Abstract
The anaphase-promoting complex/cyclosome (APC) is a tightly cell cycle-regulated ubiquitin-protein ligase that targets cyclin B and other destruction box-containing proteins for proteolysis at the end of mitosis and in G1. Recent work has shown that activation of the APC in mitosis depends on CDC20, whereas APC is maintained active in G1 via association with the CDC20-related protein CDH1. Here we show that the mitotic activator CDC20 is the only component of the APC ubiquitination pathway whose expression is restricted to proliferating cells, whereas the APC and CDH1 are also expressed in several mammalian tissues that predominantly contain differentiated cells, such as adult brain. Immunocytochemical analyses of cultured rat hippocampal neurons and of mouse and human brain sections indicate that the APC and CDH1 are ubiquitously expressed in the nuclei of postmitotic terminally differentiated neurons. The APC purified from brain contains all core subunits known from proliferating cells and is tightly associated with CDH1. Purified brain APCCDH1 has a high cyclin B ubiquitination activity that depends less on the destruction box than on the activity of mitotic APCCDC20. On the basis of these results, we propose that the functions of APCCDH1 are not restricted to controlling cell-cycle progression but may include the ubiquitination of yet unidentified substrates in differentiated cells.
Keywords: cyclosome, cell cycle, differentiation, proteolysis, ubiquitin
The anaphase-promoting complex/cyclosome (APC) is a multisubunit complex that was first discovered as a cell cycle-regulated ubiquitin-protein ligase specific for B-type cyclins, the activating subunits of the mitotic cyclin-dependent kinase 1 (CDK1) (1–3). Together with ubiquitin-activating (E1) and ubiquitin-conjugating (E2) enzymes, the APC catalyzes the assembly of polyubiquitin chains on cyclin B and thus targets this protein for degradation by the 26S proteasome. Subsequent work has shown that the APC also ubiquitinates several other cell-cycle proteins, including inhibitors of anaphase, mitotic protein kinases, the APC activator CDC20, spindle-associated proteins, and inhibitors of DNA replication (reviewed in refs. 4, 5). Many of these proteins contain a short sequence element that is required for the ubiquitination by the APC and is therefore called the destruction box (D box) (6).
The ability of the APC to support ubiquitination reactions is tightly regulated during the cell cycle, being low in S, G2, and early M phase but high from late metaphase until the end of G1 (2, 3, 7, 8). The activation of the APC in mitosis depends on a protein known as CDC20, p55CDC or Fizzy, whereas the APC is kept active between the end of mitosis and the end of G1 via association with a protein related to CDC20, called CDH1, HCT1 or Fizzy-related (reviewed in refs. 4, 5). In yeast, the maintenance of APC activity in G1 has been found to be important to prevent premature entry into S phase (9, 10).
In addition to the APC, eukaryotic cells utilize a second ubiquitination complex to control cell-cycle progression, the Skp1-cullin-F box protein complex (SCF; reviewed in ref. 11). SCF-dependent ubiquitination reactions have been shown to be important for the G1/S transition and for controlling the half-life of several cell-cycle proteins such as CDK inhibitors and G1-specific cyclins. Genes encoding several subunits and cofactors of both the APC (CDC16, CDC23, CDC20, CDC27) and the SCF (CDC4, CDC34, CDC53) were first identified through the isolation of cell division cycle (cdc) genes in budding yeast (12), a fact that emphasizes the essential function of these protein complexes in the cell cycle. Recent work has shown, however, that SCF complexes also target noncell-cycle proteins for ubiquitin-dependent proteolysis and therefore have a much broader variety of cellular functions than previously anticipated (11). In contrast, the APC pathway is presently known only as a mitosis and G1-specific proteolysis system of proliferating cells. This view is supported by the fact that almost all known APC substrates are proteins that function in mitosis and by the observation that the expression of mammalian CDC20 is restricted to proliferating cells (13).
Two previous observations hinted at the possibility that the function of the APC may not be restricted to controlling mitosis and G1: first, a fusion protein of cyclin B and chloramphenicol acetyl transferase, but not the corresponding D box mutant, was unstable when ectopically expressed in cultured myoblasts differentiated in vitro, consistent with the idea that the APC may be active in such cells (8). Second, the mRNA levels of the mammalian APC subunits Tsg24 (APC1) and CDC23 (APC8) are high in brain tissue (14, 15), raising the possibility that some APC subunits may be expressed in terminally differentiated cells. Here we have systematically analyzed the expression of the APC and its activator proteins in different mammalian tissues. We find that APC and CDH1, but not CDC20, are expressed in a variety of tissues that are predominantly composed of differentiated cells, such as adult brain. We show that APCCDH1 exists in nuclei of postmitotic neurons and can be purified from brain extracts as a highly active ubiquitin–protein ligase. These results raise the unexpected possibility that the functions of APCCDH1, like the functions of certain SCF complexes, may not be restricted to controlling cell-cycle progression.
MATERIALS AND METHODS
Cells and Antibodies.
HeLa, EpH4, and MDCK cells and primary rat hippocampal neurons were cultured as described (16, 17). Monoclonal CDC27 and polyclonal CDK1 and CDC20 (C-terminal peptide) antibodies were from Santa Cruz Biotechnology. Antibodies against CDH1, CDC16, CDC23, and APC10 have been described (17, 18). Monoclonal mouse APC2–30 antibodies were raised against a recombinant C-terminal fragment of human APC2 by using standard procedures. Additional polyclonal rabbit antibodies were raised against a recombinant C-terminal fragment of human APC7 and against recombinant full length human CDC20 and CDH1.
Extract Preparation and Fractionation.
Extracts from cultured cells and from tissues of adult mice were prepared in immunoprecipitation (IP) buffer (20 mM Tris⋅HCl, pH 7.5/100 mM NaCl/0.2% Nonidet P-40/20 mM β-glycerophosphate/10% glycerol/1 mM NaF/0.5 mM DTT). Fresh cow brain was homogenized in QA buffer (17) by using a Potter–Elvejem glass–Teflon homogenizer. High-speed supernatants of brain extracts were fractionated on a 60 ml Source Q anion exchange column.
IP.
CDC27 antibody beads (17) were incubated with extracts diluted in IP buffer. Beads were washed four times with IP buffer, and bound proteins were eluted with 100 mM glycine⋅HCl, pH 2.0, and analyzed by SDS/PAGE and immunoblot analysis. For silver staining, beads were washed three times with IP buffer and six times with IP buffer + 500 mM NaCl before elution and SDS/PAGE.
Immunofluorescense and Immunohistology.
For immunofluorescence analysis, mouse brain sections and cultured cells grown on coverslips were fixed with 4% paraformaldehyde in PBS for 10 min at room temperature and incubated with 50 mM NH4Cl for 5 min. Specimens were subsequently washed, permeabilized with PBS + 0.1% (vol/vol) Triton X-100 for 10 min, and incubated with primary antibodies diluted in PBS + 1% BSA for 20 min. Secondary antibodies were labeled with FITC or Cy3 (Sigma; Molecular Probes). Specimens were embedded in Moviol 4–88 (Hoechst Pharmaceuticals) supplemented with 1 μg/ml 4′,6-diamidino-2-phenylindole (DAPI). For immunohistological analysis, adult mouse brain sections were fixed with 4% paraformaldehyde and processed as described by using peroxidase-conjugated secondary antibodies (19). Paraffin sections were deparaffinized before incubation with antibodies. All tissue sections were counterstained with eosin/hematoxilin.
Ubiquitination Assays.
Ubiquitination assays were performed as described (17). As substrates, we used either an iodinated N-terminal recombinant fragment of sea-urchin cyclin B (amino acids 13 to 110; ref. 20) or a similar fragment of Xenopus cyclin B1 (amino acids 1–102; ref. 21) or the corresponding D box mutants. Quantitative radioactive immunoblotting with APC2 and APC10 antibodies was used to calculate specific ubiquitination activities.
RESULTS
Expression of APC Core Subunits and CDC20 and CDH1 in Mammalian Tissues.
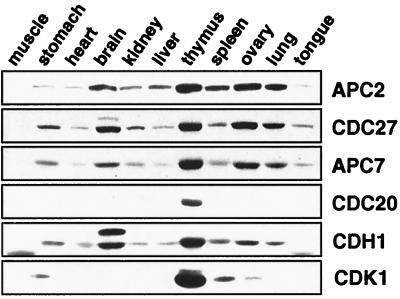
The cell-cycle functions of the APC and its activator proteins CDC20 and CDH1 have been analyzed in proliferating animal cells and in unicellular fungi, but whether the APC is expressed in differentiated tissues of multicellular organisms, and if so where, is not known. To address these questions, we analyzed protein extracts from 11 different adult mouse tissues by immunoblotting with antibodies to several core subunits of the human APC and to CDC20 and CDH1 (Fig. 1). In agreement with the original report on human CDC20 (13) and as expected for a nonabundant regulatory cell-cycle protein, CDC20 was detectable only in thymus, a tissue that contains many proliferating cells. In contrast, CDH1 and the APC core subunits APC2, CDC27, and APC7 could be detected in all tissue extracts, although in variable amounts. These proteins were barely detectable in skeletal muscle, heart, and tongue extract and were present in low amounts in stomach, kidney, and liver, whereas the highest amounts were found in extracts from thymus, spleen, ovary, lung, and brain (longer exposure times of the immunoblot in Fig. 1 revealed APC reactivity also in skeletal muscle extracts [not shown]). The expression level of CDH1 and of APC core subunits therefore differs significantly from that of other cell-cycle proteins such as CDK1 and PLK1 (Fig. 1 and not shown) whose expression is restricted to tissues with high proliferative indices (22, 23). This observation raised the unexpected possibility that the expression of APC and CDH1 may not be restricted to proliferating cells.
Figure 1.
Analysis of APC expression in mouse tissues. Equal amounts of protein from 10,000 × g supernatant fractions from different mouse tissue extracts were separated by SDS/PAGE and analyzed by immunoblotting by using antibodies to the indicated proteins. The CDH1 crossreactive band of slower mobility detected in brain extract neither cofractionated nor coimmunoprecipitated with APC (see Fig. 4B and not shown).
APC and CDH1 Are Expressed in Terminally Differentiated Neurons.
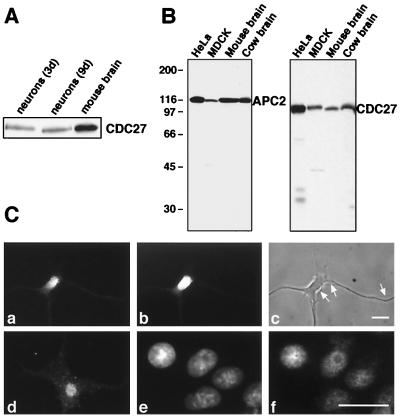
The lack of correlation between APC levels and proliferative index was particularly striking in the case of adult brain, a tissue in which cell division occurs only very rarely (24). We therefore further investigated the presence of APC core subunits and CDH1 in brain. To analyze whether these proteins are expressed in postmitotic neurons, we first used a cell-culture system that allows the in vitro differentiation of neuronal precursor cells explanted from embryonic rat hippocampus. In the absence of mitogens, these neuronal precursors never divide but differentiate into cells that resemble hippocampal neurons by numerous morphological and molecular criteria (16). Immunoblot analyses of cell lysates prepared from hippocampal neurones differentiated for 3 and 9 days in culture showed a constant expression level of the APC core subunit CDC27 (Fig. 2A), suggesting that APC expression is not down-regulated during postmitotic neuronal differentiation.
Figure 2.
Expression of APC in hippocampal neurons. (A) Immunoblot analysis of CDC27 in extracts from rat hippocampal neurons differentiated in vitro for 3 or 9 days and in mouse brain extract. (B) Characterization of monoclonal APC antibodies. Protein extracts from HeLa and MDCK cells and from mouse and cow brain were analyzed by immunoblotting with antibodies APC2–30 and CDC27. (C) Immunofluorescence microscopy of rat hippocampal neurons differentiated in vitro for 1 wk (a–d) and of cultured MDCK cells (e and f), by using APC2–30 (a and f) and CDC27 antibodies (d). b and c show the same neuron as in a stained with DAPI and visualized by phase contrast microscopy, respectively. The cell in c shows the typical morphology of a stage 3–4 neuron (16) with three dendrites that are thick at the base and taper with distance from the cell body, whereas thin axons of uniform diameter (indicated by arrows) are running along the dendrites. Cells in e are the same as in f, stained with DAPI. Size bars = 10 μm.
To analyze the intracellular distribution of the APC, we next performed immunofluorescence microscopy. For this purpose, we raised monoclonal antibodies to APC2. The antibody APC2–30 reacted exclusively with APC2 in whole-cell extracts from HeLa and MDCK cells and in tissue extracts from mouse and cow brain (Fig. 2B) and thus allowed reliable immunolocalization of the APC. In addition, we used a monoclonal CDC27 antibody that showed only minor nonspecific crossreactions in immunoblots (Fig. 2B). In proliferating cultured cells such as MDCK, EpH4, and HeLa, both the APC2–30 and the CDC27 antibodies yielded a diffuse staining in interphase nuclei (Fig. 2C-f and not shown). The staining was reduced in nucleolar regions, and only little staining was observed in the cytoplasm. In agreement with the previous localization of CDC27 and CDC16 in LLC-PK cells (25), these data suggest that APC resides predominantly in the nuclei of proliferating cells.
In cultured postmitotic hippocampal neurons, the APC2–30 antibodies also showed a strong nuclear staining, whereas little or no staining was observed in the neuronal cytoplasm and in dendrites and axons (Fig. 2C-a). Similar results were obtained with the monoclonal CDC27 antibodies, except that these antibodies also showed a weak punctate staining in the neuronal cytoplasm (Fig. 2C-d). The significance of the cytoplasmic CDC27 signal remains presently unclear because the APC2–30 antibodies did not reveal cytoplasmic staining. We conclude from these results that APC subunits are expressed in the nuclei of postmitotic hippocampal neurons.
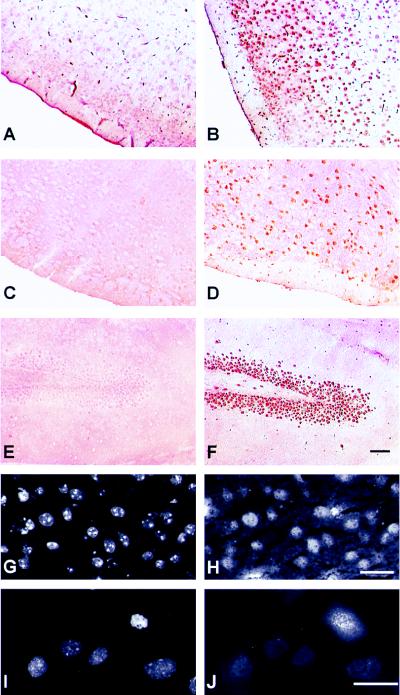
To rule out that the expression of APC subunits in hippocampal neurons is an artifact of the in vitro cell-culture conditions, we next analyzed tissue sections of adult mouse brain. Immunohistochemical peroxidase reactions with either paraffin embedded or frozen sections incubated with the APC2–30 and the CDC27 monoclonal antibodies showed a nuclear staining pattern that was absent when secondary antibodies alone were used (Fig. 3B). The nuclear staining was observed in different parts of the brain, including the cortex, the cerebellum, and the hippocampus (Fig. 3B and not shown). A similar staining pattern was observed when sections from human adult cortex were analyzed (not shown). Double staining of mouse brain sections with DAPI and with fluorescently labeled antibodies revealed that most if not all nuclei present in these sections reacted with APC antibodies (Fig. 3 G and H).
Figure 3.
Expression of APC and CDH1 in adult mouse brain. Paraffin sections (A–F) or frozen sections (G and H) of mouse brain were analyzed by peroxidase immunohistochemistry (A–F) or by immunofluorescence microscopy (G and H). Sections were incubated with antibodies to CDC27 (B), CDC20 (C and E), CDH1 (D and F), APC2 (H), or with secondary antibodies alone (A). G shows the same section as in H stained with DAPI. Sections in A–F were counterstained with hematoxilin/eosin. I and J show cultured mouse EpH4 cells stained with DAPI (I) or with CDC20 antibodies (J). Size bars = 10 μm for G–J, 50 μm for A–F.
We also performed immunohistochemical reactions on mouse brain sections incubated with either CDC20 or CDH1 antibodies. As predicted by our biochemical results (Fig. 1), no staining was observed by using the CDC20 antibodies (Fig. 3 C and E), although these antibodies are well suited for the immunolocalization of CDC20 in proliferating mouse cells where they stain kinetochores in mitosis (Fig. 3J). In contrast, strong nuclear staining was obtained with the CDH1 antibodies in different regions of the brain section, including the cortex (Fig. 3D) and the hippocampus (Fig. 3F). In many regions of the sections, layers of cells were stained that are typical for the spatial organization of neurons in the brain (for example, Fig. 3F shows neurons of the dentate gyrus in the hippocampus). These results suggest that the APC and CDH1 are ubiquitously expressed in the nuclei of neurons in the adult mammalian brain.
Subunit Composition of Brain APC.
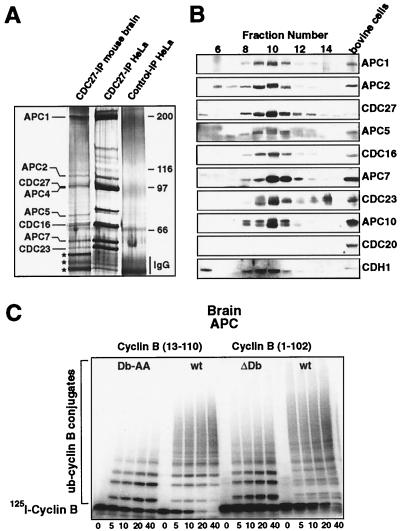
To address whether the APC subunits detected in brain are part of a multisubunit complex as they are in proliferating cells, and to investigate whether this complex is physically associated with CDH1, we immunopurified the APC from adult mouse brain extracts and compared its subunit composition with the APC purified from logarithmically growing HeLa cells. After SDS/PAGE and silver staining, polypeptide bands that comigrated with the HeLa APC subunits APC1, APC2, CDC27, APC4, APC5, CDC16, APC7, and CDC23 were clearly present in the purified brain APC fraction (Fig. 4A). These subunits cosedimented in density-gradient centrifugation experiments corresponding to an S value of 20–25S (not shown). In addition, a few unidentified polypeptide bands coimmunoprecipitated with brain APC (marked by stars in Fig. 4A), but the significance of this observation is presently unclear, because the amounts of these proteins varied in different experiments.
Figure 4.
Biochemical and enzymatic characterization of brain APCCDH1. (A) Identification of APC subunits by silver staining: immunoprecipitates obtained with affinity-purified rabbit CDC27 antibodies from logarithmically growing HeLa cells or total mouse brain extracts were analyzed by SDS/PAGE and silver staining. The three proteins marked with stars could not reproducibly be precipitated. (B) Immunoblot analysis of cow brain proteins separated by Q-column anion exchange chromatography, by using antibodies to the indicated proteins. As a positive control, proteins from bovine cells enriched in mitosis by nocodazole treatment were analyzed side by side. (C) Brain APC is an active ubiquitin ligase. Immunopurified cow brain APC was used to ubiquitinate radiolabeled cyclin B. Samples taken at the indicated time points were analyzed by SDS/PAGE and PhosphorImaging (Molecular Dynamics). N-terminal recombinant fragments of sea urchin (cyclin B 13–110) and Xenopus (cyclin B 1–102) cyclin B were used as substrates. The corresponding D box mutants 1–102 ΔDb and 13–110 Db-AA were analyzed side by side.
Neither CDH1 nor the recently identified low molecular weight subunit APC10 could be detected in these experiments (these proteins are also difficult to detect in APC from proliferating cells; ref. 18). We therefore enriched the APC by anion exchange chromatography from cow brain extracts and analyzed each fraction by immunoblotting with specific antibodies to these proteins. Fig. 4B shows that APC10 and CDH1 coeluted with the other APC core subunits, indicating that they are also part of the brain APC. This was confirmed by immunoprecipitating the APC from these column fractions that showed that APC10 and CDH1 are physically associated with other APC core subunits (not shown, but see Fig. 5B for a similar experiment).
Figure 5.
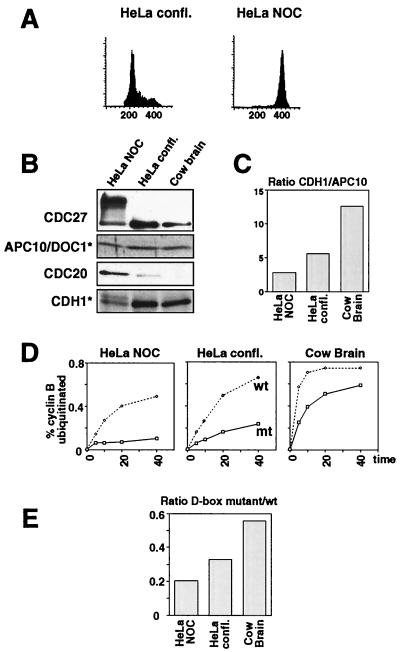
Analysis of the D box dependence of brain APC. (A) Flow cytometric analysis of HeLa cells: HeLa cells were enriched in G1 phase by growth to confluency or arrested in mitosis by treatment with nocodazole, and their DNA contents was analyzed by flow cytometry. (B) Analysis of CDC20 and CDH1 association with APC: APC immunoprecipitates from extracts of confluent and mitotic HeLa cells and from cow brain Q10 fraction were analyzed by immunoblotting with CDC20, CDH1, APC10/DOC1, and CDC27 antibodies. Immunoblots marked with * (CDH1 and APC10) were performed by using an iodinated secondary antibody. (C) Relative ratio of CDH1/APC10 by using the data of the immunoblot shown in B, quantitated by PhosphorImaging. (D) Kinetics of ubiquitination reactions: ubiquitination assays by using immunoprecipitated APC from cow brain, confluent and mitotic HeLa cells were performed with cyclin B 13–110 wild type (wt) or 13–110 Db-AA mutant as described for Fig. 4C. The percentage of cyclin B-ubiquitin conjugates was quantitated for each time point. (E) Ratios of ubiquitination activities toward cyclin B Db-AA vs. activities toward wt cyclin B, calculated for the time point at 10 min.
Although our CDC20 antibodies recognized bovine CDC20 in proliferating cells, no CDC20 signal could be detected in the enriched APC fractions from cow brain (Fig. 4B), further confirming that CDC20 is not expressed in brain. We conclude that all known core subunits of the APC are part of the brain APC and that CDH1 is physically associated with this complex. We refer to this complex as brain APCCDH1.
Ubiquitination Activity of Brain APCCDH1.
To address whether brain APCCDH1 is a functional ubiquitin–protein ligase, we analyzed complexes immunopurified from extracts of adult mouse and cow brain for their ability to ubiquitinate other proteins in the presence of purified E1 and E2 enzymes. Because no physiologic substrates of brain APCCDH1 are known, we used radiolabeled N-terminal fragments of cyclin B as model substrates in these experiments. Fig. 4C shows that immunopurified brain APCCDH1 was able to rapidly and completely catalyze the polyubiquitination of two different cyclin B fragments, indicating that this complex is a functional ubiquitin-protein ligase.
We noticed in these experiments that brain APCCDH1 is able to ubiquitinate not only wild-type cyclin B fragments but also fragments in which the D box has been mutated (Fig. 4C). It has recently been reported that addition of in vitro translated CDH1 to Xenopus APC allows the ubiquitination of mutated cyclin B fragments that lack an intact D box, but whether native APCCDH1 complexes can also ubiquitinate substrates in a D box-independent manner is not known. To address this question, we compared the abilities of brain APCCDH1 and of APCCDC20 and APCCDH1 isolated from HeLa cells to ubiquitinate wild-type and mutant cyclin B fragments (Fig. 5).
To isolate native active APCCDC20, we enriched HeLa cells in metaphase by nocodazole treatment (Fig. 5A; although cyclin B is stable in nocodazole-treated cells, APC immunoprecipitated from extracts of such cells is active, presumably because the inhibitory MAD2 protein is removed under these conditions; ref. 17). As a source for APCCDH1, we used confluent HeLa cells that are predominantly in G1 (Fig. 5A). APC was immunoprecipitated from the resulting cell extracts and analyzed for the presence of CDC20 and CDH1 (Fig. 5 B and C). As expected, the highest amounts of CDC20 were associated with APC from mitotically arrested HeLa cells, whereas only little or no CDC20 could be detected in APC from confluent cells and from brain, respectively. Contrary to this, more CDH1 was associated with the APC from confluent cells and from brain than with the APC from mitotic cells (Fig. 5B). Using radiolabeled secondary antibodies, we quantitated the amounts of CDH1 and of the APC core subunit APC10 in these preparations and calculated the CDH1 to APC10 ratios. This analysis revealed that cow brain APC is associated with the highest relative amount of CDH1, followed by APC from confluent and from mitotic HeLa cells (Fig. 5C). Similar results were obtained when the CDH1 to APC2 ratio was measured in the same way (not shown).
To analyze to what degree the activities of these complexes depend on a functional D box in the substrate, we determined the ubiquitination activities of all three complexes by using either a wild-type cyclin B fragment or a fragment containing two point mutations in the D box as substrates (Fig. 5D). All three complexes ubiquitinated the wild-type cyclin better than the D box mutant, but APC from confluent HeLa cells and from brain also showed significant activities toward the D box mutant. We also observed repeatedly in these experiments that brain APCCDH1 had a higher specific activity than APC complexes from HeLa cells (compare Fig. 5 B and D). To be able to directly compare the D box dependence of all three complexes, we calculated the ratio of activity toward the D box mutant to the activity obtained with wild-type substrate (Fig. 5E). Early data points from the linear parts of the activity curves shown in Fig. 5D were chosen for the analysis. This calculation revealed that APC from mitotic HeLa cells had the lowest relative activity toward the cyclin D box mutant, whereas brain APC showed the highest relative activity toward this substrate. The D box dependence (Fig. 5E) of the complexes analyzed in this experiment did therefore correlate with the amounts of CDH1 associated with the different forms of the APC (Fig. 5C). Similar results were obtained when a cyclin B mutant was used in which the entire D box has been deleted (not shown). These results suggest that the activity of brain APCCDH1 is not strictly dependent on the D box, and they are consistent with the notion that the amounts of CDH1 determine the D box dependence of the APC.
DISCUSSION
We have found that the CDH1-associated form of the APC is expressed in a variety of mammalian tissues and that the levels of APC in these tissues do not correlate with the proliferative indices of these tissues, suggesting that the expression of APCCDH1 is not restricted to proliferating cells. Consistent with this idea, our histologic and biochemical data clearly show that APCCDH1 exists in the nuclei of postmitotic neurons. Our observation that APCCDH1 purified from brain is highly active in vitro, together with the previous observation that ectopically expressed model substrates of the APC are unstable in another postmitotic cell type, cultured C2 myoblasts (8), strongly suggests that APCCDH1 functions as a ubiquitin–protein ligase in at least some differentiated cell types in vivo. Whether APCCDH1 is expressed only in a subset of differentiated cell types or is ubiquitously found in postmitotic cells is not yet known, but our detection of APC subunits in all mammalian tissues tested so far (Fig. 1) would be consistent with the latter possibility.
These results modify our current view of the APC pathway. The APC and its activator protein CDC20 have been shown to trigger the initiation of anaphase, whereas CDH1 was so far believed to maintain APC activity from the end of mitosis until the end of G1 in proliferating cells. Consistent with this view, all presently known APC substrates function in the cell cycle and in several cases are expressed only in proliferating cells. Our results confirm that the expression of CDC20 is restricted to proliferating cells (13) and are thus consistent with the model that the main function of CDC20 is to initiate anaphase. However, our characterization of postmitotic neurons raises the unexpected possibility that APCCDH1 may also have a role in differentiated cells. Although we cannot formally exclude that APCCDH1 exists in postmitotic neurons purely as a nonfunctional remnant that has been left over from the mitotic past of the cells, we consider this possibility unlikely because we were able to detect APC even in neurons of adult human patients, i.e., in cells that had presumably lived for decades since their last mitotic division. Also the observation that not all components of the basic cell-cycle machinery are expressed in postmitotic neurones (see below) argues against this possibility.
Why then is APCCDH1 expressed in postmitotic neurons and perhaps in other differentiated cell types? The answer to this question is not known, but we can envision two possibilities that are not mutually exclusive. One hypothesis is that the function of APCCDH1 in differentiated cells would not be any different from its function in G1 in proliferating cells. In fission and budding yeast, genetic experiments indicate that APC function is required to prevent premature entry into S phase, perhaps by helping to keep the levels of mitotic cyclins low (9, 10). Whether APC performs a similar function in G1 in metazoans is not known, but it is conceivable that the APC would help to maintain the differentiated state by keeping the levels of proteins low that could otherwise activate entry into the cell cycle. Obvious candidates for such activators would be cyclin A and B, both of which activate CDKs and both of which are believed to be APC substrates. The finding that the levels of CDK inhibitors such as p27 are up-regulated in many differentiated cells (26) supports the notion that keeping CDKs inactive may be of crucial importance for differentiated cells.
If this hypothesis were true and APCCDH1 and CDK inhibitors collaborated in keeping CDKs inactive, it would raise another puzzling question, namely why cell-cycle activators such as cyclins would not simply be down-regulated at the transcriptional level during differentiation. In many differentiated cell types, including neurons, only some cell-cycle regulators are transcriptionally down-regulated, for example CDK1, CDK2, cyclin A, and PLK1 (22, 23), whereas many others, including cyclin B, are not (27, 28). On the basis of this surprising finding and the observation that the activation of apoptosis often correlates with and in some cases even depends on the activation of mitotic kinases (reviewed in refs. 28, 29), it has been proposed that some cell-cycle genes continue to be expressed in differentiated cells to allow rapid induction of apoptosis if required (28). This hypothesis has not yet been critically tested, but it predicts that APCCDH1 would have to be inactivated to allow apoptosis to occur in differentiated cells. Indeed, caspase-dependent cleavage of APC subunits and subsequent APC inactivation have been found to be early events during apoptosis and to correlate with an increase in CDK activity (29). A second situation in which mitotic kinases appear to be reactivated in postmitotic cells has been observed in neurons of Alzheimer’s patients (30). It will therefore be interesting to analyze whether the activity of APCCDH1 is altered under these pathologic conditions.
In general, it is worth mentioning that a large variety of human neurodegenerative diseases, including Alzheimer’s, coincide with the accumulation of neuronal proteins in inclusion bodies (reviewed in ref. 31). Because the ubiquitin system apparently fails to degrade these proteins, it has been proposed that defects in the ubiquitin system contribute to the formation of these diseases (discussed in ref. 31). However, it is not known whether abnormal protein accumulation is a cause or a consequence of these diseases. Whether neuronal APC may have a role in any of these pathologic situations remains to be seen.
The second hypothesis of why APCCDH1 may be expressed in postmitotic neurons is that there could be one or several classes of APC substrates that are not yet known and that may not have any roles in the cell cycle. Although this hypothesis is purely speculative, there are two observations that make it conceivable: first, the characterization of the SCF provides clear precedence for a case in which a ubiquitin–protein ligase originally discovered as a cell-cycle regulator has more pleiotropic functions than could be predicted from the original genetic and biochemical phenotypes (reviewed in ref. 11). Second, addition of in vitro-translated CDH1 to the APC has been found to allow the ubiquitination of cyclin B destruction box mutants in vitro (32), and we have here extended these observations by showing that native APCCDH1 complexes also show a reduced dependence on the D box (Fig. 5). These results do not imply that cyclin B can be destroyed in vivo in a D box-independent manner, but they argue for a difference in the substrate specificity between CDC20 [which acts strictly D box dependent (32)] and CDH1 and would therefore be consistent with the idea that APCCDH1 may target unknown noncell-cycle proteins for destruction in differentiated cells. The identification of APCCDH1-specific substrates and the analysis of APCCDH1 mutants in multicellular organisms will be required to test these hypotheses.
Acknowledgments
We are grateful to I. Waizenegger and I. Botto for help with the generation of monoclonal antibodies, to K.-H. Heider for providing human brain sections, to M. Sibilia, U. Mühlner, and W. Jochum for help with mouse tissues and sections, to B. Hellias for help with the neuronal cell cultures, to W. Franke for primary bovine cells, and to C. Höög for APC1 antibodies. We thank K. Nasmyth, K. Wirth, and W. Zachariae for comments on the manuscript. This work was supported by a grant from the Austrian Industrial Research Promotion Fund (FFF 3/12801) to J.-M.P.
ABBREVIATIONS
- APC
anaphase-promoting complex
- CDK
cyclin-dependent kinase
- D box
destruction box
- SCF
Skp1-cullin-F box protein complex
- DAPI
4′,6-diamidino-2-phenylindole
- IP
immunoprecipitation
References
- 1.Irniger S, Piatti S, Michaelis C, Nasmyth K. Cell. 1995;81:269–278. doi: 10.1016/0092-8674(95)90337-2. [DOI] [PubMed] [Google Scholar]
- 2.King R W, Peters J-M, Tugendreich S, Rolfe M, Hieter P, Kirschner M W. Cell. 1995;81:279–288. doi: 10.1016/0092-8674(95)90338-0. [DOI] [PubMed] [Google Scholar]
- 3.Sudakin V, Ganoth D, Dahan A, Heller H, Hershko J, Luca F C, Ruderman J V, Hershko A. Mol Biol Cell. 1995;6:185–197. doi: 10.1091/mbc.6.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peters J-M. Exp Cell Res. 1999;248:339–349. doi: 10.1006/excr.1999.4443. [DOI] [PubMed] [Google Scholar]
- 5.Morgan D O. Nat Cell Biol. 1999;1:E47–E53. doi: 10.1038/10039. [DOI] [PubMed] [Google Scholar]
- 6.Glotzer M, Murray A W, Kirschner M W. Nature (London) 1991;349:132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- 7.Amon A, Irniger S, Nasmyth K. Cell. 1994;77:1037–1050. doi: 10.1016/0092-8674(94)90443-x. [DOI] [PubMed] [Google Scholar]
- 8.Brandeis M, Hunt T. EMBO J. 1996;15:5280–5289. [PMC free article] [PubMed] [Google Scholar]
- 9.Kumada K, Su S, Yanagida M, Toda T. J Cell Sci. 1995;108:895–905. doi: 10.1242/jcs.108.3.895. [DOI] [PubMed] [Google Scholar]
- 10.Irniger S, Nasmyth K. J Cell Sci. 1997;110:1523–1531. doi: 10.1242/jcs.110.13.1523. [DOI] [PubMed] [Google Scholar]
- 11.Patton E E, Willems A R, Tyers M. Trends Genet. 1998;14:236–243. doi: 10.1016/s0168-9525(98)01473-5. [DOI] [PubMed] [Google Scholar]
- 12.Pringle J R, Hartwell L H. In: The Molecular Biology of the Yeast Saccharomyces. Strathern J N, Jones E W, Broach J R, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1981. pp. 97–142. [Google Scholar]
- 13.Weinstein J, Jacobsen F W, Hsu-Chen J, Wu T, Baum L G. Mol Cell Biol. 1994;14:3350–3363. doi: 10.1128/mcb.14.5.3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao N, Lai F, Fernald A A, Eisenbart J D, Espinosa R, Wang P W, Le Beau M M. Genomics. 1998;53:184–190. doi: 10.1006/geno.1998.5473. [DOI] [PubMed] [Google Scholar]
- 15.Starborg M, Brundell E, Gell K, Höög C. J Biol Chem. 1994;269:24133–24137. [PubMed] [Google Scholar]
- 16.De Hoop M, Meyn L, Dotti C G. In: Cell Biology: A Laboratory Handbook. Celis J E, editor. Vol. 1. San Diego: Academic; 1998. [Google Scholar]
- 17.Kramer E R, Gieffers C, Hölzl G, Hengstschläger M, Peters J-M. Curr Biol. 1998;8:1207–1210. doi: 10.1016/s0960-9822(07)00510-6. [DOI] [PubMed] [Google Scholar]
- 18.Grossberger R, Gieffers C, Zachariae W, Podtelejnikov A V, Schleiffer A, Nasmyth K, Mann M, Peters J-M. J Biol Chem. 1999;274:14500–14507. doi: 10.1074/jbc.274.20.14500. [DOI] [PubMed] [Google Scholar]
- 19.Aguzzi A, Wagner E F, Williams R L, Courtneidge S A. New Biol. 1990;2:533–543. [PubMed] [Google Scholar]
- 20.Holloway S L, Glotzer M, King R W, Murray A W. Cell. 1993;73:1393–1402. doi: 10.1016/0092-8674(93)90364-v. [DOI] [PubMed] [Google Scholar]
- 21.Yu H, King R W, Peters J-M, Kirschner M W. Curr Biol. 1996;6:455–466. doi: 10.1016/s0960-9822(02)00513-4. [DOI] [PubMed] [Google Scholar]
- 22.Hayes T E, Valtz N L, McKay R D. New Biol. 1991;3:259–269. [PubMed] [Google Scholar]
- 23.Clay F J, McEwen S J, Bertoncello I, Wilks A F, Dunn A R. Proc Natl Acad Sci USA. 1993;90:4882–4886. doi: 10.1073/pnas.90.11.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eriksson P S, Perfilieva E, Bjork-Eriksson T, Alborn A M, Nordborg C, Peterson D A, Gage F H. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 25.Tugendreich S, Tomkiel J, Earnshaw W, Hieter P. Cell. 1995;81:261–268. doi: 10.1016/0092-8674(95)90336-4. [DOI] [PubMed] [Google Scholar]
- 26.Raff M C, Durand B, Gao F B. Int J Dev Biol. 1998;42:263–267. [PubMed] [Google Scholar]
- 27.Freeman R S, Estus S, Johnson E M., Jr Neuron. 1994;12:343–355. doi: 10.1016/0896-6273(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 28.Gao C Y, Zelenka P S. BioEssays. 1997;19:307–315. doi: 10.1002/bies.950190408. [DOI] [PubMed] [Google Scholar]
- 29.Zhou B B, Li H, Yuan J, Kirschner M W. Proc Natl Acad Sci USA. 1998;95:6785–6790. doi: 10.1073/pnas.95.12.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vincent I, Rosado M, Davies P. J Cell Biol. 1996;132:413–425. doi: 10.1083/jcb.132.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayer R J, Landon M, Lowe J. In: Ubiquitin and the Biology of the Cell. Peters J-M, Harris J R, Finley D, editors. NY: Plenum; 1998. pp. 429–452. [Google Scholar]
- 32.Fang G, Yu H, Kirschner M W. Mol Cell. 1998;2:163–171. doi: 10.1016/s1097-2765(00)80126-4. [DOI] [PubMed] [Google Scholar]