upon experiments utilizing Fos immunoreactivity as a marker of neuronal activity as well as MCH intraventricular microinjections, Verret et al. suggested that MCH is a powerful hypnogenic factor that plays a crucial role in the control of active (REM) sleep (AS) in the rat (Verret, et al., 2003). In hamsters and rats, MCHergic neurons are intermingled with hypocretinergic neurons and make numerous synaptic contacts with these cells (Bayer, et al., 2002, Guan, et al., 2002, Khorooshi and Klingenspor, 2005). The hypocretinergic system has an anatomical organization similar to the MCHergic system (Peyron, et al., 1998), and it has been proposed to be involved in the regulation of behavioral states; deficits in this system have been associated with narcolepsy/cataplexy (Siegel, 2004, Sutcliffe and de Lecea, 2002, Taheri, et al., 2002). In the present report, as a foundation for furthering understanding of the function of the MCHergic system and its interactions with the hypocretinergic system, we determined the location of the neuronal somata of MCH-containing neurons and their anatomical relationship with the hypocretinergic system in the cat. In addition, we studied Fos immunoreactivity in MCH-containing neurons during quiet and active waking as well as during quiet (NREM) sleep (QS) and AS, in order to reveal the activity of these cells vis a vis behavioral states.
2. Results
2.1. Location of MCH-containing somata
Neuronal somata and processes were observed after immunostaining against MCH and/or hypocretin. MCHergic neurons were located in the hypothalamus (Fig. 1). These neurons were intermingled with and were in close proximity to hypocretinergic neurons; however, these neuropeptides were not co-localized in the same cells (Fig. 2). The absence of co-localization was observed throughout the hypothalamus.
Fig. 1. MCHergic neurons are located in the hypothalamus of the cat.
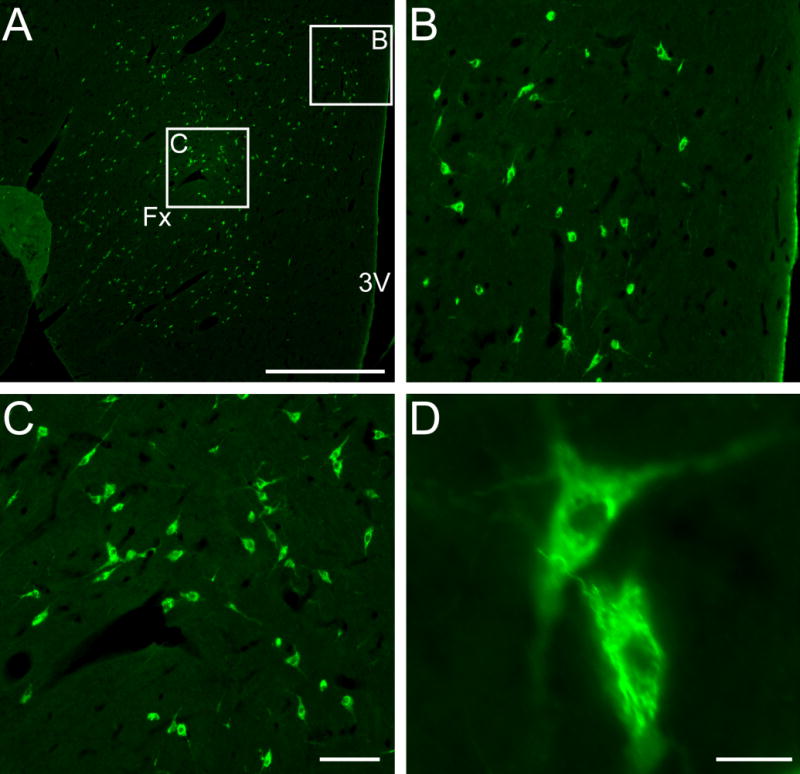
A. Low magnification photomicrographs that exhibits MCHergic neurons at the tuberal level of the hypothalamus. B–C. The insets in A are shown at higher magnification. These photomicrographs show MCHergic neurons of the dorsal hypothalamic area (B) and perifonical region (C). D. Two MCHergic neurons are shown at high magnification. The photomicrographs were taken from 20 μm-thick sections that were processed for immunoflourescence. FITC was used as fluorescent agent. Fx, fornix; 3V, third ventricle. Calibration bars: A, 1 mm; B–C, 100 μm; D, 20 μm.
Fig. 2. MCH and hypocretin are not co-localized in the cat hypothalamus.
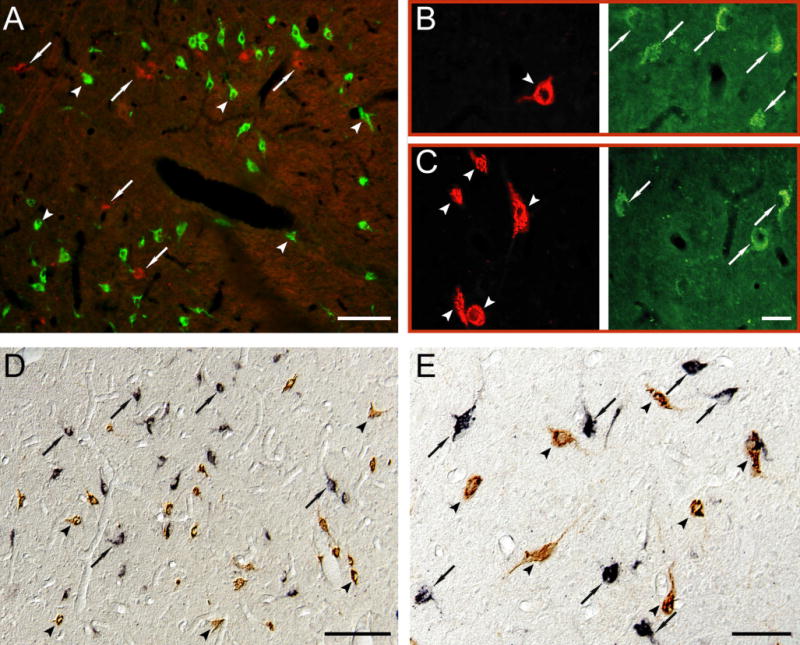
A. MCHergic (in green, examples in arrowheads) and hypocretinergic neurons (in red, examples in arrows) are intermingled in the postero-lateral hypothalamic area of the cat. The picture was obtained by superimposing photomicrographs that had been taken using green and red filters. B–C. Photomicrographs have been taken with two different filters in order to recognize MCHergic (left column, in red, arrowhead) and hypocretinergic neurons (right column, in green, arrows) in the postero-lateral hypothalamic area of the cat. The location of both neuropeptides is clearly different. These photomicrographs were taken from 20 μm-thick sections that were processed for immunoflourescence. Rhodamine and FITC were used as fluorescent agents. D and E. Photomicrographs exhibit MCHergic neurons (in brown, arrowheads) intermingled with hypocretinergic neurons (in black, arrows) in the postero-lateral hypothalamic area. The photomicrographs were obtained using Nomarski optics from 20 μm-thick sections, which were processed for immunohistochemistry utilizing the ABC-DAB method. Hypocretin immunostaining was enhanced by nickel. Calibration bars: A, 50 μm; B–C, 30 μm; D, 100 μm; E, 50 μm.
MCHergic neurons were concentrated in the perifornical region of the lateral hypothalamic area, dorsal hypothalamic area, the posterior hypothalamic area and the zona incerta. An example of the distribution of both MCH and hypocretin-containing neurons of a representative cat is presented in Fig. 3. Both groups of neurons were located principally at the tuberal level, where there were 591.3 ± 39.4 MCHergic vs. 197.9 ± 12.1 hypocretinergic neurons per section (P < 0.0001; two-tailed, paired, Student’s t test, analyzed in seven cats). At this level, the peak density of MCHergic neurons was more lateral (Fig. 3F) and ventral (Fig. 3G) than that of hypocretinergic neurons. Compared with hypocretinergic neurons, the distribution of MCHergic neurons was less extensive in the antero-posterior axis; almost no MCHergic neurons were present at the mammillary, supraoptic and preoptic levels of the hypothalamus (Fig. 3). The total number of MCHergic and hypocretinergic neurons in the whole hypothalamus (bilateral) was estimated to be 25,000 and 11,200, respectively.
Fig. 3. Location of MCHergic and hypocretinergic neurons in the hypothalamus of a representative cat.
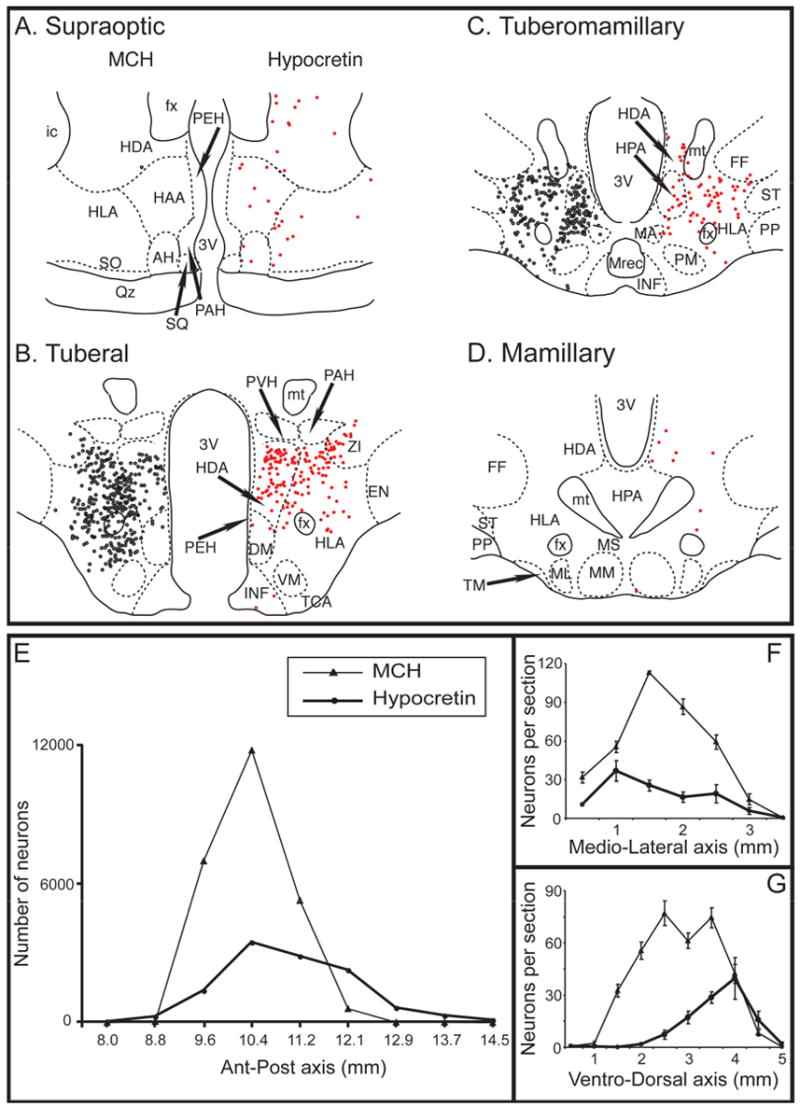
Camera lucida drawings of MCHergic (on the left, black circles) and hypocretinergic neuronal bodies (on the right, red circles) at different levels (A to D) of the hypothalamus are shown. The neurons are from the same hemi-hypothalamus (reflected in the figure). Both types of neurons were intermingled and were more concentrated at the tuberal level. There were no MCHergic neurons and very few hypocretinergic neurons at the preoptic level (not shown). Camera lucida drawings were obtained from adjacent sections, one was immunostained for MCH and the other was immunostained for Hcrt-2; these sections were counterstained with Pyronin-Y. The demarcation and nomenclature of cell groups in the cat hypothalamus are based on Berman and Jones, as well as Bleier's work (Berman and Jones, 1982, Bleier, 1961). E. Chart displaying the estimation of MCHergic (thin lines, triangles) and hypocretinergic neurons (thick lines, circles) in the antero-posterior axis of the whole hypothalamus (bilateral count). In F and G, the mean (± SE) number of neurons per hemisection in the medio-lateral and ventro-dorsal axes (analyzed at the tuberal level) is shown. In F, the horizontal axis is the distance from the medial surface of the hypothalamus to the lateral region. In G, the horizontal axis is the distance from the ventral surface of the hypothalamus to the dorsal region. AH, anterior hypothalamus; DM, dorsomedial nucleus; EN, entopeduncular nucleus; fx, fornix; FF, nucleus of the fields of Forel; HAA, anterior hypothalamic area; HDA, dorsal hypothalamic area; HLA, lateral hypothalamic area; HPA, posterior hypothalamic area; ic, internal capsule; INF, infundibular nucleus; MA, anterior mammillary nucleus; MM, medial mammillary nucleus; ML, lateral mammillary nucleus; M. Rec., mammillary recess of the third ventricle; MS, supramammillary nucleus; mt, mammillothalamic tract; PAH, paraventricular nucleus; PEH, periventricular complex; PM, premammillary nucleus; PP, pes pedunculi; PVH, parvocellular nucleus; Qz, optic chiasm; SQ, suprachiasmatic nucleus; SO, supraoptic nucleus; ST, subthalamic nucleus; TCA, area of the tuber cinereum; TM, tuberomammillary nucleus; VM, ventromedial nucleus; ZI, zona incerta; 3V, third ventricle.
Fusiform, bipolar and multipolar MCHergic and hypocretinergic neurons were observed. The mean largest diameter of the MCHergic and hypocretinergic neurons was similar (30.7 ± 1.3 vs. 30.3 ± 1.0 μm, respectively; analyzed in the perifornical area of the tuberal level). However, MCHergic neurons had a statistically significant smaller mean diameter (12.3 ± 0.7 vs. 15.0 ± 0.7 μm; two-tailed, unpaired, Student’s t test, P < 0.01).
MCHergic and hypocretinergic varicose terminals were observed throughout the hypothalamus. Close apposition of hypocretinergic fibers onto MCHergic somata or proximal dendrites was frequently observed (Fig. 4A). MCHergic axodendritic and axosomatic fiber appositions onto hypocretinergic neurons were also present (Fig. 4B-E).
Fig. 4. Reciprocal axodendritic and axosomatic contacts between MCHergic and hypocretinergic neurons.
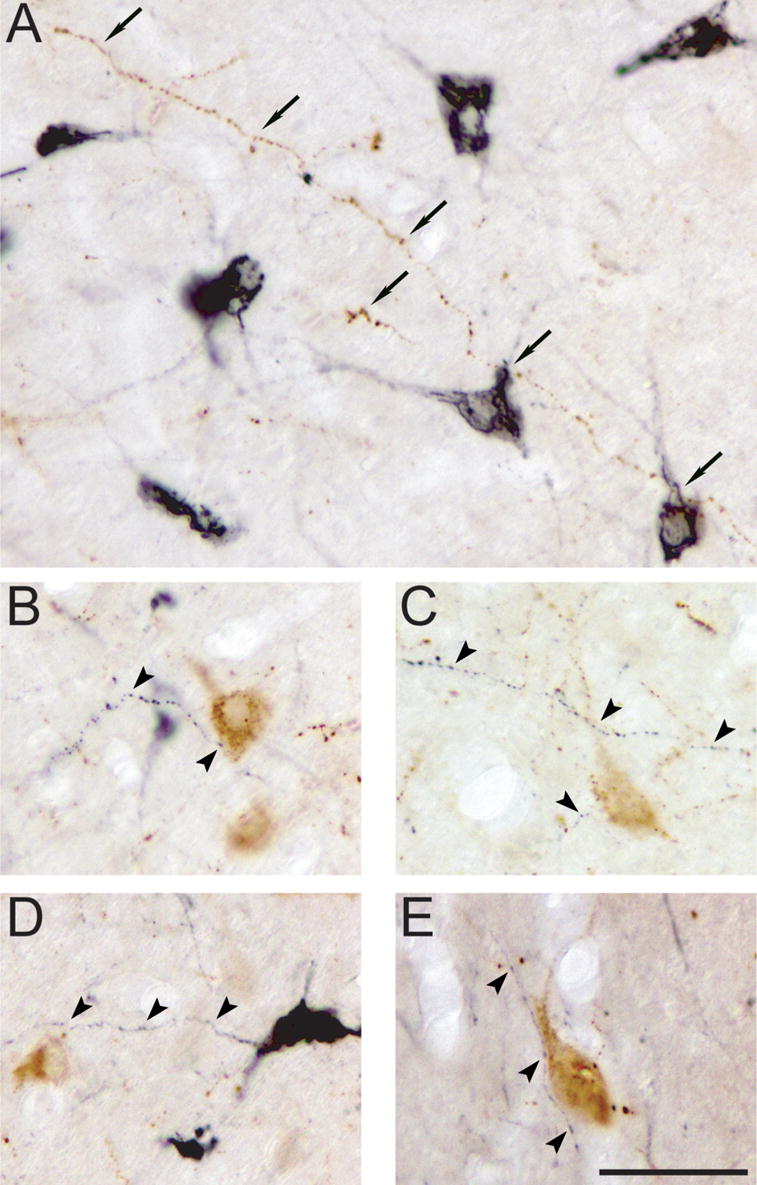
A. Apposition between a long hypocretinergic fiber (in brown, arrows) and MCHergic somata and primary dendrites (in black). B to E. MCHergic fibers (in black, arrowheads) were in close relationship with hypocretinergic somata and/or primary dendrites (in brown). All photomicrographs were taken from 20 μm-thick sections and processed with the ABC-DAB method. MCH immunostaining was enhanced by nickel. Calibration bar: 50 μm.
2.2. Fos immunoreactivity of MCH-containing neurons
The photomicrographs in Fig. 5 are examples of MCH and Fos immunoreactivity in the postero-lateral hypothalamic area during different behavioral states. MCH was stained brown while Fos, which was stained black, concentrated in the nucleus. In view of published reports of Fos labeling in MCH-containing cells of the rat during AS rebound (Verret, et al., 2003) or total sleep rebound paradigms (Modirrousta, et al., 2005), it was an unexpected finding that, in our preparation, MCHergic neurons did not exhibit Fos immunoreactivity throughout the hypothalamus in any of the conditions studied (Fig. 5). This was true in spite of the presence of non-MCHergic Fos+ neurons that were intermingled with MCHergic cells. In very few sections, one or two MCHergic neurons presented Fos immunoreactivity (less than 1% of the MCHergic neurons of the sections); there were no statistical differences between the conditions studied. Fig. 6 presents data obtained from a cat that exhibited an AS induced by carbachol state (AS-carbachol). The AS-carbachol latency was 2 minutes and the episode lasted for almost 2 hours (Fig. 6A); immediately thereafter, the animal was euthanized. A camera lucida drawing of a section of the hypothalamus of this animal shows that although a considerable number of Fos+ neurons were intermingled with MCH+ neurons, there were no double-labeled MCH+Fos+ neurons (Fig. 6B). Fig. 7 is an example of a cat euthanized after one hour of QS rebound; in this period the animal state consisted of 90% QS and 10% quiet wakefulness (QW). A camera lucida drawing of a representative section illustrates that MCHergic neurons were not Fos immunoreactive (Fig 7b).
Fig. 5. MCHergic neurons do not show Fos immunoreactivity during wakefulness or sleep.
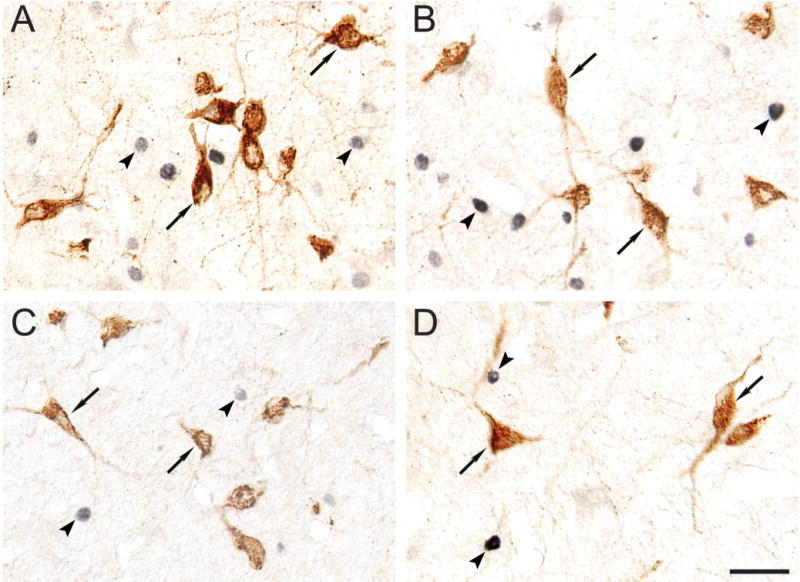
A–D. Immunoreactivity for Fos and MCH in sections of animals euthanized during A) active wakefulness with motor activity, B) AS induced by carbachol, C) active wakefulness without motor activity and D) quiet sleep. MCH-containing neurons exhibit brown immunoreactivity (examples are indicated by arrows); Fos immunoreactivity, which was restricted to nuclei, stained in black (examples are indicated by arrowheads). MCHergic neurons did not exhibit Fos immunoreactivity in any of the behavioral states that were examined. All photomicrographs were taken using Nomarski optics from 20 μm-thick sections and processed with the ABC-DAB method. Fos immunostaining was enhanced by nickel. Calibration bar: 40 μm.
Fig. 6. MCHergic neurons do not show Fos immunoreactivity during AS-carbachol.
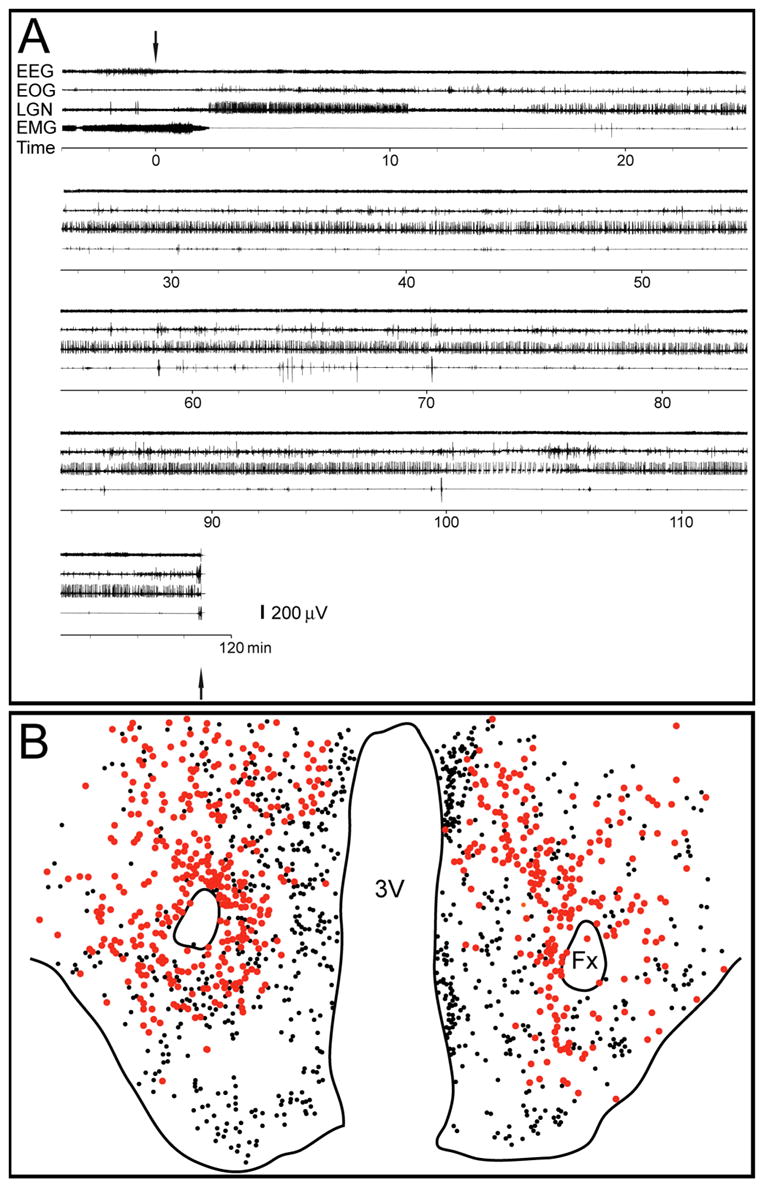
A. Polygraphic recording of an episode of AS-carbachol of a representative cat. Arrows signal the beginning of the microinjection of carbachol into the NPO as well as pentobarbital injection for euthanasia. EEG, electroencephalogram; EOG, electrooculogram; LGN, lateral geniculate nucleus electrogram (PGO waves); EMG, electromyogram. This figure was modified from Torterolo et al. (Torterolo, et al., 2001), with permission. B. Camera lucida drawings of a section from the same cat. Red circles represent MCHergic neurons; smaller black circles represent Fos+ neurons. There were no double-labeled MCH+Fos+ neurons in this representative section. 3V, third ventricle; Fx, fornix.
Fig. 7. MCHergic neurons do not express c-fos during quiet sleep.
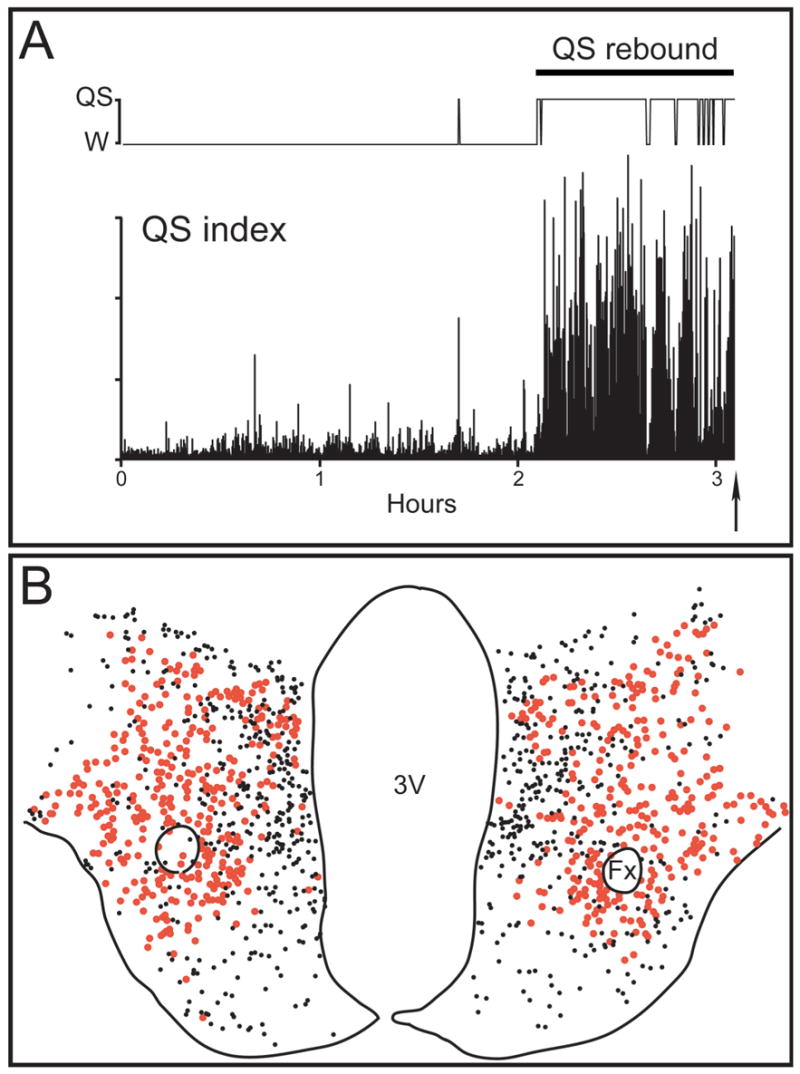
A. QS rebound after two hours of sleep deprivation. The hypnogram and a QS index [(delta x sigma)/gamma power band, in 10 sec bins] of the EEG recording from the frontal cortex are shown. As was readily observed, QS was present most of the time one hour previous to euthanasia; AS was prevented from occurring during this period. The arrow indicates the moment of the injection of pentobarbital (for euthanasia). B. Camera lucida drawings of a section from the same cat. Red circles represent MCHergic neurons; smaller black circles represent Fos+ neurons. There were no double-labeled MCH+Fos+ neurons in this representative section. 3V, third ventricle; Fx, fornix.
2.3. Fos immunoreactivity of non-MCHergic, non-hypocretinergic neurons
Fos immunoreactivity during sleep and waking were analyzed in the hypocretinergic neurons in previous studies (Torterolo, et al., 2001, Torterolo, et al., 2003). In the present report we analyzed in sections double immunostained for hypocretin and Fos, the single-labeled non-hypocretinergic Fos+ neurons that were present in the hypocretinergic field (Fig. 8A), which was defined as the area that contained most hypocretinergic neuronal somata at the tuberal level (Fig. 8B). Because MCHergic neurons did not express c-fos, the single-labeled Fos+ neurons that were counted were neither hypocretinergic nor MCHergic. Fig. 8C shows that there was a larger number of non-MCHergic, non-hypocretinergic Fos+ neurons during active wakefulness with motor activity (AW with M) compared to other behavioral states. A reduction in the number of non-MCHergic, non-hypocretinergic Fos+ neurons during QS, compared with other behavioral states, was also observed. The following are the number of non-MCHergic, non-hypocretinergic Fos+ neurons per hemisection: AW with M, 199.7 ± 21.1; AW without M, 80.8 ± 12.8; QW, 86.8 ± 13.4; QS, 40.7 ± 6.9; AS-carbachol, 99.4 ± 6.2 (*, P< 0.05; **, P < 0.01 compared with the other conditions; ANOVA-Fisher).
Fig. 8. Fos immunoreactivity in non-hypocretinergic, non-MCHergic neurons.
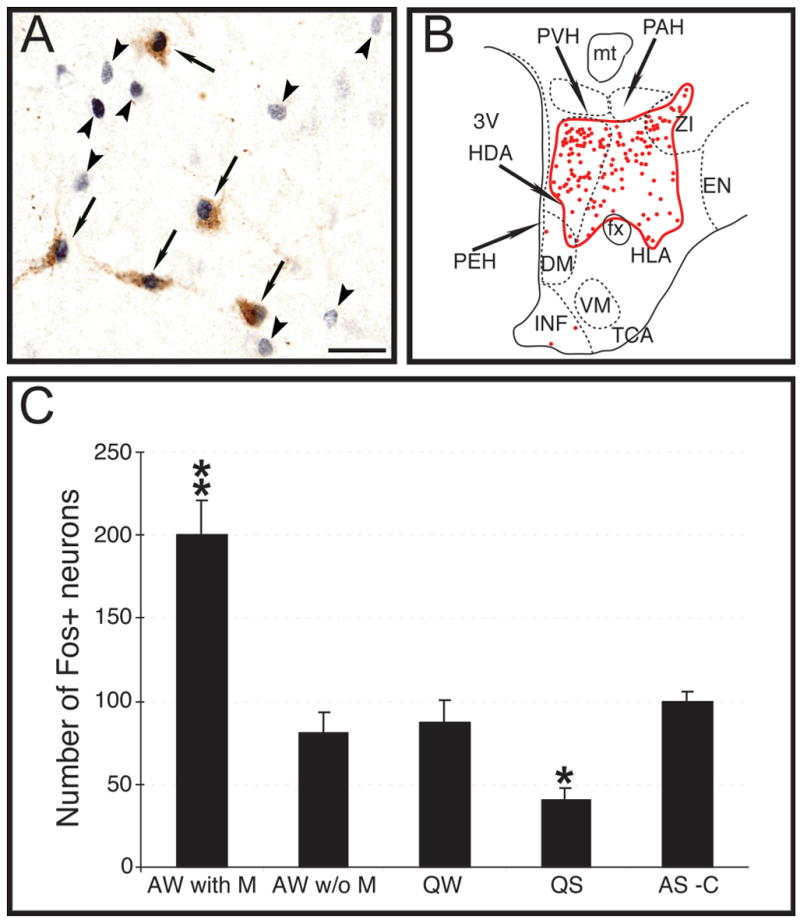
A. Photomicrograph showing the immunoreactivity for hypocretin and Fos in a section of a cat euthanized after a period of active wakefulness with motor activity. Arrows indicate Fos immunoreactive hypocretinergic neurons; arrowheads point to non-hypocretinergic Fos+ neurons. In fact, these neurons were neither hypocretinergic nor MCHergic because almost all MCH+ neurons did not express c-fos in the analyzed behavioral states. Calibration bar: 30 μm. B. The camera lucida drawing of Fig. 3 was used as an example of the hypocretinergic field (circled in red) where non-hypocretinergic, non-MCHergic Fos+ neurons were counted. See Fig. 3 for abbreviations. C. The bar chart exhibits non-hypocretinergic, non-MCHergic Fos+ neurons counted in the hypocretinergic neuronal field at the tuberal level. The number of non-hypocretinergic, non-MCHergic Fos+ neurons increased during AW with M and decreased during QS. AW with M, active wakefulness with motor activity; AW w/o M, active wakefulness without motor activity; QW, quiet wakefulness; QS, quiet sleep; AS-C, active sleep induced by carbachol.
3. Discussion
Since the pioneering work of von Economo, the hypothalamus has been the object of many studies dealing with sleep physiology and pathology (von Economo, 1930). Renewed interest in the hypothalamus has emerged due to the discovery of the hypocretinergic system which has been suggested to be involved in the control of motor activity, sleep and wakefulness (reviewed by (Siegel, 2004, Sutcliffe and de Lecea, 2002, Taheri, et al., 2002)). MCHergic neurons are intermingled with the hypocretinergic neurons in the hypothalamus and their projections are strikingly similar (Bayer, et al., 2002, Bittencourt, et al., 1992, Guan, et al., 2002, Peyron, et al., 1998, Skofitsch, et al., 1985); this anatomical data suggest that the MCHergic neurons may also contribute to the regulation of motor activity, sleep and waking functions. However, very few reports examined the role of MCH in relation to these functions.
In the present study, we present the first report of the distribution of MCHergic neuronal bodies in the cat. These neurons were intermingled with the hypocretinergic neurons in the lateral, dorsal and posterior hypothalamic areas, as well as in the zona incerta. Although the number of MCHergic neurons was larger than that of hypocretinergic neurons, the hypocretinergic neuronal field extended to a larger degree in the antero-posterior axis; this distribution differs from that observed in rodents, wherein there is a greater presence of MCHergic neurons than hypocretinergic neurons along this axis (Bayer, et al., 2002, Knigge, et al., 1996). MCHergic fibers were in close relationship with the hypocretinergic neurons, and vice versa, suggesting synaptic contacts between them; however, confocal and electron microscopic studies will be necessary to confirm synaptic contacts between these cells. Such reciprocal synaptic contacts have been demonstrated in rats (Guan, et al., 2002). This anatomical relationship as well as the presence of hypocretinergic receptors on MCHergic neurons suggests an important functional interaction between these systems (Backberg, et al., 2002). In fact, hypocretin modulates MCH mRNA expression in culture, and hypocretin has a profound excitatory effect on MCHergic neurons (Bayer, et al., 2002, van den Pol, et al., 2004).
We previously determined that 80% and 33% of the hypocretinergic neurons respectively, were active during waking with motor activity and AS-carbachol (Torterolo, et al., 2001, Torterolo, et al., 2003). In contrast, we did not observe Fos immunoreactivity in the MCHergic neurons in the conditions studied. One of the problems with the Fos technology is that the lack of Fos immunoreactivity does not necessarily indicate that these neurons were not active (false negative) (Dragunow and Faull, 1989). However, recordings in vitro showed that while hypocretinergic neurons are in an intrinsic state of membrane depolarization, which promote their spontaneous activation, MCHergic neurons are hyperpolarized and are inactive in their resting state (Eggermann, et al., 2003, van den Pol, et al., 2004). This data suggests that there is a restricted activation of MCHergic neurons.
MCHergic neurons did not present Fos immunoreactivity during AS-carbachol; because this is a pharmacological model of AS, these results should be confirmed in cats during naturally-occurring AS. However, this cholinergically-induced state resembles naturally-occurring AS; i.e., there is EEG desynchronization, as well as PGO waves, rapid eye movements, and muscle atonia. Hippocampal theta rhythm, in addition to the postsynaptic inhibition of motoneurons are also present during AS-carbachol (Lopez-Rodriguez, et al., 1994, Morales, et al., 1987).
Recently, Bayer et al. demonstrated that carbachol hyperpolarizes MCHergic neurons by direct postsynaptic action (Bayer, et al., 2005). These data suggest that MCHergic neurons may be under cholinergic inhibitory control during AS, a state in which mesopontine cholinergic neurons are activated (Siegel, 2005). However, a recent report has shown an increase in Fos immunoreactivity in MCHergic neurons of the rat during an AS hypersomnia produced by the deprivation-rebound method (Verret, et al., 2003). In addition, microinjections of MCH in the nucleus pontis oralis of the cat, a critical area for AS generation and maintenance, produces a small increase in the time spent in AS and a decrease in the latency to AS (Torterolo, et al., 2003). This is in agreement with the increase in the percentage of AS observed in rats following intraventricular injections of MCH (Verret, et al., 2003).
We did not observe Fos immunoreactivity in MCHergic neurons in cats that spent 60 to 90 minutes in QS prior to euthanasia. This result also differs from the findings of Modirrousta et al.; they described an increase in the number of Fos immunoreactive MCHergic neurons in a paradigm of sleep rebound (mainly QS) after sleep deprivation in rats compared to wakefulness (Modirrousta, et al., 2005). However, it is interesting to note that the number of Fos expressing MCHergic neurons during the sleep rebound was small (3% of the total number of MCHergic neurons). In this regard, we can not discount the fact that in our study, increasing the time the animal spent in QS, induced a small, but statistically significant increase in Fos immunoreactivity, as was found in rats. Modirrousta et al. have also hypothesized that MCH-containing and hypocretinergic neurons play opposing roles in regulating sleep, waking and energy metabolism, suggesting that MCHergic may have a role in sleep and behavioral quiescence (Modirrousta, et al., 2005).
In view of the present data is still not possible to establish a comprehensible role for the MCHergic system in the control of sleep and waking. As mentioned above, Fos immunoreactivity in MCHergic neurons appears to yield different results in cats and rats, and no obvious effect on sleep was observed in mice that were genetically modified to overexpress MCH (Cronin, et al., 2002). In addition, MCH mRNA levels increase at night, during the period of activity of rats, which suggests a role for these cells during wakefulness (Presse and Nahon, 1993). Furthermore, in vitro electrophysiological data are difficult to interpret because MCHergic neurons are inhibited by acetylcholine, noradrenaline and serotonin, but strongly excited by hypocretin; all of these neurotransmitter/neuromodulators play an important role in promoting arousal (Bayer, et al., 2005, van den Pol, et al., 2004). We consider that in vivo unit recordings from identified MCHergic neurons as well as microdialysis studies will shed light on the role and the temporal pattern of activation of MCHergic neurons vis a vis sleep and wakefulness.
A phenotype-unidentified neuronal group that was activated during active wakefulness with motor activity was found intermingled with hypocretinergic and MCHergic neurons. These data are comparable with those obtained by Alam et al. (Alam, et al., 2005), which demonstrated that the majority of neurons expressing Fos during active waking induced by bicuculline application into the lateral hypothalamus of the rat were non-hypocretinergic non-MCHergic neurons (ibid.). The postero-lateral and adjacent hypothalamic areas are known to be involved in the control of motor activity. In this regard, electrolytic and chemical lesions in this region, as well the injection of GABA agonists, produce a decrease in locomotion while electrical and chemical stimulation increases locomotor activity (Blake and Gladfelter, 1986, Gladfelter and Brobeck, 1962, Levy and Sinnamon, 1990, Marciello and Sinnamon, 1990, Shekhar and DiMicco, 1987, Sinnamon, 1984, Sinnamon, 1990, Sinnamon, et al., 1987, Sinnamon, et al., 1984, Sinnamon and Sklow, 1990, Sinnamon and Stopford, 1987, Wardas, et al., 1988). In addition, unit recordings have revealed non-hypocretinergic neurons that increase their firing rate in conjunction with motor activity (Lee, et al., 2005). Although hypocretinergic neurons seem to be primarily involved in somatomotor activity (Kiyashchenko, et al., 2002, Lee, et al., 2005, Martins, et al., 2004, Mileykovskiy, et al., 2005, Torterolo, et al., 2001, Torterolo, et al., 2003), our results suggest that additional, as-yet-unidentified neurons in this region may also be involved in this function. The activity of these unidentified neurons decreased during QS, which is in agreement with electrophysiological studies (Alam, et al., 2002). Although the phenotype of these neurons is unknown, there are several types of candidate neurons present in this region of the hypothalamus. For example, glutamatergic neurons that are present in this area regulate the excitability of hypocretinergic and other local neurons (Li, et al., 2002, Ziegler, et al., 2002), and nitrergic neurons of the postero-lateral hypothalamus of the guinea pig project to the hypoglossal and trigeminal motor nuclei (McGregor, et al., 2004, McGregor, et al., 2005). In this regard, we have observed nitrergic cells intermingled with MCHergic and hypocretinergic somata in this region of the cat (unpublished). In addition, nitric oxide has been shown to regulate motoneurons activity (Abudara, et al., 2002). Other candidate local neuronal types that utilize GABA or neuropeptides might constitute the unidentified cells that express c-fos that we observed during motor activation (Mugnaini and Oertel, 1985, Saper, 1995).
In conclusion, in the present report we describe the location of MCHergic neurons in the cat and their anatomical relationships with hypocretinergic neurons. Unexpectedly, and in contrast to hypocretinergic neurons, no Fos immunoreactivity was observed in MCHergic neurons during states of sleep or wakefulness. We also observed that in addition to hypocretinergic neurons, another neuronal group in the postero-lateral hypothalamus might be involved in the control of somatomotor activity.
4. Experimental procedure
Sixteen adult cats, which were used in the present study, were obtained from and determined to be in good health by the UCLA Division of Laboratory Animal Medicine. All experimental procedures were conducted in accord with the “Guide to the care and use of laboratory animals” (7th edition, National Academy Press, Washington D. C., 1996) and were approved by the Animal Research Committee of the UCLA Office for the Protection of the Research Subjects; adequate measures were taken to minimize pain or discomfort of the animals.
4.1. Surgical procedures
Details of the preparation of cats to monitor behavioral states and microinject drugs into the rostral pontine tegmentum have been previously reported (Torterolo, et al., 2001). Briefly, under isofluorane anesthesia, screw electrodes were placed in the calvarium for the recording of the frontal and parietal electroencephalogram (EEG), and in the orbital bone to record the electro-oculogram; strut electrodes were directed stereotaxically to the lateral geniculate nuclei in order to monitor ponto-geniculo-occipital (PGO) waves. All electrodes were connected to a female Winchester plug. Two plastic tubes, which were used to maintain the animal's head in a stereotaxic position without pain or pressure, as well as the Winchester plug, were fixed to the skull with acrylic resin. In four animals, a 5-mm diameter hole was trephined overlying the posterior fossa in order to provide access for a microsyringe to microinject carbachol or saline into the NPO. The hole was filled with a bone-wax plug. At the completion of all surgical procedures, antibiotics were administered parenterally for three days. The skin margins surrounding the implant were kept clean and topical antibiotics were administered on a daily basis. After the animals had recovered from the preceding surgical procedures, they were adapted to the recording environment for a period of at least two weeks. After this period of adaptation, the animals did not show any signs of stress or discomfort in this restrained condition, and if not disturbed, rapidly fall asleep.
4.2. Behavioral experiments and Fos immunoreactivity
The EEG, EOG, PGO and the electromyogram (EMG, recorded from two stainless steel wires inserted acutely into the posterior cervical muscles) were monitored, stored and employed for the determination of behavioral states (for details see Torterolo, et al., 2001).
In order to study Fos immunoreactivity during sleep and waking states, the animals spent one to two hours (i.e., the time needed for Fos proteins to reach an optimal degree of concentration (Dragunow and Faull, 1989, Yamuy, et al., 1993)) prior to euthanasia either in a state of 1) quiet wakefulness (QW, n = 3), 2) active wakefulness with motor activity (AW with M, n = 3), 3) active wakefulness without motor activity (AW without M, n = 3), 4) quiet sleep (QS, n = 4) and 5) AS induced by carbachol (AS-carbachol, n = 3). Details of the procedures required to maintain the animals in these behavioral states have been previously presented (Torterolo, et al., 2001, Torterolo, et al., 2003). Briefly, in experimental sessions held between 10 AM to 2 PM, cats were maintained in a head-restraining device, with continuous electrophysiological monitoring of their behavioral states during QW, AW-without M, QS and AS-carbachol. For the QW animals, if slow wave activity or spindles appeared, low noise and/or mild somesthetic stimulation (gentle touching of the animal’s face) were immediately applied. In one animal, saline (0.2 μl) was microinjected in the NPO during quiet wakefulness, using the same procedures that are described below for the AS-carbachol animals. As previously noted, no clear differences were observed in the pattern of Fos expression in this cat compared with the other QW animals (Torterolo, et al., 2001).
The AW-without M group was maintained awake by continuous bilateral loud clicks. The QS group was sleep-deprived for two to three hours; during the following 60–90 minutes period (prior to euthanasia) there was a sleep rebound, but AS was prevented from occurring. This group of animals spent about 90% (86 to 90%) of the time in QS during the 60–90 minutes prior to euthanasia. In the AS-carbachol group of cats, carbachol (0.8 μg in 0.2 μl of saline) was microinjected into the nucleus pontis oralis with a 2 μl- Hamilton microsyringe during QS (NPO, at stereotaxic coordinates AP −2 to −3, L 1.5 to 2.5, H −3.5 to −5 according to (Berman, 1968)). The period of microinjection lasted for approximately 2 minutes. Following the administration of carbachol, AS episodes occurred with a latency of 6.7 ± 2.2 minutes and 82.7 ± 20.6 minutes of duration.
The AW-with M animals (which were not restrained) were introduced to the experimental room for the first time on the day they were schedule to be euthanized; this room contained a variety of objects to attract their attention and induce motor (exploratory) activity. These animals were not recorded during this procedure.
4.3. Immunohistochemical procedures
All animals were euthanized with an overdose of sodium pentobarbital (60 mg/kg) and perfused for subsequent immunohistochemical analyses (see (Torterolo, et al., 2001) for details). Thereafter, the brain was cryoprotected, frozen and serial sections of 20 μm were obtained. Single immunostaining procedures were performed to identify MCH or hypocretin-2 (Hcrt-2). Double immunohistochemical processes were undertaken to identify Fos and Hcrt-2, Fos and MCH, MCH and Hcrt-2, as well as MCH and Hcrt-1. Immunohistochemistry with Hcrt-1 or Hcrt-2 antibodies led, as expected, to similar results because these neuropeptides are co-localized in the cat (Zhang, et al., 2002).
Details regarding the immunohistochemical procedures that were used to identify Fos and Hcrt-2 have been presented previously (Torterolo, et al., 2001). In order to identify MCH immunoreactivity, sections were incubated overnight with rabbit MCH antibody (Phoenix Pharmaceuticals, 1:40,000) and normal donkey serum (NDS, 3%). Thereafter, the sections were rinsed and incubated for 90 minutes with biotinylated donkey anti-rabbit antibody (1:300) plus NDS. After another rinse, the tissue was incubated in the ABC complex (1:200) for 60 minutes and then exposed to diaminobenzidine tetrahydrochloride (DAB, 0.02%) and hydrogen peroxide (0.0015%); occasionally this reaction was enhanced using 0.6% nickel ammonium sulfate. Control omission experiments were performed by not exposing the tissue to the primary antibody. In addition, antiserum preabsorded with an excess amount of MCH peptide (50 times), was also utilized as a control.
Pyronin-Y counterstaining was utilized in immunolabeled sections, or thionin staining was utilized on adjacent sections to identify the cytoarchitecture of the analyzed areas.
In order to examine the potential co-localization of MCH and Hcrt, the following double immunoflourescence procedures were performed. Free-floating sections were rinsed several times in PBST (0.1 M phosphate buffer saline with 0.25% Triton X-100). Thereafter, they were incubated simultaneously with goat anti-Hcrt-1 (Diagnostic systems laboratories, 1:500) and rabbit anti-MCH antibodies (Phoenix Pharmaceuticals; 1:2000) in PBST solutions for 3 days at 4°C. After the sections were rinsed four times in PBST for a total duration of 30 min, they were simultaneously incubated for 3 hs in PBST containing donkey anti-rabbit IgG conjugated with Rhodamine (Jackson laboratories; 1;300) and donkey anti-goat IgG conjugated with flourescein isothiocyanate (FITC, Jackson laboratories, 1:200). We also employed the reverse procedure; donkey anti-rabbit IgG conjugated with flourescein isothiocyanate (FITC, Jackson Laboratories: 1:200) and donkey anti-goat IgG conjugated with Rhodamine (Jackson Laboratories: 1:200). Finally, the sections were rinsed for 45 minutes with PBST and coverslipped with Vectashield fluorescent mounting media (Vector laboratories).
4.4. Histological data analysis
Brain sections were examined either with conventional or fluorescent light microscopy; photomicrographs were obtained using a SPOT digital camera. Images were analyzed using Adobe PhotoShop software with a Power Macintosh G4 computer. The distribution of immunolabeled neurons was determined from drawings, which were obtained with a camera lucida.
Hypocretinergic, MCHergic and MCHergic neurons that displayed Fos immunoreactivity (MCH+ Fos+) were analyzed in two representative coronal sections from each cat at the mammillary (AP 8–9), tuberomammillary (AP 9–10), tuberal (AP 10–11), supraoptic (AP 13–14) and preoptic (AP 14–15) levels of the hypothalamus (according to (Berman and Jones, 1982)). Therefore, the analysis was performed in 10 representative sections, per cat and in 3 to 4 cats, per condition. Single labeled Fos+ neurons were also analyzed in sections that were double-immunostained for both hypocretin and Fos at the tuberal level (AP 10–11), where MCHergic and hypocretinergic neurons were most highly concentrated (see Results); two representative sections per cat (four hemisections) and 3 to 4 cats per condition were utilized in this analysis. The results are presented as the mean ± S.E.M of the number of neurons. Differences in the number of single-labeled Fos+ neurons or MCH+Fos+ neurons per condition were evaluated with the ANOVA and Fisher post hoc tests. Differences in the number of MCHergic and hypocretinergic neurons were determined by employing the paired two-tailed Student’s t test in seven cats (the number of each group of cell per cat was paired); the differences in neuronal size were evaluated with the unpaired Student's t test. The criterion chosen to discard the null hypothesis was P < 0.05. An estimate of the total number of MCHergic and hypocretinergic neurons was obtained utilizing the Abercrombie method (Abercrombie, 1946); for this analysis, in one representative cat, counts were performed from coronal sections that were obtained every 100 μm for the study of hypocretinergic neurons, and every 200 μm for the examination of MCHergic neurons.
Acknowledgments
We thank Dr. J. K. Engelhardt for his critical comments regarding the manuscript. This study was supported by USPHS grants MH43362, MH 69372, NS09999, NS23426, HL602969 and AG04307.
References
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Abudara V, Alvarez AF, Chase MH, Morales FR. Nitric oxide as an anterograde neurotransmitter in the trigeminal motor pool. J Neurophysiol. 2002;88:497–506. doi: 10.1152/jn.2002.88.1.497. [DOI] [PubMed] [Google Scholar]
- Alam MN, Gong H, Alam T, Jaganath R, McGinty D, Szymusiak R. Sleep-waking discharge patterns of neurons recorded in the rat perifornical lateral hypothalamic area. J Physiol. 2002;538:619–31. doi: 10.1113/jphysiol.2001.012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam MN, Kumar S, Bashir T, Suntsova N, Methippara MM, Szymusiak R, McGinty D. GABA-mediated control of hypocretin- but not melanin-concentrating hormone-immunoreactive neurones during sleep in rats. J Physiol. 2005;563:569–82. doi: 10.1113/jphysiol.2004.076927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backberg M, Hervieu G, Wilson S, Meister B. Orexin receptor-1 (OX-R1) immunoreactivity in chemically identified neurons of the hypothalamus: focus on orexin targets involved in control of food and water intake. Eur J Neurosci. 2002;15:315–28. doi: 10.1046/j.0953-816x.2001.01859.x. [DOI] [PubMed] [Google Scholar]
- Bayer L, Eggermann E, Serafin M, Grivel J, Machard D, Muhlethaler M, Jones BE. Opposite effects of noradrenaline and acetylcholine upon hypocretin/orexin versus melanin concentrating hormone neurons in rat hypothalamic slices. Neuroscience. 2005;130:807–11. doi: 10.1016/j.neuroscience.2004.10.032. [DOI] [PubMed] [Google Scholar]
- Bayer L, Mairet-Coello G, Risold PY, Griffond B. Orexin/hypocretin neurons: chemical phenotype and possible interactions with melanin-concentrating hormone neurons. Regul Pept. 2002;104:33–9. doi: 10.1016/s0167-0115(01)00320-2. [DOI] [PubMed] [Google Scholar]
- Berman AL. The brain stem of the cat. A cytoarquitectonic atlas with stereotaxic coordinates. University of Wisconsin; Madison: 1968. [Google Scholar]
- Berman AL, Jones EG. The thalamus and basal telencephalum of the cat. A cytoarquitectonic atlas with stereotaxic coordinates. University of Wisconsin; Madison: 1982. [Google Scholar]
- Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, Vale W, Sawchenko PE. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol. 1992;319:218–45. doi: 10.1002/cne.903190204. [DOI] [PubMed] [Google Scholar]
- Blake DJ, Gladfelter WE. Wheel-running activity after kainic acid injection into lateral hypothalamus of rats. Physiol Behav. 1986;36:1009–16. doi: 10.1016/0031-9384(86)90472-5. [DOI] [PubMed] [Google Scholar]
- Bleier R. The hypothamus of the cat. The John Hopkins Press; Baltimore, MD: 1961. [Google Scholar]
- Borowsky B, Durkin MM, Ogozalek K, Marzabadi MR, DeLeon J, Heurich R, Lichtblau H, Shaposhnik Z, Daniewska I, Blackburn TP, Branchek TA, Gerald C, Vaysse PJ, Forray C. Antidepressant, anxiolytic and anorectic effects of a melanin- concentrating hormone-1 receptor antagonist. Nat Med. 2002;8:825–30. doi: 10.1038/nm741. [DOI] [PubMed] [Google Scholar]
- Cronin SJ, Mochizuki T, Papadopoulou M, Tromby D, Maratos-Flier E, Scammell TE. Sleep/wake behavior in MCH overexpressing mice. Sleep. 2002;25:A168. [Google Scholar]
- Dragunow M, Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods. 1989;29:261–5. doi: 10.1016/0165-0270(89)90150-7. [DOI] [PubMed] [Google Scholar]
- Eggermann E, Bayer L, Serafin M, Saint-Mleux B, Bernheim L, Machard D, Jones BE, Mulethaler M. The wake-promoting hypocretin-orexin neurons are in an intrinsic state of membrane depolarization. J Neurosci. 2003;23:1557–1562. doi: 10.1523/JNEUROSCI.23-05-01557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forray C. The MCH receptor familiy: feeding brain disorders? Curr Opin Pharmacol. 2003;3:85–9. doi: 10.1016/s1471-4892(02)00013-9. [DOI] [PubMed] [Google Scholar]
- Gladfelter WE, Brobeck JR. Decreased spontaneous locomotor activity in the rat induced by hypothalamic lesions. Am J Physiol. 1962;203:811–17. doi: 10.1152/ajplegacy.1962.203.5.811. [DOI] [PubMed] [Google Scholar]
- Guan JL, Uehara K, Lu S, Wang QP, Funahashi H, Sakurai T, Yanagisawa M, Shioda S. Reciprocal synaptic relationship between orexin-and melanin-concentrating hormone-containing neurons in the rat lateral hypothalamus: a novel circuit implicated in feeding regulation. Int J Obes Relat Metab Disord. 2002;26:1523–32. doi: 10.1038/sj.ijo.0802155. [DOI] [PubMed] [Google Scholar]
- Jones B. In: Basic mechanisms of sleep-wake states. In Principles and practices of sleep medicine. Kryger MH, Roth T, Dement WC, editors. Elsevier-Saunders; Philadelphia: 2005. pp. 136–53. [Google Scholar]
- Kawauchi H, Kawazoe I, Tsubokawa M, Kishida M, Baker BI. Characterization of melanin-concentrating hormone in chum salmon pituitaries. Nature. 1983;305:321–323. doi: 10.1038/305321a0. [DOI] [PubMed] [Google Scholar]
- Khorooshi RM, Klingenspor M. Neuronal distribution of melanin-concentrating hormone, cocaine- and amphetamine-regulated transcript and orexin B in the brain of the Djungarian hamster (Phodopus sungorus. J Chem Neuroanat. 2005;29:137–48. doi: 10.1016/j.jchemneu.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Kilduff TS, De Lecea L. Mapping of the mRNAs for the hypocretin/orexin and melanin-concentrating hormone receptors: networks of overlapping peptide systems. J Comp Neurol. 2001;435:1–5. doi: 10.1002/cne.1189. [DOI] [PubMed] [Google Scholar]
- Kiyashchenko LI, Mileykovskiy BY, Maidment N, Lam HA, Wu MF, John J, Peever J, Siegel JM. Release of hypocretin (orexin) during waking and sleep states. J Neurosci. 2002;22:5282–6. doi: 10.1523/JNEUROSCI.22-13-05282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knigge KM, Baxter-Grillo D, Speciale J, Wagner J. Melanotropic peptides in the mammalian brain: the melanin-concentrating hormone. Peptides. 1996;17:1063–73. doi: 10.1016/0196-9781(96)00131-3. [DOI] [PubMed] [Google Scholar]
- Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–20. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DI, Sinnamon HM. Midbrain areas required for locomotion initiated by electrical stimulation of the lateral hypothalamus in the anesthetized rat. Neuroscience. 1990;39:665–74. doi: 10.1016/0306-4522(90)90251-x. [DOI] [PubMed] [Google Scholar]
- Li Y, Gao XB, Sakurai T, van den Pol AN. Hypocretin/Orexin excites hypocretin neurons via a local glutamate neuron-A potential mechanism for orchestrating the hypothalamic arousal system. Neuron. 2002;36:1169–81. doi: 10.1016/s0896-6273(02)01132-7. [DOI] [PubMed] [Google Scholar]
- Lopez-Rodriguez F, Kohlmeier K, Morales FR, Chase MH. State dependency of the effects of microinjection of cholinergic drugs into the nucleus pontis oralis. Brain Res. 1994;649:271–81. doi: 10.1016/0006-8993(94)91074-x. [DOI] [PubMed] [Google Scholar]
- Ludwig DS, Tritos NA, Mastaitis JW, Kulkarni R, Kokkotou E, Elmquist J, Lowell B, Flier JS, Maratos-Flier E. Melanin-concentrating hormone overexpression in transgenic mice leads to obesity and insulin resistance. J Clin Invest. 2001;107:379–86. doi: 10.1172/JCI10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciello M, Sinnamon HM. Locomotor stepping initiated by glutamate injections into the hypothalamus of the anesthetized rat. Behav Neurosci. 1990;104:980–90. doi: 10.1037//0735-7044.104.6.980. [DOI] [PubMed] [Google Scholar]
- Martins PJ, D'Almeida V, Pedrazzoli M, Lin L, Mignot E, Tufik S. Increased hypocretin-1 (orexin-a) levels in cerebrospinal fluid of rats after short-term forced activity. Regul Pept. 2004;117:155–8. doi: 10.1016/j.regpep.2003.10.003. [DOI] [PubMed] [Google Scholar]
- McGregor R, Damian A, Fabbiani G, Torterolo P, Morales FR, Chase MH. Hypothalamic Innervation of the Trigeminal and Hypoglossal Motor Nuclei: a Retrograde Tracer Study. Program No 755.5, 2004 Abstract viewer/itinerary planner. San Diego: Society for Neuroscience CD-ROM; 2004. [Google Scholar]
- McGregor R, Damian A, Fabbiani G, Torterolo P, Morales FR, Chase MH. Direct hypothalamic innervation of trigeminal motor nucleus: a retrograde tracer study. Neuroscience. 2005 doi: 10.1016/j.neuroscience.2005.08.028. in press. [DOI] [PubMed] [Google Scholar]
- Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–98. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modirrousta M, Mainville L, Jones BE. Orexin and MCH neurons express c-Fos differently after sleep deprivation vs. recovery and bear different adrenergic receptors. Eur J Neurosci. 2005;21:2807–16. doi: 10.1111/j.1460-9568.2005.04104.x. [DOI] [PubMed] [Google Scholar]
- Morales FR, Engelhardt JK, Soja PJ, Pereda AE, Chase MH. Motoneuron properties during motor inhibition produced by microinjection of carbachol into the pontine reticular formation of the decerebrate cat. J Neurophysiol. 1987;57:1118–29. doi: 10.1152/jn.1987.57.4.1118. [DOI] [PubMed] [Google Scholar]
- Mugnaini E, Oertel WH. An atlas of the distribution of GABAergic neurons and terminals. In: Bjorklund A, Hokfelt T, editors. Handbook of Chemical Neuroanatomy. Vol. 4. GABA and Neuropeptides in the CNS; Elsevier, Amsterdam: 1985. pp. 436–608. [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presse F, Nahon JL. Differential regulation of melanin-concentrating hormone gene expression in distinct hypothalamic areas under osmotic stimulation in rat. Neuroscience. 1993;55:709–20. doi: 10.1016/0306-4522(93)90436-j. [DOI] [PubMed] [Google Scholar]
- Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, Mathes WF, Przypek R, Kanarek R, Maratos-Flier E. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380:243–7. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- Rossi M, Choi SJ, O'Shea D, Miyoshi T, Ghatei MA, Bloom SR. Melanin-concentrating hormone acutely stimulates feeding, but chronic administration has no effect on body weight. Endocrinology. 1997;138:351–5. doi: 10.1210/endo.138.1.4887. [DOI] [PubMed] [Google Scholar]
- Rovere C, Viale A, Nahon J, Kitabgi P. Impaired processing of brain proneurotensin and promelanin-concentrating hormone in obese fat/fat mice. Endocrinology. 1996;137:2954–8. doi: 10.1210/endo.137.7.8770919. [DOI] [PubMed] [Google Scholar]
- Saper CB. Central autonomic system. In: Paxinos G, editor. The rat nervous system. Academic Press; London: 1995. pp. 107–135. [Google Scholar]
- Shekhar A, DiMicco JA. Defense reaction elicited by injection of GABA antagonists and synthesis inhibitors into the posterior hypothalamus in rats. Neuropharmacology. 1987;26:407–17. doi: 10.1016/0028-3908(87)90020-7. [DOI] [PubMed] [Google Scholar]
- Shi Y. Beyond skin color: emerging roles of melanin-concentrating hormone in energy homeostasis and other physiological functions. Peptides. 2004;25:1605–11. doi: 10.1016/j.peptides.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998;396:670–4. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- Siegel JM. Hypocretin (orexin): role in normal behavior and neuropathology. Annu Rev Psychol. 2004;55:125–48. doi: 10.1146/annurev.psych.55.090902.141545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM. REM Sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and practices of sleep medicine. Elsevier-Saunders; Philadelphia: 2005. pp. 120–135. [Google Scholar]
- Sinnamon HM. Forelimb and hindlimb stepping by the anesthetized rat elicited by electrical stimulation of the diencephalon and mesencephalon. Physiol Behav. 1984;33:191–9. doi: 10.1016/0031-9384(84)90099-4. [DOI] [PubMed] [Google Scholar]
- Sinnamon HM. Locomotor stepping elicited by electrical stimulation of the hypothalamus persists after lesion of descending fibers of passage. Physiol Behav. 1990;48:261–6. doi: 10.1016/0031-9384(90)90310-z. [DOI] [PubMed] [Google Scholar]
- Sinnamon HM, Ginzburg RN, Kurose GA. Midbrain stimulation in the anesthetized rat: direct locomotor effects and modulation of locomotion produced by hypothalamic stimulation. Neuroscience. 1987;20:695–707. doi: 10.1016/0306-4522(87)90120-5. [DOI] [PubMed] [Google Scholar]
- Sinnamon HM, Lee SH, Adams DB, Stopford CK. Locomotor stepping elicited by electrical stimulation of the lateral hypothalamus requires an ipsilateral descending pathway. Physiol Behav. 1984;33:209–15. doi: 10.1016/0031-9384(84)90101-x. [DOI] [PubMed] [Google Scholar]
- Sinnamon HM, Sklow B. Latency to initiate locomotion elicited by stimulation of the diencephalon positively correlates in awake and anesthetized rats. Pharmacol Biochem Behav. 1990;36:725–8. doi: 10.1016/0091-3057(90)90067-r. [DOI] [PubMed] [Google Scholar]
- Sinnamon HM, Stopford CK. Locomotion elicited by lateral hypothalamic stimulation in the anesthetized rat does not require the dorsal midbrain. Brain Res. 1987;402:78–86. doi: 10.1016/0006-8993(87)91049-3. [DOI] [PubMed] [Google Scholar]
- Skofitsch G, Jacobowitz DM, Zamir N. Immunohistochemical localization of a melanin concentrating hormone- like peptide in the rat brain. Brain Res Bull. 1985;15:635–49. doi: 10.1016/0361-9230(85)90213-8. [DOI] [PubMed] [Google Scholar]
- Sutcliffe JG, de Lecea L. The hypocretins: setting the arousal threshold. Nat Rev Neurosci. 2002;3:339–49. doi: 10.1038/nrn808. [DOI] [PubMed] [Google Scholar]
- Taheri S, Zeitzer JM, Mignot E. The role of hypocretins (orexins) in sleep regulation and narcolepsy. Annu Rev Neurosci. 2002;25:283–313. doi: 10.1146/annurev.neuro.25.112701.142826. [DOI] [PubMed] [Google Scholar]
- Torterolo P, Rojas M, Sampogna S, Morales FR, Chase MH. MCH-containing neurons and the control of sleep and wakefulness. Sleep. 2003;26:A20. doi: 10.1016/j.brainres.2006.08.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torterolo P, Yamuy J, Sampogna S, Morales FR, Chase MH. Hypothalamic neurons that contain hypocretin (orexin) express c-fos during active wakefulness and carbachol-induced active sleep. Sleep Res Online. 2001;4:25–32. http://www.sro.org/2001/Torterolo/25. [Google Scholar]
- Torterolo P, Yamuy J, Sampogna S, Morales FR, Chase MH. Hypocretinergic neurons are primarily involved in activation of the somatomotor system. Sleep. 2003;1:25–28. [PubMed] [Google Scholar]
- van den Pol AN, Acuna-Goycolea C, Clark KR, Ghosh PK. Physiological properties of hypothalamic MCH neurons identified with selective expression of reporter gene after recombinant virus infection. Neuron. 2004;42:635–52. doi: 10.1016/s0896-6273(04)00251-x. [DOI] [PubMed] [Google Scholar]
- Verret L, Goutagny R, Fort P, Cagnon L, Salvert D, Leger L, Boissard R, Salin P, Peyron C, Luppi PH. A role of melanin-concentrating hormone producing neurons in the central regulation of paradoxical sleep. BMC Neurosci. 2003;4:19. doi: 10.1186/1471-2202-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Economo C. Sleep as a problem of localization. J Nerv Ment Dis. 1930;71:249–259. [Google Scholar]
- Wardas J, Ossowska K, Wolfarth S. Evidence for the independent role of GABA synapses of the zona incerta- lateral hypothalamic region in haloperidol-induced catalepsy. Brain Res. 1988;462:378–82. doi: 10.1016/0006-8993(88)90569-0. [DOI] [PubMed] [Google Scholar]
- Yamuy J, Mancillas JR, Morales FR, Chase MH. C-fos expression in the pons and medulla of the cat during carbachol- induced active sleep. J Neurosci. 1993;13:2703–18. doi: 10.1523/JNEUROSCI.13-06-02703.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Sampogna S, Morales F, Chase M. Co-localization of hypocretin-1 and hypocretin-2 in the cat hypothalamus and brainstem. Peptides. 2002;23:1479. doi: 10.1016/s0196-9781(02)00084-0. [DOI] [PubMed] [Google Scholar]
- Ziegler DR, Cullinan WE, Herman JP. Distribution of vesicular glutamate transporter mRNA in rat hypothalamus. J Comp Neurol. 2002;448:217–29. doi: 10.1002/cne.10257. [DOI] [PubMed] [Google Scholar]


