Abstract
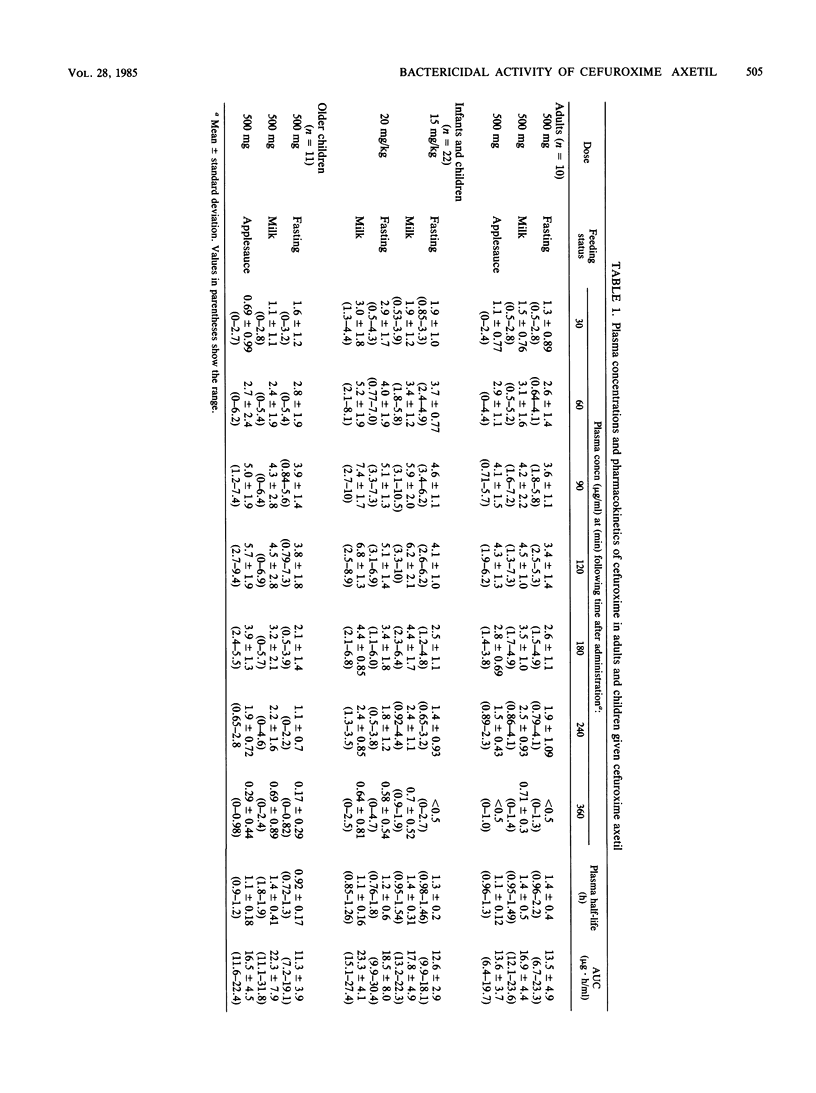
The pharmacokinetics of cefuroxime axetil were studied in 10 adult volunteers aged 24 to 31 years (mean age, 27), 22 infants and children aged 11 to 68 months (mean age, 33 months), and 11 children aged 7 years, 7 months to 12 years, 3 months (mean age, 11 years, 1 month). Mean peak plasma concentrations of cefuroxime occurred between 90 and 120 min in all study patients and were independent of the fasting or feeding status. The areas under the concentration-time curves were significantly higher in adult volunteers who received cefuroxime axetil with milk than in those who received the drug while fasting or with applesauce. The bioavailability of cefuroxime axetil was significantly enhanced in children by the concomitant ingestion of cefuroxime axetil and infant formula or whole milk. The areas under the concentration-time curves were 25 to 88% higher when cefuroxime axetil and milk were administered simultaneously than when the same dose was given to all fasting patients. The plasma bactericidal activities of cefuroxime against beta-lactamase-positive and -negative strains of Haemophilus influenzae and Staphylococcus aureus at the time of peak plasma concentrations were independent of feeding status and were similar in adults and in children. Against these strains, 52% of the children and 38% of the adults had peak bactericidal levels of 1:8 or greater.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ginsburg C. M., McCracken G. H., Jr Pharmacokinetics of cephradine suspension infants and children. Antimicrob Agents Chemother. 1979 Jul;16(1):74–76. doi: 10.1128/aac.16.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding S. M., Williams P. E., Ayrton J. Pharmacology of Cefuroxime as the 1-acetoxyethyl ester in volunteers. Antimicrob Agents Chemother. 1984 Jan;25(1):78–82. doi: 10.1128/aac.25.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken G. H., Jr, Ginsburg C. M., Clahsen J. C., Thomas M. L. Pharmacologic evaluation of orally administered antibiotics in infants and children: effect of feeding on bioavailability. Pediatrics. 1978 Nov;62(5):738–743. [PubMed] [Google Scholar]
- Sommers D. K., Van Wyk M., Williams P. E., Harding S. M. Pharmacokinetics and tolerance of cefuroxime axetil in volunteers during repeated dosing. Antimicrob Agents Chemother. 1984 Mar;25(3):344–347. doi: 10.1128/aac.25.3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. E., Harding S. M. The absolute bioavailability of oral cefuroxime axetil in male and female volunteers after fasting and after food. J Antimicrob Chemother. 1984 Feb;13(2):191–196. doi: 10.1093/jac/13.2.191. [DOI] [PubMed] [Google Scholar]


