Abstract
Prudent use of antibiotics is mandatory to control antibiotic resistance. The objective of this study was to determine if prevalence surveys are useful tools to determine the appropriateness of antimicrobial therapy (AMT) and determinants of inappropriate AMT. The study was performed in a 1,350-bed teaching hospital including all medical specialities. Six consecutive 1-day prevalence surveys of in-patients were performed twice yearly from 2001 to 2004. Data on the demographics, infections, and AMT were gathered. The appropriateness of AMT was assessed according to a standardized algorithm based on the local AMT prescription guidelines. On average, 684 patients were included in each survey (total, 4,105). The use of AMT as determined in the prevalence survey corresponded to the annual data from the pharmacy department. Nine hundred thirty-eight (22.9%) of the patients received AMT, and in 351 (37.4%) of these patients AMT was inappropriate. Only 25 (0.6%) patients did not receive AMT, although it was indicated. After multivariate analysis, the use of quinolones was the only statistically significant variable associated with inappropriate use. Prevalence surveys proved to be useful tools to judge the appropriateness of AMT and to identify determinants of inappropriate use. This study shows that in a setting with a low use of AMT, there are few patients who inadvertently do not receive AMT. On the other hand, a substantial number of the patients are treated inappropriate.
Resistance to antimicrobial drugs is a serious and increasing problem throughout the world (9, 10). Hospitals play a key role in the development of antimicrobial resistance. To control the development of resistance, a restrictive antimicrobial policy in combination with effective infection control measures to prevent the spread of resistant microorganisms is advisable. Therefore, local or national guidelines for antimicrobial therapy (AMT) have been developed (6, 11, 13, 14). The implementation of these guidelines and their effectiveness is questionable regarding the ever-increasing problem of resistance. More insight into the actual implementation of the prescription guidelines is needed. Investigating the consumption of antimicrobial agents from the pharmacy department is often used and provides an estimate of the total use AMT. Using this method, it is clear that huge variations exist between countries and between hospitals within countries (4). However, it does not provide insight into the appropriateness of AMT and about determinants of inappropriate use.
The objectives of this study were to determine the usefulness of prevalence surveys to measure antimicrobial consumption in the hospital, to determine the appropriateness of AMT, and to identify determinants of inappropriate use.
MATERIALS AND METHODS
Setting.
The Amphia hospital is a 1,350-bed teaching hospital with three locations. All medical specialties are available. In 2004 there were 39,704 admissions and 273,125 bed days. The average length of stay was 6.9 days.
Prevalence surveys.
Prevalence surveys are performed twice a year, in the spring and in the autumn. All patients that are present in the hospital at 6 a.m. on the day of the survey are included. Patients in day care, in psychiatric wards, or on hemodialysis are excluded. Infection control practitioners (ICP) collect the data from the medical and nursing records and by conversation with the nursing staff. All data are registered using standardized forms. There are six ICPs participating, who are all trained in national surveillance workshops to gather the data in a standardized way. From each patient the following demographic variables are recorded: age, sex, medical specialty, medical ward, and presence of infection on admission. Nosocomial infections are recorded using the Centers for Disease Control and Prevention definitions (8, 7), as is whether patients are still symptomatic or are still being treated on the day of the survey. Judgment of the infection data (infection on admission and kind of nosocomial infection) is performed by the ICPs. Furthermore, the use of antibiotics and variables like dose-related issues are noted. The pharmacy dispensing data were not validated on a patient level and therefore are not suitable for this purpose. If more than one antibiotic is prescribed for one patient, all antibiotics, with a maximum of three, are registered. Antifungal and antiviral therapy as well as medication for tuberculosis were excluded from the study.
Appropriateness of antimicrobial therapy.
The appropriateness of AMT is determined using a standardized method developed by Gyssens et al. (5). The following classifications are used: correct decision, incorrect decision, incorrect choice, incorrect use, or insufficient data. This score system only takes into account patients that are on AMT. Using prevalence surveys it is also possible to examine the appropriateness of not receiving AMT. Antibiotic use categorized as “correct decision” is deemed appropriate. Antibiotic use categorized as “incorrect decision,” “incorrect choice,” or “incorrect use” is deemed inappropriate. The criteria for evaluation are summarized in Table 1. The use of antibiotics is judged according to the local AMT prescription guidelines. The local AMT prescription guidelines are written by a local team of consultant microbiologists, infectious disease physicians, and pharmacists based on national and international guidelines adapted to the local susceptibility patterns of pathogens. All medical specialists working in the hospital are invited to comment on a draft version, and finally the local committee on antimicrobial therapy sanctions these guidelines. The hospital pharmacist performs the first screening of the appropriateness of AMT, while more complicated cases are judged by a consultant microbiologist. Complicated cases included all ICU patients, patients who received antibiotics without having an active infection, patients who did not receive antibiotics and did have an active infection, patients who received an antibiotic that was not indicated by the local AMT prescription guidelines, and all cases that were considered questionable by the person who performed the initial screening (hospital pharmacist or study coordinator).
TABLE 1.
Score system for the appropriateness of antimicrobial therapy
| Action and score | Description |
|---|---|
| Correct decision | |
| 1 | No AMT; no infection; no AMT needed |
| 2 | No AMT; infection; no AMT needed |
| 3 | AMT; infection; APa choice; AP use |
| Incorrect decision | |
| 1 | No AMT; infection; AMT needed |
| 2 | AMT; no infection; no prophylaxis; no AMT needed |
| 3 | AMT; no infection; prophylaxis; no AMT needed |
| Incorrect choice | |
| 1 | Divergence from guideline |
| Incorrect use | |
| 1 | IAb dosage |
| 2 | IA timing |
| 3 | IA administration |
| 4 | IA duration of therapy |
| Missing data | |
| 1 | No AMT; not enough diagnostic information about infection |
| 2 | Infection; not enough diagnostic information if AMT is needed |
| 3 | AMT; not enough diagnostic information about infection |
| 4 | Infection; not enough information about AMT |
AP, appropriate.
IA, inappropriate.
General data on antimicrobial use.
The annual data on antimicrobial use from the pharmacy department were used to validate the observations in the prevalence surveys (the annual consumption data of antibiotic use from the pharmacy department have been validated since 2002). In addition, the number of admissions and the average length of stay are obtained from the hospital administration. The antibiotic consumption is calculated to defined daily doses (DDD)/100 patient days according to the ATC/DDD index 2005 from the WHO Collaborating Centre for Drug statistics Methodology (16).
Data analyses, quality control, and statistics.
Privacy of patients is provided by decoding all data according to the requirements of the privacy regulation in the Amphia hospital. The data were entered in a database, double checked by the investigator and ICP of the project, and analyzed using the Statistical Package for Social Sciences software (SPSS, version 12.0). Before as well as during the project, the case-finding methods and interpretation of the medical information by the ICP are validated for intra- and interobserver reproducibility by discussing all nosocomial infections with another ICP, and if they disagree the case is resolved by plenary discussion. The ICP and the consultant microbiologist discuss all completed forms from ICU patients. Categorical variables were analyzed by Fisher's exact test or the chi-square test when appropriate, and continuous variables were analyzed using a t test or Mann-Whitney U test when appropriate. Trends over time were examined using linear regression analysis. Binary logistic regression analysis was performed to control for confounding. All variables with a P value below 0.1 were entered into the model. Statistical significance was accepted when the chance for coincidence was less than 5%.
RESULTS
Demographics and infections.
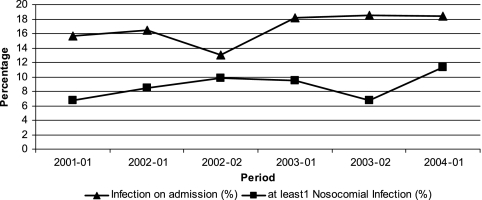
Between 2001 and 2004, six surveys were performed, and a total of 4,105 patients were included. Overall, 1,894 (46.1%) were male, and the mean age was 59.9 years (standard deviation, 22.7); both variables were constant over time. An infection on admission was present in 685 patients (16.7%), and 359 patients (8.7%) had at least one active nosocomial infection on the day of the survey. Figure 1 shows the trends over time of infection on admission and nosocomial infections. There was a significant increase in the number of patients with an infection on admission in the hospital (P = 0.02) and in the overall proportion of patients with nosocomial infections (P = 0.03).
FIG. 1.
Trends over time of infections on admission and nosocomial infections in six surveys between 2001 and 2004.
Antimicrobial therapy by prevalence surveys.
A total of 938 patients (22.9%) were on AMT. Of those 938 patients, 48 (5.1%) were treated with two antibiotics, and 10 (1.1%) were treated with three antibiotics. The prevalence of AMT was consistent over time, and no significant trend was observed.
Antimicrobial therapy by pharmacy department.
The PDD/100 patient days increased from 22.5 in 2002 to 26.5 in 2003 to 29.5 in 2004 (corresponding with a DDD/100 patient days from 32.1 in 2002, 37.7 in 2003, and 42.6 in 2004).
Appropriateness of AMT.
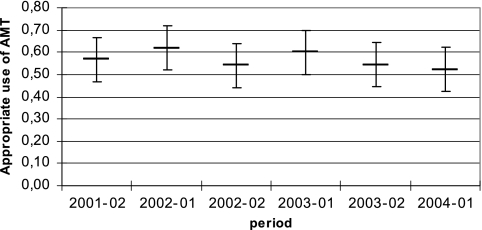
In 351 (37.4%) patients of the total of 938 who were on AMT, AMT was deemed inappropriate. More specifically, in 123 patients (13.0%) AMT was unjustified, in 140 patients (14.9%) an incorrect choice was made, and in 88 patients (9.4%) the correct antibiotic was used but it was used incorrectly. There were no significant differences in the appropriateness of AMT between the six surveys, and there was no significant trend over time (Fig. 2). Twenty-five patients (0.6%) did not receive AMT, although this was indicated. Finally, 71 (1.7%) patients could not be judged because of insufficient information.
FIG. 2.
Appropriateness of use of AMT (95% confidence interval) in six surveys between 2001 and 2004.
Determinants of inappropriate use of antibiotics.
In the univariate analysis, the use of quinolones and co-amoxicillin-clavulanic acid were statistically significantly associated with more frequent inappropriate use of AMT (Table 2). The use of cephalosporines, small-spectrum penicillins, meropenem, metronidazole, and rifampin were significantly associated with more frequent appropriate use of AMT (Table 2). Considering the use of AMT in the different medical specialties, urology; ear, nose, and throat; geriatrics; and neurology proved to be statistically significantly associated with more frequent inappropriate use, and pediatrics was statistically significantly associated with more frequent appropriate use (Table 3).
TABLE 2.
The appropriateness of antimicrobial therapy in different groups of antibioticsf
| Antimicrobial agent(s) | APa use (n) | IAb use (n) | % of total use | RRg for IA use (95% confidence interval) |
|---|---|---|---|---|
| Narrow-spectrum penicillinc | 117 | 37 | 15.1 | 0.55 (0.41-0.74) |
| Co-amoxicillin-clavulanic acid | 181 | 158 | 33.2 | 1.30 (1.11-1.53) |
| Narrow- and expanded-generation cephalosporinsd | 85 | 30 | 11.3 | 0.62 (0.45-0.85) |
| Broad-spectrum cephalosporins | 38 | 14 | 5.1 | 0.66 (0.42-1.04) |
| Piperacillin-tazobactam | 6 | 1 | 0.7 | 0.36 (0.06-2.19) |
| Meropenem | 12 | 0 | 1.2 | NAe (P = 0.005) |
| Aminoglycosides | 14 | 6 | 2.0 | 0.75 (0.38-1.46) |
| Quinolones | 42 | 71 | 11.1 | 1.72 (1.45-2.03) |
| Trimethoprim-sulfamethoxazole | 30 | 21 | 5.0 | 1.03 (0.74-1.45) |
| Macrolides/lincosamides | 29 | 16 | 4.4 | 0.88 (0.59-1.32) |
| Metronidazole | 44 | 14 | 5.7 | 0.59 (0.37-0.93) |
| Vancomycin | 7 | 4 | 1.1 | 0.91 (0.41-2.00) |
| Doxycycline | 7 | 6 | 1.3 | 1.16 (0.64-2.09) |
| Furadantin | 7 | 9 | 1.6 | 1.42 (0.91-2.20) |
| Rifampin | 14 | 0 | 1.4 | NA (P = 0.001) |
| Total | 633 | 387 | 100 |
AP, appropriate.
IA, inappropriate.
Narrow-spectrum penicillin means penicillin, amoxicyclin, and (flu)cloxacillin.
Narrow- and expanded-spectrum cephalosporins means cefazolin, cefuroxim, and cefamandol.
NA, not applicable. In these cases a P value is given.
Sixty patients were not included in this table because of insufficient information, and 58 patients were treated with more than one antibiotic.
RR, relative risk.
TABLE 3.
The appropriateness of antimicrobial therapy by medical specialtyc
| Medical specialty | APa use (n) | IAb use (n) | % of total use | RRd for IA use (95% confidence interval) |
|---|---|---|---|---|
| Surgery | 114 | 76 | 21.6 | 1.00 (0.82-1.22) |
| Internal medicine | 97 | 74 | 19.5 | 1.11 (0.91-1.34) |
| Lung diseases | 104 | 55 | 18.1 | 0.84 (0.67-1.06) |
| Orthopedics | 85 | 51 | 15.5 | 0.93 (0.73-1.17) |
| Cardiology | 34 | 18 | 5.9 | 0.86 (0.59-1.26) |
| Pediatrics | 35 | 10 | 5.1 | 0.54 (0.31-0.94) |
| Neurology | 18 | 21 | 4.4 | 1.37 (1.01-1.85) |
| Urology | 11 | 21 | 3.6 | 1.68 (1.29-2.19) |
| Gynecology | 13 | 5 | 2.1 | 0.69 (0.33-1.46) |
| Geriatrics | 4 | 10 | 1.6 | 1.81 (1.29-2.55) |
| Other specialties | 12 | 10 | 2.5 | 1.14 (0.72-1.82) |
| Total | 527 | 351 | 100 |
AP, appropriate.
IA, inappropriate.
60 patients were not included, because of insufficient information.
RR, relative risk.
Other factors that were statistically significantly associated with more appropriate use were younger age and the presence of an infection on admission (Table 4). After multivariate analysis, the use of quinolones was the only statistically significant factor associated with inappropriate use.
TABLE 4.
Appropriateness of use of antimicrobial therapy by age and presence of infection
| Age and infection status | APa use | IAb use | P | RRc for IA use (95% Cl) |
|---|---|---|---|---|
| Mean age (yr) | 60.3 | 64.3 | 0.007 | |
| Infection on admission (n) | 262 | 141 | 0.006 | 0.79 (0.67-0.94) |
| At least 1 nosocomial infection (n) | 127 | 90 | 0.63 | 1.05 (0.87-1.26) |
| Total (n) | 527 | 351 |
AP, appropriate.
IA, inappropriate.
RR, relative risk.
DISCUSSION
The mean prevalence of AMT by prevalence surveys was 0.26. The prevalence of AMT was stable over time, and none of the point prevalence estimates differed significantly from any other. There were no significant differences between the annual data from the pharmacy department and the estimates from the separate prevalence surveys. Only a small fraction of the patients could not be judged (1.7%) due to insufficient information. Therefore, a single prevalence survey offers a reliable estimate on the current use of AMT. However, this estimate by itself offers no advantage over the data from the pharmacy department, which are easier to acquire. The added value of prevalence surveys is the possibility to relate AMT to an individual patient. First, the appropriateness of AMT can be determined. Second, by collecting demographic variables and infection-related information, it provides the determinants of inappropriate use of AMT. Third, it provides an estimate of the proportion of patients that did not receive AMT while this was indicated. Finally, point prevalence surveys are efficient methods, which are performed relatively easily and rapidly. The added values of repeated prevalence surveys are to observe trends over time and the effects of interventions.
In our study, an infection on admission was present in 16.7% of the patients, and 8.7% had at least one nosocomial infection on the day of the survey. For both types of infections there was a slight but significant increase over time. It is possible that this reflects a true increase, but it can also be due to a better recognition of the infections by the ICP who performed the survey over time. The reported prevalence of nosocomial infections varies widely. In a large national prevalence survey in the United Kingdom and Republic of Ireland, the average prevalence was 9.0%. In teaching hospitals it was 11.2% (1).
The prevalence of AMT was 0.26. To judge this figure, it is important to realize that The Netherlands has among the lowest use of AMT in Europe (4). A recent study by Filius (2) showed that the average use in Dutch hospitals was 55 DDD/100 patient days. The mean use in our hospital between 2002 and 2004 was 37 DDD/100 patient days, which is on the lower edge for Dutch hospitals. Still, 37.4% of all patients on AMT were treated inappropriately. In 13% of those, AMT was not indicated at all. The latter comprises 3.0% of the total group of patients and may seem relatively unimportant. However, this means that annually more than 8,000 days of unjustified AMT are given in our hospital.
As indicated, the total use of AMT in The Netherlands in general and in our hospital in particular is low. This could lead to a situation in which patients who need AMT are not treated. The prevalence surveys provide information on the clinical situation of the patient, including infection-related information. Therefore, it is possible to identify those patients who inadvertently did not receive AMT (0.6%). Six of these patients were treated with AMT shortly after the day of the survey, and seven suffered from minor infections and were discharged within 1 week after the survey. Although AMT was indicated, their outcome seemed not adversely affected at discharge. Four of the remaining were deliberately not treated. It can be concluded that this situation of restrictive AMT is not accompanied by frequent abstinence of indicated treatment.
The use of quinolones especially proved to be an independent risk factor for inappropriate use of AMT in this study. Meropenem, piperacillin-tazobactam, and vancomycin were used rarely, and the use was highly appropriate. These antibiotics are classified as restricted agents in our hospital, and the pharmacy and microbiology departments closely monitor their application. After multivariate analysis, the use of quinolones was the only statistically significant factor associated with inappropriate use. The areas of the hospitals where quinolones were used most inappropriately were identified as well. When patients in orthopedic surgery, urology, or neurology were treated with quinolones, more than 75% of the time it was inappropriate.
There was a significant relationship between more appropriate use of AMT and the presence of an infection on admission. The presence of nosocomial infections was not associated with more appropriate use. This could indicate that physicians are more aware of the correct antibiotic choice for community-acquired infections than for nosocomial infections. Also, it could be that an infection on admission is judged more carefully than when it develops during hospitalization (3, 15).
The results from prevalence surveys offer a possibility for targeted interventions in problem areas. Subsequently, repeated prevalence surveys can be used to measure the effect of the intervention. During the study, from 2001 to 2004 no interventions in antibiotic use were initiated. Interim data were not used to direct antimicrobial therapy. After interpretation of the results of the study, several interventions for improvement of the use of antibiotics were started. The first intervention concerned the standardization of the drugs for perioperative prophylaxis. Before the intervention, eight different antibiotics were used for this purpose, and after the intervention only three were used (cefazolin, metronidazole, and clindamycin). This standardization resulted in a significant improvement of the timing of prophylaxis and a cost reduction of at least €40,000 per year. The second intervention aimed to improve the use of ciprofloxacin by switching from intravenous to oral administration as soon as possible. Six months after the start, the use of intravenous ciprofloxacin has been decreased more than 50%. This offers an annual saving of at least €65,000. A project to reduce the total use of ciprofloxacin will start soon. Repeated prevalence surveys will be used as a tool to measure the effects of the interventions. In conclusion, prevalence surveys offer an effective tool to improve the quality of AMT.
Acknowledgments
We are indebted to the infection control practitioners Gonny Moen, Henk Coertjens, Karin van Dijk, Miranda van Rijen, and Yvonne Hendriks for the collection of the data and Anja Boele for assistance with the data files.
Footnotes
Published ahead of print on 8 January 2007.
REFERENCES
- 1.Emmerson, A., J. Enstone, and M. Kelsey. 1995. The second national prevalence survey of infection in hospitals: methodology. J. Hosp. Infect. 30:7-29. [DOI] [PubMed] [Google Scholar]
- 2.Filius, P. 2005. An additional measure for quantifying antibiotic use in hospitals. J. Antimicrob. Chemother. 55:805-808. [DOI] [PubMed] [Google Scholar]
- 3.Gastmeier, P. M. D., D. Sohr, D. Forster, G. Schulgen, M. Schumacher, F. Dascher, and H. Ruden. 2000. Identifying outliers of antibiotic usage in prevalence studies on nosocomial infections. Infect Control Hosp. Epidemiol. 21:324-328. [DOI] [PubMed] [Google Scholar]
- 4.Goossens, H., M. Ferech, R. Vander Stichele, and M. Elseviers. 2005. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet 365:579-587. [DOI] [PubMed] [Google Scholar]
- 5.Gyssens, I. C., P. J. van den Broek, B. Kullberg, Y. A. Hekster, and J. W. M. van der Meer. 1992. Optimizing antimicrobial therapy. A method for antimicrobial drug use evaluation. J. Antimicrob. Chemother. 30:724-727. [DOI] [PubMed] [Google Scholar]
- 6.Gyssens, I. C. 2005. International guidelines for infectious diseases: a practical guide. Neth. J. Med. 63:291-299. [PubMed] [Google Scholar]
- 7.Horan, T. C., R. P. Gaynes, W. J. Martone, W. R. Jarvis, and T. G. Emori. 1992. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp. Epidemiol. 13:606-608. [PubMed] [Google Scholar]
- 8.Horan, T. C., and R. P. Gaynes. 2004. Surveillance of nosocomial infections, p. 1659-1702. In C. G. Mayhall (ed.), Hospital epidemiology and infection control, 3rd ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 9.Kunin, C. 1993. Resistance to antimicrobial drugs. Ann. Int. Med. 118:557-561. [DOI] [PubMed] [Google Scholar]
- 10.Neu, H. 1992. The crisis in antibiotic resistance. Science 257:1064-1073. [DOI] [PubMed] [Google Scholar]
- 11.Peetermans, W. E., and D. Ramaekers. 2002. Clinical practice guidelines in infectious diseases. Neth. J. Med. 60:343-348. [PubMed] [Google Scholar]
- 12.Reference deleted.
- 13.Stobberingh, E., R. Janknegt, and G. Wijnands. 1993. Antibiotic guidelines and antibiotic utilization in Dutch hospitals. J. Antimicrob. Chemother. 32:153-161. [DOI] [PubMed] [Google Scholar]
- 14.Van Kasteren, M. E. E., J. W. M. Van der Meer, E. E. Stoberingh, H. A. Verbrugh, and R. Janknegt. 1997. Richtlijnen voor het gebruik van antimicrobiele middelen. Ned. Tijdschr. Geneeskd. 142:949-952. [Google Scholar]
- 15.Weinstein, J., D. Mazon, E. Pantelick, P. Reagan-Cirincione, L. Dembry, and W. Hierholzer. 1999. A decade of prevalence surveys in a tertiary-care center: trends in nosocomial infection rates, device utilization, and patient acuity. Infect Control Hosp. Epidemiol. 20:543-548. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. 2002. Guidelines for ATC classification and DDD assignment. WHO Collaborating Centre for Drug Statistics Methology, Norwegian Institute of Public Health, Oslo, Norway.