Abstract
The interplay between peritoneal mesothelial cells and ovarian cancer cells is critical for the initiation and peritoneal dissemination of, and ascites formation in, ovarian cancer. The production of lysophosphatidic acid (LPA) by both peritoneal mesothelial cells and ovarian cancer cells has been shown to promote metastatic phenotype in ovarian cancer. Herein, we report that exogenous addition or ectopic overexpression of the matricellular protein SPARC (secreted protein acidic and rich in cysteine) significantly attenuated LPA-induced proliferation, chemotaxis, and invasion in both highly metastatic SKOV3 and less metastatic OVCAR3 ovarian cancer cell lines. SPARC appears to modulate these functions, at least in part, through the regulation of LPA receptor levels and the attenuation of extracellular signal-regulated kinase (ERK) 1/2 and protein kinase B/AKT signaling. Moreover, our results show that SPARC not only significantly inhibited both basal and LPA-induced interleukin (IL) 6 production in both cell lines but also attenuated IL-6-induced mitogenic, chemotactic, and proinvasive effects, in part, through significant suppression of ERK1/2 and, to a lesser extent, of signal transducers and activators of transcription 3 signaling pathways. Our results strongly suggest that SPARC exerts a dual inhibitory effect on LPA-induced mesothelial-ovarian cancer cell crosstalk through the regulation of both LPA-induced IL-6 production and function. Taken together, our findings underscore the use of SPARC as a potential therapeutic candidate in peritoneal ovarian carcinomatosis.
Keywords: SPARC, LPA, IL-6, ovarian cancer, mesothelial cells
Introduction
Epithelial ovarian cancer is the leading cause of death from malignant gynecologic tumors. Currently, more than 75% of ovarian cancers are diagnosed at International Federation of Gynecology and Obstetrics stages III and IV [1], with solid tumor masses growing either as peritoneal implants or as floating tumor cells within ascitic fluid. High mortality is predominantly due to occult progression of tumors in the peritoneal cavity—a condition known as peritoneal ovarian carcinomatosis. The mesothelial lining of the peritoneal cavity, as a host for tumor cells, provides the appropriate “soil” for the shedding of ovarian cancer cells, which are “seeds.” Cancer cells can induce changes in the surrounding microenvironment that actively support tumor progression, creating the so-called “reactive tumor microenvironment.” In the context of ovarian cancer, this reactive supporting microenvironment is composed of the mesothelial lining of the peritoneal cavity, extracellular matrix (ECM) components, inflammatory and immune cells, and ascitic fluid exuded from hyperpermeable blood vessels.
Lysophosphatidic acid (LPA) has been shown to be an essential microenvironmental factor in ovarian cancer. It is elevated in the blood and ascites of patients with ovarian cancer [2,3]. Several lines of evidence suggest that LPA signaling is involved in the initiation, progression, and metastasis of ovarian cancer [4,5], and imparts a cytoprotective effect to ovarian cancer cells exposed to cisplatin [6]. LPA also induces the production of potent inflammatory cytokines, which further facilitate tumor survival and a more aggressive behavior of tumor cells [7]. LPA is produced by a variety of cells, including mesothelial cells, macrophages, activated platelets, endothelial cells, and ovarian cancer cells [8,9]. Recently, peritoneal mesothelial cells have been reported to constitutively produce LPA, highlighting them as an important source not only of elevated LPA levels found in ovarian cancer ascites but, more importantly, of the initiation and maintenance of the pathogenic cascade of peritoneal ovarian carcinomatosis through preservation of a reactive microenvironment that is supportive of tumor progression [10].
Interleukin (IL) 6 is a potent pleiotropic inflammatory cytokine that mediates a plethora of physiological functions [11] and has been implicated in tumor growth, metastasis, and resistance to chemotherapy in a variety of tumor cells [12]. Depending on cell type, IL-6 is able to act through several classic protein kinase cascades, such as mitogen-activated protein kinase and phosphatidylinositol triphosphate kinase (PI-3K)/AKT [13]. Moreover, the ability of IL-6 to directly activate signal transducers and activators of transcription (STAT) 3 produces serious unintended consequences when examined in the context of tumor progression [11,14,15]. Recently, IL-6 was suggested to be an independent marker of health-related quality of life in ovarian cancer patients. High levels of IL-6 in the sera and ascitic fluid of ovarian cancer patients correlated with the psychosocial stress and depression frequently encountered in ovarian cancer patients [16].
SPARC (secreted protein acidic and rich in cysteine), also known as osteonectin and BM-40, is a matricellular glycoprotein that mediates cell-matrix interactions. In addition to its counteradhesive and antiproliferative functions, SPARC modulates angiogenesis and regulates the production, assembly, and organization of the ECM [17]. The role of SPARC in tumorigenesis is complex due to its diverse functions in a given microenvironment. We and others have shown that SPARC functions as a tumor suppressor in ovarian cancer [18,19]. However, information regarding the effect of SPARC on the normalization of the reactive tumor microenvironment, rendering it unfavorable for tumor growth, is limited. Herein, we studied the role of SPARC in the regulation of crosstalk between ovarian cancer cells and peritoneal mesothelial cells. Particular emphasis was given to the interaction of SPARC with LPA, the key player in the initiation and maintenance of the reactive microenvironment in ovarian cancer.
Materials and Methods
Antibodies and Reagents
SPARC from mouse parietal yolk sac was purchased from Sigma (St. Louis, MO). Human and bovine osteonectin, and mouse monoclonal anti-human osteonectin antibody were purchased from Hematologic Technologies (Essex, VT). 1-Oleoyl-LPA was purchased from Sigma. LPA was dissolved in vehicle [phosphate-buffered saline (PBS) containing 1% fatty acid-free bovine serum albumin (BSA); Sigma]. The phospholipase inhibitor AACOCF3 was purchased from Calbiochem (San Diego, CA). Recombinant human IL-6 and human IL-6-neutralizing antibodies were purchased form Peprotech (Rocky Hill, NJ). Human plasma fibronectin (FN) was from BD Biosciences (Bedford, MA). Hemacolor 3 stain was purchased from Fisher Scientific (Fairlawn, NJ). Antibodies against AKT/PKB, extracellular signal-regulated kinase (ERK) 2, STAT3, phospho-AKT/PKBSer473, phospho-ERK1/2, and phospho-STAT3 were purchased from Cell Signaling (Beverly, MA). Horseradish peroxidase (HRP)-conjugated secondary antibodies were from Jackson ImmunoResearch (West Grove, PA). All other chemicals were of analytical grade and were purchased from Sigma and Fisher Scientific.
Cell Culture
An immortalized human mesothelial cell line, Meso 301, originally obtained from a premenopausal woman with endometrial cancer and normal human ovarian surface epithelial (HOSE) cells were isolated under a protocol approved by the internal review board of the Brigham and Women's Hospital (Boston, MA). Both cell lines were immortalized with HPVE6E7 and maintained in Medium 199/MCDB110 (1:1 mixture) supplemented with 2 mM l-glutamine and 15% fetal bovine serum (FBS; Atlanta Biologicals, Norcross, GA). The ovarian cancer cell lines SKOV3 (originally isolated from the ascitic fluid of a patient with recurrent platinum-resistant ovarian cancer) and NIH:OVCAR3 were obtained from the American Type Culture Collection (Manassas, VA) and maintained in McCoy's 5A and RPMI 1460 medium supplemented with 15% FBS, which was in turn supplemented with 100 µU/ml penicillin and 100 µg/ml streptomycin. All media and supplements were purchased from Sigma.
Generation of SPARC Adenovirus
The gene encoding a conserved sequence of murine and human SPARC was isolated from murine aorta through reverse transcription-polymerase chain reaction (RT-PCR). In brief, total RNA was extracted from frozen tissues using TRIzol (Invitrogen, Carlsbad, CA). cDNA was synthesized from oligo-dT primed total RNA (1 µg) using Superscript II (Invitrogen). The 0.91-kb gene for SPARC was amplified using primers (accession no. BC004638; sense 5′-CACCATGAGGGCCTGGATCTTCTTTC-3′ and antisense 5′-GATCACCAGATCCTTGTTGATG-3′) and subcloned into the adenoviral shuttle vector pAdTrack (Stratagene, La Jolla, CA). Subsequent clones were verified for integrity through bidirectional DNA sequencing and protein expression. Replication-deficient adenoviruses expressing either SPARC or green fluorescent protein (GFP), under the control of the cytomegalovirus (CMV) promoter, were generated using the pAdTrack-CMV vector and AdEasy System (Stratagene). Viruses were amplified in HEK293 cells, purified using CsCl2, titrated using a cytopathic effect assay, and stored in PBS containing 5% sucrose/2 mM MgCl2. SKOV3 cells were transduced with SPARC and control (GFP) adenoviruses at a multiplicity of infection of 15 to 25, and SPARC production was confirmed in cell lysates and conditioned media by Western blot analysis after 24 hours. The levels and activities of SPARC secreted into the conditioned medium of transduced ovarian cancer cell lines were found to be within the range of concentrations of exogenous SPARC used in the experiments described herein, as determined by semiquantitative Western blot analysis and proliferation assays, respectively.
Preparation of Mesothelial Cell Conditioned Medium
Subconfluent cultures of Meso 301 cells were washed thrice with PBS and incubated with serum-free medium (SFM) for 24 hours. Conditioned media were collected and centrifuged to remove cell debris, and half of the conditioned media were heated at 95°C for 5 minutes. Heated and unheated conditioned media were then sterilized through 0.22-µm filters and stored at -80°C.
Migration Assays
Migration of ovarian cancer cells was assayed in 24-well plates and transwell inserts (8-µm pore-size polycarbonate filters, Corning Costar; Fisher Scientific) as described previously [18]. The lower surface of the filter was coated with FN at a concentration of 10 µg/ml. SKOV3 and OVCAR3 cells were starved overnight, and 1 x 105 cells in 100 µl were added to the upper chamber. Migration assays were carried out for 5 hours, at the end of which the contents of the top chambers were aspirated. Cells attached to the upper surface of the inserts were scraped with cotton swabs (Fisher Scientific), and cells attached to the bottom surface of the inserts were stained with Hemacolor 3 stain, as recommended by the manufacturer. Cells were counted in six fields per insert using an inverted microscope equipped with a DFC 320 digital camera (Leica Microsystems, Wetzlar, Germany; original magnification, x200).
Mesothelial Cell Invasion
Peritoneal mesothelial cells were grown to confluent monolayers in transwell inserts (8-µm pore-size polycarbonate filters). SKOV3 and OVCAR3 cells (1 x 105 cells in 100 µl) were added to the upper chamber and allowed to invade mesothelial monolayers for 72 hours. The contents of the upper chambers were aspirated, cells attached to the upper surface of the inserts were scraped, and cells attached to the bottom surface were stained and counted as described above.
RT-PCR
Total RNA was isolated from cultured cell lines using TRIzol reagent (Invitrogen) according to the instructions of the manufacturer. RNA was further purified with RNeasy isolation kit (Qiagen, Valencia, CA). Total RNA (2 µg) was reverse-transcribed in a 20-µl reaction system using an Improm II RT enzyme kit (Promega, Madison, WI) as described by the supplier. cDNA was prepared from 2 µg of total RNA with oligo-dT primers according to the cDNA synthesis ImProm-II protocol (Promega). cDNA was amplified in a 20-µl reaction system containing 200 µM of each dNTP (Promega) and 25 pmol of each primer, with the standard buffer containing 1 U of Jumpstart Taq polymerase (Sigma) and 1.5 mM MgCl2. Specific oligonucleotide primer pairs used for LPA receptors were derived from a published report [7] and were as follows: LPA1/Edg-2, 5′-CAAAATGAGGCCTTACGACGCCA-3′ and 5′-TCCCATTCTGAAGTGCTGCGTTC; LPA2/Edg-4, 5′-GCGCGCGGATCCACCATGGTCATCATGGGCCAGTGCT-3′ and 5′-GCGCGGTCGACTCAGTCCTGTTGGTTGGGTTGA-3′; LPA3/Edg-7, 5′-CTGATGTTTAACACAGGCCC-3′ and 5′-GACGTTGGTTTTCCTCTTGA-3′; and GAPDH, 5′-CACTGGCGTCTTCACCACCATG-3′ and 5′-GCTTCACCACCTTCTTGATGTCA-3′ (450 bp). Reaction conditions were as follows: initial denaturation at 95°C for 4 minutes, followed by 30 cycles (25 cycles for GAPDH) of 95°C for 30 seconds, 56°C for 45 seconds, and 72°C for 1 minute, and a final elongation at 72°C for 8 minutes. PCRproducts were resolved on 2% agarose gels containing ethidium bromide and were visualized under UV light. Gel imaging, documentation, and analysis were performed with a Kodak digital camera linked to Kodak D 3.6 software (Fisher Scientific).
Nonradioactive Cell Proliferation Assay
CellTiter96 kit (Promega) was used according to the manufacturer's instructions. SKOV3 and OVCAR3 cells that were serum-starved overnight were harvested by mild trypsinization. Cells (1 x 105) were seeded in each well of a 96-well plate in a complete growth medium and allowed to attach for 4 hours. Cells were then switched to a growth medium containing 5% FBS, with the indicated concentrations of LPA, in the presence of PBS vehicle or SPARC (10 µg/ml), which was replenished every 24 hours. After 72 hours, 20 µl of MTS was added to each well for an additional 3 hours. The number of proliferating cells was determined colorimetrically by measuring the absorbance at 590 nm (A590) of the dissolved formazan product. All experiments were carried out in triplicate, and the results were expressed as mean ± SEM.
Immunoblotting
Subconfluent monolayers of SKOV3 and OVCAR3 cells were serum-starved for 20 hours and pretreated with SPARC (20 µg/ml) for 2 hours. Cells were then stimulated as described in figure legends and harvested in lysis buffer (20mM Tris, pH 7.4, 150 mM NaCl, 1 mM EDTA, 50 mM NaF, 0.5% sodium deoxycholate, 1% NP-40, 1 mM Na3VO4, and 1 x protease inhibitor cocktail mixture). Lysates were cleared by centrifugation at 12,000g for 20 minutes at 4°C. Twenty-five to 40 µg of cell lysates was resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto PVDF membranes (BioRad, Hercules, CA). The membranes were incubated overnight with phospho-specific antibodies against ERK1/2, AKT, and STAT3 at 4°C. Protein detection was carried out using HRP-conjugated anti-mouse or anti-rabbit secondary antibodies and a Super-Signal West Dura Chemiluminescence kit (Pierce, Rockford, IL). Membranes were then stripped (Restore Western Blot Stripping Buffer; Pierce) and reprobed with antibodies against total ERK2, AKT, and STAT3 to ensure equal protein loading.
Cytokine Antibody Array
Subconfluent serum-starved SKOV3 cells grown in 60-mm plates were pretreated with PBS (control) or SPARC (20 µg/ml) for 2 hours before stimulation with LPA (50 µM) for an additional 24-hour period. Conditioned media were then collected, centrifuged to remove cell debris, and concentrated by 10-kDa cutoff centricons (Millipore, Billerica, MA). Forty inflammatory cytokines were assayed using RayBio Human Inflammation Antibody Array III (RayBiotech, Norcross, GA). After exposing the membranes to blocking buffer, they were incubated with the collected conditioned medium from SKOV3 cells treated with LPA and/or SPARC for 24 hours. Membranes were then processed to visualize protein levels of various inflammatory cytokines according to the manufacturer's instructions.
Measurement of IL-6 Production by Enzyme-Linked Immunosorbent Assay (ELISA)
Culture supernatants were collected and used for the determination of IL-6 concentration by ELISA using a human IL-6 ELISA kit (RayBiotech) according to the manufacturer's instructions. Each measurement was performed in duplicate, and average values were recorded as picograms per milliliter.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism version 3.1 for Windows (GraphPad Software, San Diego CA). The significance of the results using different concentrations of LPA and IL-6 on ovarian cancer cell lines was determined by one-way analysis of variance followed by Newman-Keuls multiple comparison post hoc test. All other statistical analyses were determined by Student's t test. Differences were considered significant at P < .05.
Results
Conditioned Medium from Meso 301 Induces Ovarian Cancer Cell Migration
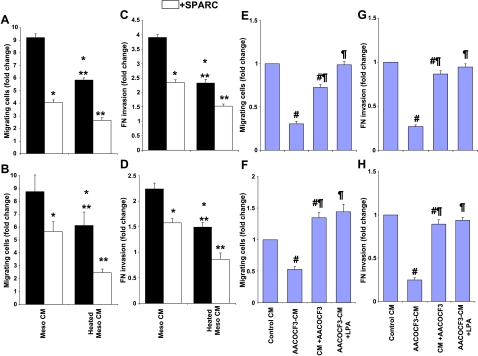
Previous studies have shown that the conditioned medium from primary human peritoneal mesothelial cells isolated from a patient with ovarian cancer stimulated the migration of ovarian cancer cells mainly through LPA in heat-resistant fractions [10]. In agreement with these findings, the conditioned medium from the immortalized cell line Meso 301, which was isolated from an otherwise healthy peritoneum, was shown to significantly (approximately nine-fold) increase the chemotaxis of SKOV3 and OVCAR3 cells, compared to the complete growth medium control (Figure 1, A and B). Heat inactivation of biologically active peptides and proteins in the conditioned medium resulted in ∼ 40% decrease in chemotactic activity compared to unheated controls. However, the chemotactic activity of the heat-sensitive fraction was still significantly (approximately six-fold) higher than that exerted by growth medium control. Pretreatment of ovarian cancer cells with SPARC resulted in a significant (35–55%) decrease in their chemotaxis toward heated or unheated conditioned medium. Addition of the unheated and heated conditioned media of Meso 301 to the upper chamber of FN-coated transwell inserts resulted in increases in the invasion of SKOV3 (3.8-fold and 2.3-fold) and OVCAR3 (2.3-fold and 1.5-fold), respectively, relative to medium controls (Figure 1, C and D). SPARC treatment of SKOV3 and OVCAR3 cells decreased conditioned medium-induced invasion by as much as 40% and 30%, respectively. These data demonstrate that SPARC significantly inhibits the chemotactic and chemokinetic effects of both heat-sensitive and heat-resistant fractions of mesothelial conditioned medium. The inhibitory effect of SPARC was observed only when it was added to ovarian cancer cells in the upper chamber of the inserts. When added to the bottom chamber with either the conditioned medium of Meso 301 or medium controls, SPARC had no effect on chemotactic activity, suggesting that it exerts its action on ovarian cancer cells rather than neutralizing the chemotactic activity of the conditioned medium. Recently, it has been shown that the biologic activity of the heat-resistant fraction of mesothelial cell conditioned medium is due to the constitutive production of LPA by the action of phospholipases, mainly phospholipase A2 (PLA2) [10]. Both cytosolic Ca2+-dependent PLA2 (cPLA2) and Ca2+-independent PLA2 (iPLA2) have been implicated in LPA production by peritoneal mesothelial cells and/or LPA-induced cell migration of ovarian cancer cells [10,20,21]. To determine whether the activity of the heat-resistant fraction of Meso-CM is due to LPA production, we included AACOCF3 (an iPLA2 and cPLA2 inhibitor; 25 µM) during the generation of the conditioned medium from Meso 301 cells (AACOCF3-CM) to inhibit LPA production. Heated AACOCF3-CM significantly reduced the migration and invasion of ovarian cancer cells (by ∼ 50–75%) compared to heated Meso-CM controls (Figure 1, E–H). The observed inhibitory effect of AACOCF3 on SKOV3 and OVCAR3 migration and invasion was concentration-dependent (data not shown), was fully rescued by the addition of LPA (50 µM) to heated AACOCF3-CM, and was not due to toxicity or reduced cell viability (as determined by LIVE/DEAD viability/cytotoxicity kit; Invitrogen data not shown). These results suggest that the chemotactic and promigratory effects of the heat-resistant fraction of Meso-CM on ovarian cancer cells were, for the most part, due to PLA2-induced LPA production/secretion in the conditioned medium. This production of LPA from Meso 301 cells appeared to be constitutive because the conditioned medium was collected from cells in SFM and in the absence of any stimuli.
Figure 1.
The conditioned medium from Meso 301 induces ovarian cancer cell motility through LPA. Subconfluent Meso 301 cells were allowed to condition in SFM for 24 hours, and the collected conditioned medium (heated or not) was used as a chemoattractant (600 µl in the bottom chambers of transwell inserts) for SKOV3 (A) and OVCAR3 (B) (1 x 105 cells/100 µl of SFM-0.4% BSA in the upper chamber) in the presence or in the absence of SPARC (10 µg/ml). Results are expressed as the mean ± SEM of the fold change in cell migration induced by a complete medium in the bottom chamber as controls. FN invasion by SKOV3 (C) and OVCAR3 (D) cells was tested in response to the conditioned medium of Meso 301 (heated or not) added with the cells to the upper chamber of transwell inserts, in the presence or in the absence of SPARC (10 µg/ml). LPA production by Meso 301 was inhibited by PLA2 inhibitor (AACOCF3). The conditioned medium of Meso 301 serum-starved for 24 hours was collected in the absence (control conditioned medium) or in the presence of AACOCF3 (25 µM) added at serum starvation (AACOCF3-CM) or after the conditioned medium had been generated (conditioned medium + AACOCF3). All collected conditioned media were heat-inactivated and added to the bottom chamber of transwell inserts, and the chemotactic activity of SKOV3 (E) and OVCAR3 (F) was tested in the aforementioned conditioned medium and after replenishment with LPA (50 µM; AACOCF3-CM + LPA). Results are expressed as the mean ± SEM of the fold change of the chemotactic activity of heated conditioned medium under experimental conditions, compared to the conditioned medium (control conditioned medium) of Meso 301 heated for 24 hours. The effect of the above-mentioned conditioned medium on FN invasion by SKOV3 (G) and OVCAR3 (H) was tested when they were added with the cells to the top chambers of the inserts, whereas the bottom chambers contained a complete growth medium. Results are expressed as the mean ± SEM of the fold change of the invasive activity of SKOV3 and OVCAR3 induced by different experimental conditions, relative to that induced by the conditioned medium (control conditioned medium) of Meso 301 heated for 24 hours. Data summarized are representative of an experiment performed in triplicate and repeated thrice with similar results. *P < .05, compared to unheated Meso-CM. **P < .05, compared to heated Meso-CM. #P < .05, compared to heated Meso-CM. ¶P < .05, compared to AACOCF3-CM.
SPARC Inhibits LPA-Induced Ovarian Cancer Cell Migration and Invasion
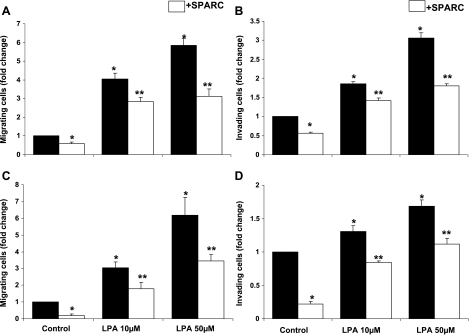
Our finding that SPARC inhibits SKOV3 and OVCAR3 chemotaxis and FN invasion induced by heated Meso-CM prompted us to test whether LPA-induced migration can also be inhibited by SPARC. In agreement with earlier reports [4], we found that the chemotactic effect of LPA (10–50 mM) on SKOV3 and OVCAR3 cell lines was concentration-dependent and resulted in up to 5.8-fold and 6-fold increases in migrating cells, compared to unstimulated controls (Figure 2, A and C, respectively). SPARC significantly inhibited the migration of SKOV3 and OVCAR3 cells toward both the control medium (∼ 45% and ∼ 70%, respectively) and tested concentrations of LPA (up to 40%). LPA-induced FN invasion by SKOV3 and OVCAR3 cells was also shown to be concentration-dependent and resulted in 1.8-fold to 3-fold increases in FN invasion by SKOV3, and to 1.4-fold to 1.9-fold increases in FN invasion by OVCAR3 (Figure 2, B and D). SPARC significantly inhibited control medium-induced (by ∼ 50–70%) and LPA-induced (25–40%) invasion of FN by SKOV3 and OVCAR3 cell lines.
Figure 2.
SPARC antagonizes LPA-induced ovarian cancer cell chemotaxis and invasion. LPA (10–50 µM in SFM, added to the bottom chamber of transwell inserts) stimulated the migration of SKOV3 (A) and OVCAR3 (C) cells in a concentration-dependent manner. SPARC (10 µg/ml) added with SKOV3 and OVCAR3 cells to the top chamber of the inserts inhibited LPA-induced migration. Results are expressed as the mean ± SEM of the fold change in migrated cells under experimental conditions, compared to controls attracted by a complete growth medium placed in the bottom chamber (assigned a value of 1). LPA (10–50 µM in SFM) added to SKOV3 (B) and OVCAR3 (D) cells in the top chamber stimulated the invasion of FN-coated inserts and the migration of both cell lines toward a complete growth medium in the bottom chamber. The effect of LPA on FN invasion was significantly inhibited by SPARC. Results are expressed as the mean ± SEM of the fold change in invading cells under experimental conditions, compared to control cells exposed to 0.4% BSA in SFM (assigned a value of 1). Represented are the results of an experiment performed in triplicate and repeated thrice with similar results. *P < .05, compared to unstimulated control. **P < .05, compared to control or LPA stimulation.
Adenoviral Transduction of SPARC Attenuates Ovarian Cancer Cell Migration and Mesothelial Cell Invasion
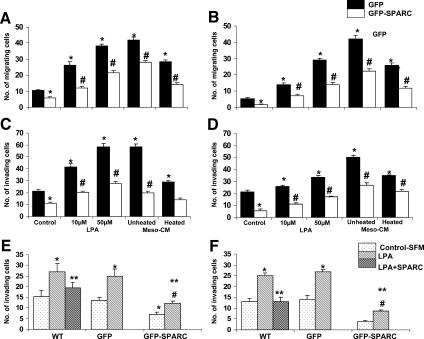
SKOV3 and OVCAR3 cells neither express nor secrete SPARC due to hypermethylation of the SPARC promoter (Motamed et al., unpublished data). Conversely, Meso 301 cells express and secrete SPARC into their conditioned medium. We have recently reported that SPARC inhibits ovarian cancer cell migration and ECM invasion [18]. Therefore, we hypothesized whether restoring SPARC expression by adenovirus gene transfer would attenuate the promigratory effects of LPA on SKOV3 and OVCAR3 cells. Interestingly, we found that ovarian cancer cells transduced with GFP-SPARC adenovirus exhibited significantly (∼ 40%) reduced migration toward the control medium, relative to cells transduced with GFP alone (Figure 3, A and B). Although LPA-induced migration was concentration-dependent for both cell lines, cells transduced with GFP-SPARC exhibited significant diminution in chemotaxis toward LPA (∼ 40–60%) and heated and unheated Meso-CM (∼ 50%), compared to controls. In all experimental conditions, OVCAR3 cells exhibited less migration than did SKOV3 cells. In agreement with these findings, LPA was shown to induce the FN invasion of both cell lines in a concentration-dependent manner (Figure 3, C and D). SKOV3-GFP-SPARC exhibited significant (∼ 40–60%) attenuation in FN invasion stimulated by LPA or Meso-CM, compared to SKOV3-GFP controls (Figure 3C). Similarly, OVCAR3-GFP-SPARC exhibited significant attenuation in FN invasion stimulated by LPA or Meso-CM, compared to OVCAR3-GFP controls (Figure 3D). Using a coculture system, LPA (50 µM) significantly (up to two-fold) stimulated the invasion of Meso 301 monolayers by SKOV3 and OVCAR3 cells in 72 hours (Figure 3, E and F). Exogenous addition (10 µg/ml) or overexpression of SPARC significantly (up to ∼ 45%) inhibited both basal and LPA-stimulated invasions of Meso 301 by ovarian cancer cell lines in thismodel system. To account for the known antiproliferative effect of SPARC on SKOV3 cells in this two-cell coculture system, the number of viable ovarian cancer cells was determined in parallel studies by counting GFP-expressing cells in the top chamber, attached to or invading through the mesothelial monolayer, and cells attached to the bottom surface of the filter. These numbers were compared and corrected according to the doubling time of SKOV3-GFP and SKOV3-GFP-SPARC (21.5 and 34.9 hours, respectively) and that of OVCAR3-GFP and OVCAR3-GFP-SPARC (39.7 and 50.8 hours, respectively). However, these parallel studies did not reveal a significant difference in the total viable number of the cell populations tested, suggesting that SPARC overexpression overexpression in this two-cell coculture system did not result in significant inhibition of SKOV3 cell proliferation. A plausible explanation that can account for this observation is the constitutive production of high levels of LPA and IL-6 by mesothelial cells, as well as the synergistic production of IL-6 (and other mitogenic/prosurvival factors) from both ovarian cancer cells and mesothelial cells in this model system.
Figure 3.
Restoration of SPARC expression in ovarian cancer cells antagonizes LPA-induced and Meso-CM-induced chemotaxis and invasion. SKOV3 and OVCAR3 cells were transduced (> 90% transduction efficiency) with GFP or GFP-SPARC adenoviruses and allowed to recover for 24 hours in a complete growth medium. LPA (10–50 µM)-induced and Meso-CM-induced chemotaxis of SKOV3 (A) and OVCAR3 (B) were tested as described in the legend to Figure 1. *P < .05, compared to unstimulated control cells attracted by a complete growth medium in the bottom chamber. #P < .05, compared to matched LPA or Meso-CM stimulation. LPA-induced and Meso-CM-induced FN invasions by SKOV3 (C) and OVCAR3 (D) were also tested as described earlier. *P < .05, compared to unstimulated control cells in SFM containing 0.04% BSA added to the top chamber of the transwell inserts. #P < .05, compared to matched LPA or Meso-CM stimulations. Results are expressed as the mean ± SEM of a representative of three independent experiments. In a two-cell coculture model described in the Materials and Methods section, LPA (50 µM)-stimulated invasion of Meso 301 monolayers by untransduced (WT) SKOV3 (E) and OVCAR3 (F) cells was significantly inhibited in the presence of exogenous SPARC (10 µg/ml), as well as by SKOV3 and OVCAR3 cells transduced with an adenovirus overexpressing SPARC. *P < .05, compared to unstimulated WT or GFP-transduced (GFP) cells. **P < .05, compared to LPA-stimulated WT or GFP-transduced cells. #P < .05, compared to unstimulated GFP-SPARC-transduced (GFP-SPARC) cells. Results are expressed as the mean ± SEM of a representative of three independent experiments performed in quadruplicate.
SPARC Diminishes LPA-Induced Proliferation and Survival Signaling in Ovarian Cancer Cells
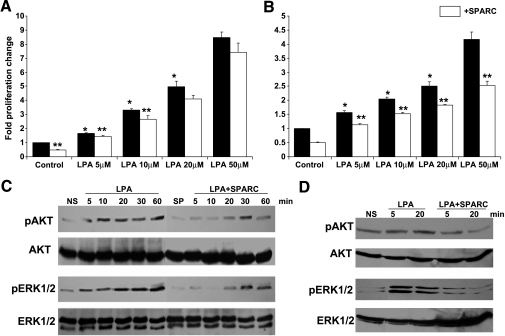
High levels of LPA (up to 80 µM) have been reported in the ascitic fluid of ovarian cancer patients [8,22–25]. At these concentrations, LPA plays a critical role in the stimulation of both anchorage-dependent proliferation and anchorageindependent proliferation of ovarian cancer cells, prevention of apoptosis and anoikis, and stimulation of the production of mitogenic and prosurvival factors, including LPA itself [26,27]. We have previously reported the inhibition of serum-induced ovarian cancer cell proliferation and survival signaling by SPARC [18,19,28]. LPA stimulation resulted in a concentration-dependent increase in the proliferation of SKOV3 (more than eight-fold; Figure 4A) and OVCAR3 (more than four-fold; Figure 4B), compared to unstimulated controls. SPARC (10 µg/ml) significantly inhibited (∼ 15–20%) the proliferation of SKOV3 at low (5–10 mM), but not high (20–50 mM), concentrations of LPA. However, the inhibitory effect of SPARC on LPA-induced OVCAR3 proliferation was significant (20–45%) at all tested concentrations of LPA. We next determined the effect of SPARC on LPA-mediated survival signaling pathways and found that SPARC significantly inhibited LPA-induced ERK1/2 and AKT phosphorylation in SKOV3 cells in as early as 5 minutes—an effect that was sustained for up to 60 min (Figure 4C). A similar inhibitory effect of SPARC on LPA-induced ERK1/2 and AKT phosphorylation was also observed in OVCAR3 cells (Figure 4D).
Figure 4.
Effect of SPARC on LPA-induced proliferation and survival signaling in ovarian cancer cells. Cell proliferation of SKOV3 (A) and OVCAR3 (B) cells in response to indicated concentrations of LPA, in the presence (open bars) and in the absence (closed bars) of SPARC, was assessed by measuring the released formazan at A590. Results are expressed as the mean ± SEM of the fold increase in proliferation relative to unstimulated control cells (assigned a value of 1). *P < .05, LPA-stimulated cells compared to control cells and between different concentrations of LPA. **P < .05, SPARC-treated versus matched unstimulated control or LPA-stimulated cells. Represented are the results of one experiment performed in quadruplicate that was representative of two independent experiments. SKOV3 (C) and OVCAR3 (D) cells starved overnight were pretreated with 20 µg/ml SPARC in SFM for 2 hours, followed by stimulation with 50 µM LPA for indicated time points. Western blot analysis of phosphorylated and total ERK1/2 and AKT was performed as described in the Materials and Methods section. Blots represent the results of three independent experiments. NS = not stimulated; SP = SPARC-treated.
Regulation of LPA Receptor Expression in SKOV3 Cells By SPARC
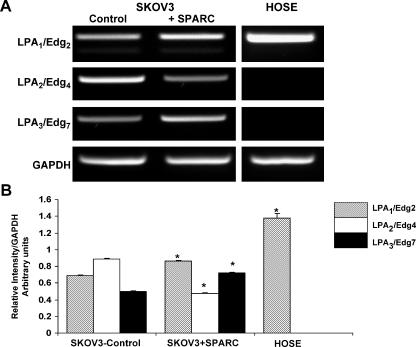
It has been suggested that the net effect of LPA on ovarian cancer cells depends on the outcome of activation of its different receptors [26]. We determined the effect of SPARC on LPA receptor expression by semiquantitative RT-PCR. Interestingly, we found that SPARC upregulated LPA1/Edg2 and downregulated LPA2/Edg4 basal expression levels (Figure 5, A and B), consistent with the role of SPARC as a negative regulator of ovarian cancer. The significance of SPARC augmenting the expression levels of LPA3/Edg7 is not known and is yet to be elucidated. Normal HOSE cells, which are known to express only LPA1/Edg2, were used as controls. These findings suggest that SPARC mediates its negative regulatory role on LPA signaling in SKOV3, at least in part, through alterations in the expression of LPA receptor repertoire.
Figure 5.
Effect of SPARC on LPA receptor expression in SKOV3 cells. SKOV3 cells serum-starved overnight were treated with PBS (control) or SPARC (10 µg/ml) for 6 hours. The expression of LPA receptors Edg2/LPA1 and Edg4/LPA2 and Edg7/LPA3 was determined by semiquantitative RT-PCR. PCR products of LPA receptors and GAPDH were run on 2% agarose gels (A). Expression levels were normalized to that of the housekeeping gene GAPDH and were represented as a bar graph (B). HOSE cells serum-starved overnight were used as controls for LPA receptor expression. Results shown are from one experiment that was representative of three independent experiments. *P < .05, compared to corresponding control SKOV3 cells.
SPARC Suppresses Secretion of IL-6 in Ovarian Cancer Cells
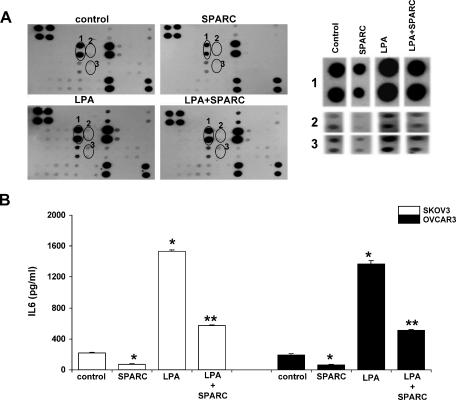
LPA has been shown to induce inflammatory cytokines that further augment peritoneal ovarian carcinomatosis [27]. IL-6 is a secreted multifunctional glycoprotein that was found to be present at high levels in the serum and ascites of ovarian cancer patients [29,30]. LPA enhances IL-6 expression and secretion by ovarian cancer cells, but not in normal ovarian epithelial cells. This selective induction was attributed to LPA2/Edg4-LPA3/Edg7 receptors in cancer cells [7]. To determine whether SPARC affects LPA-induced IL-6 production in SKOV3, we used a growth factor/cytokine array to identify factors secreted by SKOV3 after LPA stimulation in the presence or in the absence of SPARC. Array spots were analyzed, and the intensity of each spot was compared with respective spots in the control array (Figure 6A). Our data showed that LPA significantly increased the production of IL-6 by SKOV3 cells, and SPARC treatment significantly decreased both basal and LPA-stimulated IL-6 production. Results from our quantitative IL-6 ELISA confirmed that SPARC was able to significantly inhibit basal (∼ 68%) and LPA-induced IL-6 production in SKOV3 and OVCAR3 cell lines (Figure 6B). In addition to significant increases in IL-6 levels, LPA stimulation also upregulated the following inflammatory cytokines and growth factors: granulocyte-macrophage colony-stimulating factor, IL-6 soluble receptor (IL-6sR), IL-8, interferon γ-inducible protein 10 (IP10), monocyte chemoattractant protein (MCP) 1, macrophage colony-stimulating factor, transforming growth factor β1, and platelet-derived growth factor (PDGF) BB relative to unstimulated SKOV3 controls. SPARC treatment of SKOV3 cells for 24 hours significantly inhibited the basal levels of all aforementioned cytokines. SPARC pretreatment of SKOV3 cells decreased levels of LPA-induced IL-1α,-6sR, IP10, MCP-1, and PDGF-BB with no noticeable effect on the levels of other upregulated cytokines. These results indicate that SPARC strongly inhibits both basal and LPA-induced production of IL-6 by ovarian carcinoma cells and, to a lesser extent, the levels of other proinflammatory cytokines and growth factors that may in turn augment the functions of IL-6 and/or contribute to the production of LPA and IL-6 by other cells in the microenvironment of peritoneal ovarian carcinomatosis.
Figure 6.
SPARC inhibits basal and LPA-induced IL-6 secretions from ovarian cancer cells. A human inflammation cytokine protein array was used to detect differences in the protein levels of inflammation-related factors secreted into the conditioned medium of serum-starved SKOV3 cells pretreated with PBS (control) or SPARC (10 µg/ml) for 2 hours and stimulated with LPA (50 µM) for 24 hours (A; left panel). A higher magnification of duplicate spots from the cytokine array depicting protein levels of selected inflammation-related factors that were significantly upregulated by LPA and attenuated by SPARC (A; right panel). (1) IL-6. (2) IL-6sR. (3) MCP-1. Quantification of IL-6 secretion by ELISA in conditioned media of SKOV3 (B; left panel) and OVCAR3 cells (B; right panel) in the presence or in the absence of LPA and SPARC. *P < .05, compared to unstimulated control cells. **P < .01, compared to LPA-stimulated cells. Results shown are from one experiment that was representative of three independent experiments.
Coculture of Meso 301 and Ovarian Cancer Cells Results in a Synergistic Increase in IL-6 Levels
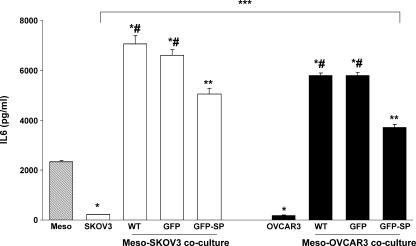
In agreement with earlier reports suggesting that peritoneal mesothelial cells may be a prominent source of IL-6 in ovarian cancer-related ascites [31,32], we found that steady-state secretion of IL-6 by Meso 301 was 10-fold higher than both SKOV3 and OVCAR3 cell lines (Figure 7). However, addition of the same number of ovarian cancer cells to confluent monolayers of Meso 301 in a coculture system resulted in synergistic augmentation of IL-6 production (32-fold and 3.5-fold higher than either cell lines and Meso 301, respectively). Restoring SPARC expression in ovarian cancer cells by adenoviral gene transfer reduced IL-6 production from Meso ovarian cancer cell cocultures relative to untransduced (by ∼ 28%) or GFP-transduced cells (by ∼ 24%). To account for the antiproliferative effect of SPARC on SKOV3 and OVCAR3 cells in this two-cell coculture system, we first determined whether proliferation plays a significant role in the invasion of mesothelial monolayers by these cancer cell lines. Results of our pilot studies performed in the presence or in the absence of the proliferation inhibitor mitomycin C (used at a concentration range that resulted in a 50–75% inhibition of LPA-induced proliferation of either cell line) did not show a statistically significant difference in the number of invading tumor cells (data not shown). Hence, we concluded that proliferation does not play a significant role in this two-cell coculture invasion assay. To further confirm these results, the number of viable ovarian cancer cells (transduced with GFP alone or GFP-SPARC) was quantified in parallel studies by counting GFP-expressing cells in the top chamber, attached to or invading through the mesothelial monolayer, as well as cells attached to the bottom surface of the filter after 72 hours. In agreement with our mitomycin C experiments, these parallel studies did not reveal a significant difference in the total number of viable cell populations tested, suggesting that SPARC overexpression in this two-cell coculture system did not result in significant inhibition of SKOV3 cell proliferation (data not shown). A plausible explanation that can account for this observation is the constitutive production of high levels of LPA and IL-6 by mesothelial cells, as well as the synergistic production of IL-6 (and other mitogenic prosurvival factors) from both ovarian cancer cells and mesothelial cells in this model system. It is also noteworthy that IL-6 production by SKOV3 cells (transduced or not) was significantly higher than that of OVCAR3 cells either alone or in coculture with Meso 301 cells. Interestingly, ectopic expression or exogenous addition of SPARC had no significant effect on the proliferation, survival, or IL-6 production of Meso 301 (data not shown).
Figure 7.
Effect of ovarian cancer-mesothelial cell coculture on IL-6 production. SKOV3 and OVCAR3 cells (WT) were transduced with GFP or GFP-SPARC in a complete growth medium and allowed to recover for 24 hours. After trypsinization, 1 x 106 cells in SFM were added to confluent monolayers of the Meso 301 cell line in 60-mm plates for an additional 24 hours. Conditioned media were collected, and IL-6 levels were determined by ELISA. *P < .01, between either SKOV3 or OVCAR3 and Meso 301. *#P < .01, between cocultured Meso and untransduced (WT) or GFP-transduced SKOV3 or OVCAR3, compared to either cell line alone. **P < .05, compared to WT or GFP-transduced cells. ***P < .05, between SKOV3 and OVCAR3 under all experimental conditions. Results shown are from one experiment that was representative of three independent experiments.
Effect of SPARC on IL-6-Mediated Proliferation and Survival Signaling of Ovarian Cancer
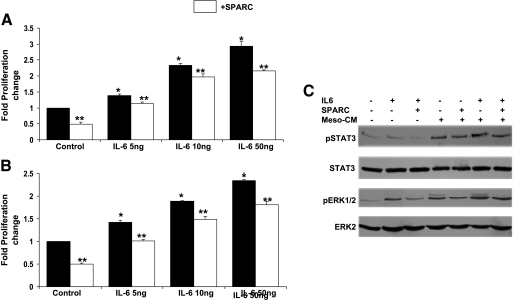
Previous studies have shown that IL-6 promotes tumor cell proliferation and metastasis of ovarian cancer cell lines [14,33], and exerts a potent proangiogenic effect that further supports progression of the disease [34]. These effects of IL-6 have been shown to be mediated through the activation of downstream signaling molecules, of which STAT3 and ERK1/2 are well characterized [7,14,27,34]. Similar to our reported results with LPA (Figure 4), the effect of IL-6 on SKOV3 and OVCAR3 proliferation was concentrationdependent. SPARC (10 µg/ml) significantly inhibited the proliferation induced by tested concentrations of IL-6 (5–50 ng/ml; Figure 8, A and B). Analysis of the regulation of IL-6-induced signaling by SPARC revealed inhibition of ERK1/2 and STAT3 activation (Figure 8B). Because cultured peritoneal mesothelial cells reportedly produce high levels of IL-6, we also tested the effect of heated Meso-CM on ERK1/2 and STAT3 phosphorylation. As expected, heated Meso-CM strongly activated both ERK1/2 and STAT3 in SKOV3 cells as early as 5 min, and this effect was attributed to heat-resistant bioactive lipids, mainly LPA. Augmented activation of ERK1/2 and STAT3 was noticed when IL-6 was replenished in heated Meso-CM. SPARC was able to significantly suppress ERK1/2 activation but had a less pronounced inhibitory effect on STAT3 activation (Figure 8C).
Figure 8.
Effect of SPARC on IL-6-induced proliferation and survival signaling pathways in ovarian cancer cells. Proliferation of SKOV3 (A) and OVCAR3 (B) cells in response to indicated concentrations of IL-6, in the presence of PBS (Control) or SPARC (20 µg/ml), was determined by MTS assay, as described previously. Results are expressed as the fold change of the proliferation of IL-6-stimulated SKOV3 and OVCAR3 cells relative to the proliferation of PBS-stimulated (control) cells (assigned a value of 1). *P < .05, from controls. **P < .05, from matched control or LPA-stimulated cells. SKOV3 cells serum-starved overnight were pretreated with PBS (control) or SPARC (20 µg/ml) for 2 hours, followed by stimulation with IL-6 (50 ng/ml), heat-inactivated Meso-CM, or heat-inactivated Meso-CM plus IL-6 (50 ng/ml) for 5 minutes (C). The expression levels of phosphorylated and total ERK and STAT3 were determined by Western blot analysis, as described previously. Results shown are from one experiment that was representative of three independent experiments.
Effect of SPARC on IL-6-Induced Chemotaxis and Invasion of Ovarian Cancer Cells
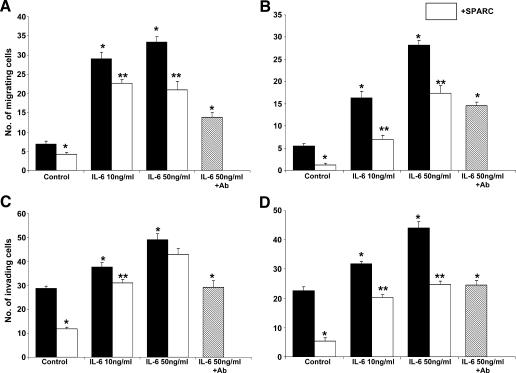
It has been reported that IL-6 stimulates ovarian cancer cell migration and that higher levels of IL-6 correlated with larger tumors, faster progression, relapses, and an overall poor prognosis [16,31,33]. We studied the effect of SPARC on IL-6-induced chemotaxis and FN invasion by SKOV3 and OVCAR3 cells. Our results revealed that SPARC significantly inhibited the concentration-dependent chemotactic effect of IL-6 (10–50 ng/ml) on SKOV3 cells by 22% to 38%, and on OVCAR3 cells by 40% to 70%, respectively, compared to the inhibitory effect (up to ∼ 58%) of IL-6-neutralizing antibody (Figure 9, A and B). The inhibitory effect of SPARC on IL-6-induced FN invasion of SKOV3 was only significant (∼ 18%) at IL-6 concentrations of up to 10 ng/ml. Higher concentrations of IL-6 (50 ng/ml) stimulated FN invasion by > 60% relative to unstimulated controls and were significantly (>38%) inhibited by the IL-6-neutralizing antibody but not by SPARC (Figure 9C). In contrast, the inhibitory effect of SPARC on IL-6-induced FN invasion of OVCAR3 was significant (30–50%) at all tested concentrations of IL-6 and was comparable to the effect of the IL-6-neutralizing antibody. These results implicate that the inhibitory role of SPARC on IL-6-induced chemotaxis and invasion was more pronounced in less invasive OVCAR3 cells compared to highly metastatic SKOV3 cells.
Figure 9.
SPARC antagonizes IL-6-induced chemotaxis and invasion of ovarian cancer cells. The chemotactic activity of SKOV3 (A) and OVCAR3 (B) cells toward IL-6 was tested as described previously. The indicated concentrations of IL-6 were used to attract ovarian cancer cells either alone or after pretreatment of cells with SPARC (10 µg/ml) for 2 hours. As control, the IL-6-neutralizing antibody (50 µg/ml) was mixed with SFM containing IL-6 (50 ng/ml) for 30 min before use in chemotaxis assay. *P < .05, from PBS-treated (control) cells. **P < .05, from matched control or IL-6-stimulated cells. FN invasion by SKOV3 (C) and OVCAR3 (D) cells was studied under the same conditions as described for the chemotaxis assay, with the exception that IL-6 was added to the cells in the upper chamber of the transwell inserts and cells were allowed to migrate toward a complete growth medium. Pretreatment of either SKOV3 or OVCAR3 cells with the IL-6-neutralizing antibody (50 µg/ml) for 30 minutes was used as control. *P < .05, from control PBS-treated cells. **P < .05, from matched control or IL-6-stimulated cells. Results are expressed as the mean ± SEM of the number of cells per field that migrated to and/or invaded the lower surface of the inserts. Experiments were performed in triplicate per experimental condition and were repeated thrice with similar results.
Discussion
The importance of the reactive tumor microenvironment is becoming increasingly appreciated. A decade ago, LPA was identified as an “ovarian cancer-activating factor,” and high levels of LPA in the ascitic fluid and serum of patients have been correlated with poor prognosis of the disease [22–24]. In addition to the crucial paracrine effects of LPA on the initiation and maintenance of the reactive tumor microenvironment required for tumor progression, a positive autocrine feedback loop between peritoneal mesothelial cells and ovarian cancer cells has been reported [10,35]. Herein, we have identified a novel function of the matricellular protein SPARC in blocking the tumor-promoting effect of LPA at multiple levels. The most pronounced effect of SPARC was found to be its inhibition of the chemotactic and proinvasive effects of both LPA and peritoneal mesothelial cell conditioned medium on ovarian cancer cells. This effect was observed not only with SPARC added exogenously to ovarian cancer cells but also when the expression of SPARC was rescued ectopically in these cells by adenoviral transduction. These results are in agreement with our recent findings on the counteradhesive effects of SPARC and its inhibitory effect on survival signaling in ovarian cancer cell lines, in response to serum and epidermal growth factor stimulation [18] (Said et al., unpublished observations).
Pleiotropic biologic effects of LPA are mediated through its interaction with cognate G protein-coupled receptors, namely, LPA1/Edg 2, LPA2/Edge4, and LPA3/Edg7. The expression of LPA2/Edg 4 and LPA3/Edge7, which are not expressed in normal ovarian epithelial cells, is upregulated in ovarian cancer cells and has been correlated with deleterious effects of the disease. However, LPA1/Edg2 has been shown to be a negative regulator of ovarian cancer progression [26,36]. Our data suggest that the effect of SPARC on the abrogation of the mitogenic, migratory, and prosurvival effects of LPA may be mediated, at least in part, through perturbation of the expression of LPA1/Edg2 and LPA2/Edg4 receptors, the balance of which was reported to determine the net effect of LPA on ovarian cancer [26]. LPA is known to induce proinflammatory cytokine production in different tumors, with IL-6 as one of the main cytokines implicated in tumor survival, migration and angiogenesis [7,15,27,34,37]. LPA-induced upregulation of IL-6 in SKOV3 cell line has been shown to be mediated through the activation of the Gi/PI-3K/AKT pathway [7]. Therefore, our finding that SPARC exerts a potent inhibitory effect on both basal and LPA-stimulated proinflammatory cytokine production not only from SKOV3 cells but also from OVCAR3 cells strongly suggests that these effects of SPARC are mediated through perturbation of the balance between LPA receptor expression and inhibition of LPA-induced AKT activation.
The high levels of IL-6 detected in the ascitic fluid of ovarian cancer patients have been correlated with poor prognosis and, until recently, the source of these high levels had remained unidentified. The high basal levels of IL-6 in the peritoneal mesothelial cell conditioned medium reported herein and in other studies [31,32] can be correlated with the constitutive production of biologically active LPA from mesothelial cells [10], augmenting the chemotactic and proadhesive effects of LPA on tumor cells. As shown in this study, a pronounced synergistic increase in IL-6 secretion occurs on mesothelial-tumor cell contact, further maintaining the vicious cycle of disease cascade. Interestingly, rescue of SPARC expression in ovarian cancer cells significantly attenuated this dramatic increase in IL-6 secretion, albeit not to that of basal levels produced by either ovarian cancer cells or mesothelial cells. In addition to the pronounced inhibitory effect of SPARC on IL-6 production by ovarian cancer cells, SPARC had a negative regulatory role on levels of IL-6sR—an IL-6 agonist implicated in IL-6 trans-signaling [38]. Moreover, SPARC inhibited the basal and LPA-induced production of MCP-1, a major chemoattractant of peritoneal macrophages, thus suppressing another significant source of LPA and IL-6 production in ascitic fluid. Collectively, our data suggest that the role of SPARC as a negative regulator of IL-6 is mainly mediated through the drastic diminution of its production from ovarian cancer cells and other cell types in the microenvironment of peritoneal ovarian carcinomatosis. Further studies are warranted for the in vivo use of SPARC, not only to block the tumor-promoting effects of IL-6 but also to alleviate associated psychosocial disturbances and to improve the quality of life of ovarian cancer patients.
In summary, we have provided evidence that through interference with mesothelial cancer cell crosstalk and concomitant attenuation of LPA activities, SPARC is implicated as a crucial player in the normalization of the reactive microenvironment of peritoneal ovarian carcinomatosis. Our finding that rescuing the expression of SPARC in ovarian cancer cells through gene transfer antagonized the tumor-promoting properties of LPA and its downstream effector IL-6, combined with its established antiproliferative, proapoptotic, and antimetastatic properties, highlights its therapeutic potential as a promising novel inhibitor of peritoneal ovarian carcinomatosis.
Acknowledgement
We thank Y. A. Daaka for critical reading of the manuscript.
Footnotes
This work was supported, in part, by the Georgia Cancer Coalition (grant GCC0023 to K.M.) and the National Institutes of Health (grant HL074279 to D.J.F. and grant K01-CA089689 to K.M.).
References
- 1.Hsia D, Lim S, Bernard-Trifilo J, Mitra S, Tanaka S, den Hertog J, Streblow D, Ilic D, Ginsberg M, Schlaepfer D. Integrin alpha4beta1 promotes focal adhesion kinase-independent cell motility via alpha4 cytoplasmic domain-specific activation of c-Src. Mol Cell Biol. 2005;25:9700–9712. doi: 10.1128/MCB.25.21.9700-9712.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz B, Hong G, Morrison B, Wu W, Baudhuin L, Xiao Y, Mok S, Xu Y. Lysophospholipids increase interleukin-8 expression in ovarian cancer cells. Gynecol Oncol. 2001;81:291–300. doi: 10.1006/gyno.2001.6124. [DOI] [PubMed] [Google Scholar]
- 3.Guo R, Kasbohm E, Arora P, Sample C, Baban B, Sud N, Sivashanmugam P, Moniri N, Daaka Y. Expression and function of lysophosphatidic acid LPA1 receptor in prostate cancer cells. Endocrinology. 2006;147:4883–4892. doi: 10.1210/en.2005-1635. [DOI] [PubMed] [Google Scholar]
- 4.Bian D, Su S, Mahanivong C, Cheng R, Han Q, Pan Z, Sun Pand Huang S. Lysophosphatidic acid stimulates ovarian cancer cell migration via a Ras-MEK kinase 1 pathway. Cancer Res. 2004;64:4209–4217. doi: 10.1158/0008-5472.CAN-04-0060. [DOI] [PubMed] [Google Scholar]
- 5.Li H, Ye X, Mahanivong C, Bian D, Chun J, Huang S. Signaling mechanisms responsible for lysophosphatidic acid-induced urokinase plasminogen activator expression in ovarian cancer cells. J Biol Chem. 2005;280:10564–10571. doi: 10.1074/jbc.M412152200. [DOI] [PubMed] [Google Scholar]
- 6.Frankel A, Mills G. Peptide and lipid growth factors decrease cis-diaminedichloroplatinum-induced cell death in human ovarian cancer cells. Clin Cancer Res. 1996;2:1307–1313. [PubMed] [Google Scholar]
- 7.Chou C, Wei L, Kuo M, Huang Y, Lai K, Chen C, Hsieh C. Up-regulation of interleukin-6 in human ovarian cancer cell via a Gi/PI3K-Akt/NF-kappaB pathway by lysophosphatidic acid, an ovarian cancer-activating factor. Carcinogenesis. 2005;26:45–52. doi: 10.1093/carcin/bgh301. [DOI] [PubMed] [Google Scholar]
- 8.Westermann A, Beijnen J, Moolenaar W, Rodenhuis S. Growth factors in human ovarian cancer. Cancer Treat Rev. 1997;23:113–131. doi: 10.1016/s0305-7372(97)90024-4. [DOI] [PubMed] [Google Scholar]
- 9.Shen Z, Belinson J, Morton R, Xu Y, Xu Y. Phorbol 12-myristate 13-acetate stimulates lysophosphatidic acid secretion from ovarian and cervical cancer cells but not from breast or leukemia cells. Gynecol Oncol. 1998;71:364–368. doi: 10.1006/gyno.1998.5193. [DOI] [PubMed] [Google Scholar]
- 10.Ren J, Xiao Y, Singh L, Zhao X, Zhao Z, Feng L, Rose T, Prestwich G, Xu Y. Lysophosphatidic acid is constitutively produced by human peritoneal mesothelial cells and enhances adhesion, migration, and invasion of ovarian cancer cells. Cancer Res. 2006;66:3006–3014. doi: 10.1158/0008-5472.CAN-05-1292. [DOI] [PubMed] [Google Scholar]
- 11.Hodge D, Peng B, Cherry J, Hurt E, Fox S, Kelley J, Munroe D, Farrar W. Interleukin 6 supports the maintenance of p53 tumor suppressor gene promoter methylation. Cancer Res. 2005;65:4673–4682. doi: 10.1158/0008-5472.CAN-04-3589. [DOI] [PubMed] [Google Scholar]
- 12.Ogata A, Chauhan D, Teoh G, Treon S, Urashima M, Schlossman R, Anderson K. IL-6 triggers cell growth via the Ras-dependent mitogen-activated protein kinase cascade. J Immunol. 1997;159:2212–2221. [PubMed] [Google Scholar]
- 13.Yang L, Wang L, Lin H-K, Kan P-Y, Xie S, Tsai M-Y, Wang P-H, Chen Y-T, Chang C. Interleukin-6 differentially regulates androgen receptor transactivation via PI3K-Akt, STAT3, and MAPK, three distinct signal pathways in prostate cancer cells. Biochem Biophys Res Commun. 2003;305:462–469. doi: 10.1016/s0006-291x(03)00792-7. [DOI] [PubMed] [Google Scholar]
- 14.Syed V, Ulinski G, Mok S, Ho SM. Reproductive hormone-induced, STAT3-mediated interleukin 6 action in normal and malignant human ovarian surface epithelial cells. J Natl Cancer Inst. 2002;94:617–629. doi: 10.1093/jnci/94.8.617. [DOI] [PubMed] [Google Scholar]
- 15.Sivashanmugam P, Tang L, Daaka Y. Interleukin 6 mediates the lysophosphatidic acid-regulated cross-talk between stromal and epithelial prostate cancer cells. J Biol Chem. 2004;279:21154–21159. doi: 10.1074/jbc.M313776200. [DOI] [PubMed] [Google Scholar]
- 16.Costanzo E, Lutgendorf S, Sood A, Anderson B, Sorosky J, Lubaroff D. Psychosocial factors and interleukin-6 among women with advanced ovarian cancer. Cancer. 2005;104:305–313. doi: 10.1002/cncr.21147. [DOI] [PubMed] [Google Scholar]
- 17.Bradshaw A, Sage EH. SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J Clin Invest. 2001;107:1049–1054. doi: 10.1172/JCI12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Said N, Motamed K. Absence of host-secreted protein acidic and rich in cysteine (SPARC) augments peritoneal ovarian carcinomatosis. Am J Pathol. 2005;167:1739–1752. doi: 10.1016/S0002-9440(10)61255-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yiu G, Chan W, Ng S, Chan P, Cheung K, Berkowitz R, Mok S. SPARC (secreted protein acidic and rich in cysteine) induces apoptosis in ovarian cancer cells. Am J Pathol. 2001;159:609–622. doi: 10.1016/S0002-9440(10)61732-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu J, Xiao Yj Y, Baudhuin L, Hong G, Xu Y. Role of ether-linked lysophosphatidic acids in ovarian cancer cells. J Lipid Res. 2002;43:463–476. [PubMed] [Google Scholar]
- 21.Sengupta S, Xiao Y, Xu Y. A novel laminin-induced LPA autocrine loop in the migration of ovarian cancer cells. FASEB J. 2003;17:1570–1572. doi: 10.1096/fj.02-1145fje. [DOI] [PubMed] [Google Scholar]
- 22.Xu Y, Gaudette D, Boynton J, Frankel A, Fang X, Sharma A, Hurteau J, Casey G, Goodbody A, Mellors A, et al. Characterization of an ovarian cancer activating factor in ascites from ovarian cancer patients. Clin Cancer Res. 1995;1:1223–1232. [PubMed] [Google Scholar]
- 23.Xu Y, Fang X, Casey G, Mills G. Lysophospholipids activate ovarian and breast cancer cells. Biochem J. 1995;309(Pt 3):933–940. doi: 10.1042/bj3090933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Y, Shen Z, Wiper D, Wu M, Morton R, Elson P, Kennedy A, Belinson J, Markman M, Casey G. Lysophosphatidic acid as a potential biomarker for ovarian and other gynecologic cancers. JAMA. 1998;280:719–723. doi: 10.1001/jama.280.8.719. [DOI] [PubMed] [Google Scholar]
- 25.Xu Y, Xiao Y, Baudhuin L, Schwartz B. The role and clinical applications of bioactive lysolipids in ovarian cancer. J Soc Gynecol Invest. 2001;8:1–13. [PubMed] [Google Scholar]
- 26.Fang X, Gaudette D, Furui T, Mao M, Estrella V, Eder A, Pustilnik T, Sasagawa T, Lapushin R, Yu S, et al. Lysophospholipid growth factors in the initiation, progression, metastases, and management of ovarian cancer. Ann NY Acad Sci. 2000;905:188–208. doi: 10.1111/j.1749-6632.2000.tb06550.x. [DOI] [PubMed] [Google Scholar]
- 27.Fang X, Yu S, Bast R, Liu S, Xu H, Hu S, LaPushin R, Claret F, Aggarwal B, Lu Y, et al. Mechanisms for lysophosphatidic acid-induced cytokine production in ovarian cancer cells. J Biol Chem. 2004;279:9653–9661. doi: 10.1074/jbc.M306662200. [DOI] [PubMed] [Google Scholar]
- 28.Mok S, Chan W, Wong K, Muto M, Berkowitz R. SPARC, an extracellular matrix protein with tumor-suppressing activity in human ovarian epithelial cells. Oncogene. 1996;12:1895–1901. [PubMed] [Google Scholar]
- 29.Plante M, Rubin S, Wong G, Federici M, Finstad C, Gastl G. Interleukin-6 level in serum and ascites as a prognostic factor in patients with epithelial ovarian cancer. Cancer. 1994;73:1882–1888. doi: 10.1002/1097-0142(19940401)73:7<1882::aid-cncr2820730718>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 30.van der Zee A, de Cuyper E, Limburg P, de Bruijn H, Hollema H, Bijzet J, Krans M, de Vries E. Higher levels of interleukin-6 in cystic fluids from patients with malignant versus benign ovarian tumors correlate with decreased hemoglobin levels and increased platelet counts. Cancer. 1995;75:1004–1009. doi: 10.1002/1097-0142(19950215)75:4<1004::aid-cncr2820750416>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 31.Offner F, Obrist P, Stadlmann S, Feichtinger H, Klingler P, Herold M, Zwierzina H, Hittmair A, Mikuz G, Abendstein B, et al. IL-6 secretion by human peritoneal mesothelial and ovarian cancer cells. Cytokine. 1995;7:542–547. doi: 10.1006/cyto.1995.0073. [DOI] [PubMed] [Google Scholar]
- 32.Yao V, Platell C, Hall J. Peritoneal mesothelial cells produce inflammatory related cytokines. ANZ J Surg. 2004;74:997–1002. doi: 10.1111/j.1445-1433.2004.03220.x. [DOI] [PubMed] [Google Scholar]
- 33.Obata N, Tamakoshi K, Shibata K, Kikkawa F, Tomoda Y. Effects of interleukin-6 on in vitro cell attachment, migration and invasion of human ovarian carcinoma. Anticancer Res. 1997;17:337–342. [PubMed] [Google Scholar]
- 34.Nilsson M, Langley R, Fidler I. Interleukin-6, secreted by human ovarian carcinoma cells, is a potent proangiogenic cytokine. Cancer Res. 2005;65:10794–10800. doi: 10.1158/0008-5472.CAN-05-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eder A, Sasagawa T, Mao M, Aoki J, Mills G. Constitutive and lysophosphatidic acid (LPA)-induced LPA production: role of phospholipase D and phospholipase A2. Clin Cancer Res. 2000;6:2482–2491. [PubMed] [Google Scholar]
- 36.Hu Y, Tee M, Goetzl E, Auersperg N, Mills G, Ferrara N, Jaffe R. Lysophosphatidic acid induction of vascular endothelial growth factor expression in human ovarian cancer cells. J Natl Cancer Inst. 2001;93:762–768. doi: 10.1093/jnci/93.10.762. [DOI] [PubMed] [Google Scholar]
- 37.Yun C, Sun H, Wang D, Rusovici R, Castleberry A, Hall R, Shim H. LPA2 receptor mediates mitogenic signals in human colon cancer cells. Am J Physiol Cell Physiol. 2005;289:C2–C11. doi: 10.1152/ajpcell.00610.2004. [DOI] [PubMed] [Google Scholar]
- 38.Kallen K-J. The role of transsignalling via the agonistic soluble IL-6 receptor in human diseases. Biochim Biophys Acta Mol Cell Res. 2002;1592:323–343. doi: 10.1016/s0167-4889(02)00325-7. [DOI] [PubMed] [Google Scholar]