Abstract
Serious infections with Pseudomonas aeruginosa are frequently treated with the combination of a β-lactam antimicrobial and an aminoglycoside. P. aeruginosa strain PA0905 was isolated in 2005 from an inpatient in Brazil. It showed a panresistant phenotype that included resistance to β-lactams, aminoglycosides, and fluoroquinolones. The β-lactam resistance was conferred by the production of the metallo-β-lactamase SPM-1. No inhibitory zone was observed when a disk diffusion test was performed with the semisynthetic aminoglycoside arbekacin, raising suspicion of 16S rRNA methylase production. A cloning experiment subsequently revealed the presence of a novel 16S rRNA methylase, RmtD, which accounted for the high-level resistance to all 4,6-disubstituted deoxystreptamine aminoglycosides, such as amikacin, tobramycin, and gentamicin. RmtD shared a moderate degree of identity with RmtA, another 16S rRNA methylase that was initially reported to occur in P. aeruginosa in Japan in 2003. This is the first identification of aminoglycoside resistance mediated by a 16S rRNA methylase in South America. This is also the first report to document coproduction of a metallo-β-lactamase and a 16S rRNA methylase, a combination that would severely compromise therapeutic options for the infected patients.
Serious infections with Pseudomonas aeruginosa are associated with substantial mortality (13). Although the merits of combination antimicrobial therapy with use of a β-lactam and an aminoglycoside have been debated, many guidelines do recommended such combinations (1). Coproduction of potent mechanisms of resistance to both β-lactams and aminoglycosides may therefore have considerable clinical impact.
A wide range of β-lactamases are known to be produced by P. aeruginosa, including metallo-β-lactamases, which may compromise all β-lactam antimicrobials except aztreonam (3). Production of a 16S rRNA methylase has recently emerged as a mechanism of high-level resistance to all 4,6-disubstituted deoxystreptamine aminoglycosides, such as amikacin, tobramycin, and gentamicin (8, 24). Therefore, coproduction of a metallo-β-lactamase and a 16S rRNA methylase may result in an extremely significant antimicrobial resistance profile.
Four 16S rRNA methyltransferases have been identified thus far. RmtA was reported to occur in Pseudomonas aeruginosa clinical isolates in Japan (24, 26), whereas ArmA and RmtB have been identified among various species belonging to the Enterobacteriaceae in Europe and East Asia (7, 8, 11, 24, 25). In addition, RmtC was most recently found from Proteus mirabilis in Japan (22). All of the responsible genes have been found in association with transposons or transposon-like elements (5, 9, 22, 23). Functionality of these transposons have been demonstrated for ArmA and RmtC, suggesting that transposase-mediated recombination events are likely responsible for the acquisition and dissemination of these genes (8, 21).
P. aeruginosa PA0905 was isolated from an inpatient at a hospital in Brazil in 2005. The strain showed panresistance, including high-level resistance to carbapenems and multiple aminoglycosides, including amikacin, tobramycin, and gentamicin (14). The strain did not form an inhibitory zone around a Kirby Bauer disk impregnated with 30 μg of arbekacin, a semisynthetic aminoglycoside. Demonstration of high-level resistance to arbekacin in gram-negative pathogens has been reported to be suggestive of aminoglycoside resistance mediated by production of 16S rRNA methylases (5). Cloning experiments were therefore conducted to identify the aminoglycoside resistance determinant in P. aeruginosa PA0905.
MATERIALS AND METHODS
Bacterial strains and plasmids.
P. aeruginosa PA0905 was isolated in 2005 from the urine of an inpatient at a hospital in the city of Sorocaba, approximately 80 km west of São Paulo, Brazil. P. aeruginosa PA01 and Escherichia coli XL1-Blue were used as the hosts in transformation experiments. pBC SK(−) and pBluescript II SK(+) (Stratagene, La Jolla, CA) were used as the cloning vectors.
Antimicrobial susceptibility testing.
Aminoglycosides were obtained from Sigma (St. Louis, MO), except for arbekacin, which was provided by Meiji Seika Kaisha Ltd. (Tokyo, Japan). The MICs of aminoglycosides were determined by use of the agar dilution method in accordance with the recommendations from the Clinical and Laboratory Standards Institute (CLSI) (4), using E. coli ATCC 25922 as the control strain. The MICs of β-lactams, ciprofloxacin, and colistin for P. aeruginosa PA0905 were determined by Etest (AB Biodisk, Solna, Sweden). Kirby Bauer disks containing 30 μg of arbekacin were obtained from Eiken Kagaku Co., Ltd. (Tokyo, Japan).
Detection of metallo-β-lactamase production.
Etest MBL (AB Biodisk) was used to screen for metallo-β-lactamase production. PCR for detection of blaSPM was conducted as previously described (6).
Transfer of aminoglycoside resistance genes.
Plasmids of P. aeruginosa PA0905 were extracted by use of both the Wizard Plus Minipreps DNA purification system (Promega, Madison, WI) and the method described by Kado and Liu (10). Electrocompetent E. coli XL1-Blue and P. aeruginosa PA01 were transformed by the plasmids. Transformants were selected on Luria-Bertani (LB) agar plates containing 10 and 50 μg/ml of amikacin for E. coli XL1-Blue and P. aeruginosa PA01, respectively.
Curing of plasmids.
Curing experiments were conducted according to the methods described by Trevors (20). Briefly, P. aeruginosa PA0905 was cultured overnight in LB broth containing 128 μg/ml of ethidium bromide, which was the highest concentration with appreciable growth. The broth was then aliquoted onto plain LB agar plates. The colonies were subsequently replicated on LB agar plates with and without 50 μg/ml of arbekacin to evaluate for loss of arbekacin resistance.
Cloning of the aminoglycoside resistance gene.
The genomic DNA of P. aeruginosa PA0905 was prepared and digested with BamHI, and the resultant fragments were ligated with plasmid vector pBC SK(−). Electrocompetent E. coli XL1-Blue was prepared and transformed with these plasmids. Transformants were selected on LB agar plates containing 30 μg/ml of chloramphenicol and 10 μg/ml of amikacin. For use in MIC determination, the recombinant plasmid obtained was partially digested with Sau3AI and ligated with pBluescript II SK(+). The transformants carrying the aminoglycoside resistance determinant were selected out on LB agar plates containing 50 μg/ml of ampicillin and 10 μg/ml of amikacin.
Nucleotide sequence accession number.
The nucleotide sequence containing rmtD determined in this study appears in the EMBL/GenBank/DDBJ databases under accession number DQ914960.
RESULTS
Aminoglycoside and carbapenem resistance of P. aeruginosa PA0905.
P. aeruginosa PA0905 showed a high level of resistance to arbekacin, amikacin, tobramycin, and gentamicin (Table 1). It was resistant to ceftazidime (MIC, >256 μg/ml), cefepime (>256 μg/ml), piperacillin-tazobactam (192 μg/ml), imipenem (>32 μg/ml), meropenem (>32 μg/ml), and ciprofloxacin (>32 μg/ml) but was susceptible to aztreonam (8 μg/ml) and colistin (1.0 μg/ml).
TABLE 1.
Results of antimicrobial susceptibility testing
| Antimicrobial agent | MIC (μg/ml) for:
|
||
|---|---|---|---|
| P. aeruginosa PA0905 | E. coli XL1-Blue (pPA95S29) | E. coli XL1-Blue [pBluescript II SK(+)] | |
| Arbekacin | >256 | >256 | 0.25 |
| Amikacin | >256 | >256 | 0.5 |
| Tobramycin | 256 | 64 | 0.06 |
| Gentamicin | >256 | 256 | 0.25 |
| Apramycin | 32 | 2.0 | 2.0 |
| Neomycin | 64 | 0.12 | 0.12 |
| Streptomycin | 128 | 1.0 | 0.5 |
Detection of metallo-β-lactamase production.
By Etest MBL, the MIC of imipenem for P. aeruginosa PA0905 was reduced by greater than eightfold in the presence of EDTA, suggesting the presence of a metallo-β-lactamase. PCR for blaSPM yielded an amplicon, which was confirmed to represent blaSPM-1 upon sequencing.
Cloning and sequencing of aminoglycoside resistance gene.
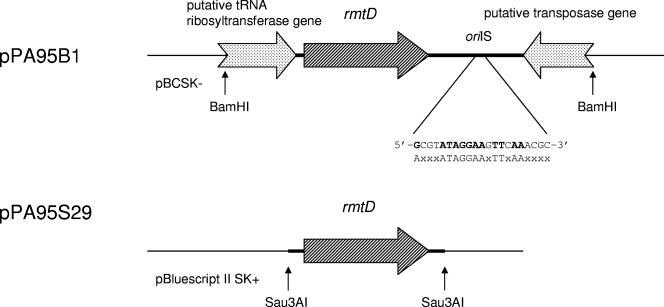
When electrocompetent E. coli XL1-Blue cells were transformed with recombinant plasmids consisting of a BamHI-digested genomic library of P. aeruginosa PA0905 ligated with pBC SK(−), two colonies were obtained by selection with amikacin and chloramphenicol. They possessed a recombinant plasmid containing a 2.3-kb BamHI insert (pPA95B1), which was then sequenced. The structure of the sequence is shown in Fig. 1. The initial 0.5 kb of the sequence contained the 3′ end of an open reading frame. It showed 70% identity to part of the putative tRNA ribosyltransferase located upstream of rmtA (23). The next open reading frame predicted a protein with 247 amino acids and molecular mass of ca. 28 kDa. Its deduced amino acid sequence showed moderate identity with those of previously reported 16S rRNA methylases that confer high-level resistance to 4,6-disubstituted deoxystreptamine aminoglycosides in gram-negative pathogens. The open reading frame was thus designated rmtD as a novel 16S rRNA methylase gene. The deduced amino acid sequence of the gene product showed 42% identity with that of RmtB, followed by 40% with that of RmtA (Fig. 2). Its GC content was 59%, similar to that of RmtA (55%) but somewhat lower than the genomic content of P. aeruginosa (67%). The last 0.4 kb of the sequenced region contained the 3′ end of an open reading frame downstream of rmtD. Its predicted amino acid sequence shared approximately 80% identity with those of putative transposases reported for the blaPSE-1-containing Tn2610 in E. coli as well as ISCR5S, located upstream of blaOXA-45 in P. aeruginosa (15, 17, 19). It also had moderate (54%) identity with Orf513, which constitutes the common region downstream of sul1-type complex class 1 integrons (ISCR1) and is assumed to encode a recombinase responsible for accumulation of various antimicrobial resistance genes (17). A putative insertion site of transposition (oriIS) was identified 224 bp downstream of the 3′ end of the putative transposase gene (Fig. 1). The oriIS contained a sequence identical to that of ISCR3, which consisted of 5′-GCGTATAGGAAGTTCAAACGC-3′ (18).
FIG. 1.
Schematic presentation of the genetic structure of the cloned regions containing rmtD. oriIS indicates the putative insertion site of transposition and its sequence. The lower sequence represents the consensus sequence of oriIS for IS91-related transposons.
FIG. 2.
Amino acid sequence of RmtD in comparison with those of known 16S rRNA methylases RmtA (GenBank accession number AB083212), RmtB (AB103506), RmtC (AB194779), and ArmA (AY220558).
Transfer of aminoglycoside resistance and curing experiments.
No transformant with resistance to amikacin and positive for the presence of the rmtD gene by PCR was obtained, despite repeated attempts. Curing experiments did not yield arbekacin-susceptible isolates.
Antimicrobial susceptibilities.
The MICs of various aminoglycosides for the parental strain P. aeruginosa PA0905 and for E. coli XL1-Blue harboring pPA95S29, a truncated derivative of pPA95B1, are shown in Table 1. The results confirmed the substantial role of rmtD in the high-level resistance of P. aeruginosa PA0905 against 4,6-disubstituted deoxystreptamine aminoglycosides, including arbekacin, amikacin, tobramycin, and gentamicin. It did not confer resistance to apramycin, neomycin, or streptomycin, which are structurally different aminoglycosides. The latter finding suggested that other mechanisms, especially aminoglycoside-modifying enzymes, may play a role in the resistance against these agents in the parental strain PA0905.
DISCUSSION
The present study for the first time identified a clinical isolate of P. aeruginosa that produced both a 16S rRNA methylase and a metallo-β-lactamase. The antimicrobial armamentarium for infections caused by multidrug-resistant or panresistant P. aeruginosa strains is extremely limited (14). Antipseudomonal cephalosporins, such as ceftazidime and cefepime; carbapenems, including imipenem and meropenem; and the antipseudomonal aminoglycosides tobramycin and amikacin are among the few therapeutic options if susceptibility is retained. Coproduction of a metallo-β-lactamase and a 16S rRNA methylase confers high-level resistance to all of the aforementioned agents. The emergence of such isolates therefore further adds to the complexity of treatment of infections caused by this species. Among all available antipseudomonal antimicrobials tested, the isolate remained susceptible only to aztreonam and colistin. Aztreonam is a monobactam that is known to be stable against the hydrolytic activity of metallo-β-lactamases (3). Aztreonam-resistant isolates of P. aeruginosa that produce SPM-1 metallo-β-lactamase have been reported, however, implying that these organisms are capable of acquiring additional resistance mechanisms targeting aztreonam (27).
This is also the first report to document the emergence of 16S rRNA methylase-mediated aminoglycoside resistance in either North or South America. This novel resistance mechanism in gram-negative pathogens is increasingly recognized worldwide since the initial reports in 2003. ArmA and RmtB have been reported from many countries in Europe and East Asia, often in association with CTX-M-type β-lactamases, whereas RmtA and RmtC are confined to Japan to date (8, 22, 24). However, it is possible that the prevalence of this resistance mechanism is underestimated. This assumption may be made due to the fact that clinical isolates are not always tested for susceptibility to multiple aminoglycosides and, even so, determinations of MICs higher than the breakpoints are not performed routinely. The hallmark of resistance by production of a 16S rRNA methylase is high-level resistance to multiple 4,6-disubstituted deoxystreptamine aminoglycosides. These agents comprise practically all parenterally formulated aminoglycosides in clinical use, and thus the presence of this resistance mechanism may not easily be detected in current routine susceptibility testing.
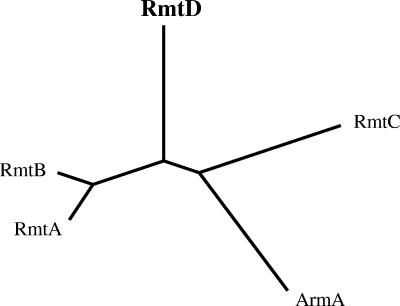
RmtD is the fifth 16S rRNA methylase identified in gram-negative organisms and the second in P. aeruginosa (after RmtA), adding to the growing family of aminoglycoside-resistance 16S rRNA methylases (Fig. 3). Its structural gene was identified in association with a putative transposase accompanied by an intact insertion site of transposition. Although the entire structure of the transposon carrying rmtD is yet to be elucidated, this finding suggests that rmtD was possibly mobilized to the genomic content of P. aeruginosa by a rolling-circle transposition event mediated by a novel IS91-like CR element (16, 18). These findings point to the possibility that rmtD, with a lower GC content than that in the P. aeruginosa genome, may have been acquired through recombination events from a group of closely related bacterial species that are yet to be identified.
FIG. 3.
Dendrogram of 16S rRNA methylases found in gram-negative bacilli, constructed with the ClustalW program provided by the Kyoto University Bioinformatics Center (http://align.genome.jp).
Two cardinal sites of methylation are known among 16S rRNA methylases produced by aminoglycoside-producing actinomycetes. Methylation of G1405 within the A site of 16S rRNA is associated with a kanamycin-gentamicin resistance phenotype, whereas that of A1408 is associated with a kanamycin-apramycin resistance phenotype (2). Though the site of methylation in RmtD was not examined, the resistance pattern that includes gentamicin but not apramycin is suggestive of the residue G1405 being the substrate of the methylation event, as was recently shown to be the case in ArmA (12).
Arbekacin served as a useful marker for screening the isolate studied here as a potential 16S rRNA methylase producer, as has been reported previously (5). Arbekacin is a semisynthetic derivative of dibekacin and is stable against most aminoglycoside-modifying enzymes. When gram-negative pathogens highly resistant to both amikacin or tobramycin and gentamicin are encountered, testing for arbekacin resistance remains a simple and effective method for screening for 16S rRNA methylase production, as was demonstrated for this RmtD-producing organism, which formed no inhibitory zone around an arbekacin-containing disk. This two-step screening method may facilitate early detection of the presence of 16S rRNA methylase-mediated aminoglycoside resistance.
In conclusion, we have reported a novel aminoglycoside resistance 16S rRNA methylase, RmtD, in a panresistant P. aeruginosa clinical isolate which coproduced the metallo-β-lactamase SPM-1. Of the 16S rRNA methylases known to date, RmtD and its genetic structure are phylogenetically close to RmtA, both of which were identified in P. aeruginosa but in geographically distant parts of the world. The use of arbekacin as a screening tool with gram-negative pathogens resistant to multiple aminoglycosides may enhance detection of this emerging resistance mechanism and thus lead to early containment through timely implementation of infection control procedures.
Acknowledgments
This study was supported in part by a Fogarty International Center Global Infectious Diseases Research Training Program grant from the National Institutes of Health (grant 5D43TW006592; principal investigator, Lee H. Harrison).
Footnotes
Published ahead of print on 11 December 2006.
REFERENCES
- 1.American Thoracic Society and Infectious Diseases Society of America. 2005. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 171:388-416. [DOI] [PubMed] [Google Scholar]
- 2.Beauclerk, A. A., and E. Cundliffe. 1987. Sites of action of two ribosomal RNA methylases responsible for resistance to aminoglycosides. J. Mol. Biol. 193:661-671. [DOI] [PubMed] [Google Scholar]
- 3.Bonomo, R. A., and D. Szabo. 2006. Mechanisms of multidrug resistance in Acinetobacter species and Pseudomonas aeruginosa. Clin. Infect. Dis. 43(Suppl. 2):S49-S56. [DOI] [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute. 2006. Performance standards for antimicrobial susceptibility testing; 16th informational supplement. CLSI document M100-S16. Clinical and Laboratory Standards Institute, Wayne, Pa.
- 5.Doi, Y., K. Yokoyama, K. Yamane, J. Wachino, N. Shibata, T. Yagi, K. Shibayama, H. Kato, and Y. Arakawa. 2004. Plasmid-mediated 16S rRNA methylase in Serratia marcescens conferring high-level resistance to aminoglycosides. Antimicrob. Agents Chemother. 48:491-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gales, A. C., L. C. Menezes, S. Silbert, and H. S. Sader. 2003. Dissemination in distinct Brazilian regions of an epidemic carbapenem-resistant Pseudomonas aeruginosa producing SPM metallo-β-lactamase. J. Antimicrob. Chemother. 52:699-702. [DOI] [PubMed] [Google Scholar]
- 7.Galimand, M., P. Courvalin, and T. Lambert. 2003. Plasmid-mediated high-level resistance to aminoglycosides in Enterobacteriaceae due to 16S rRNA methylation. Antimicrob. Agents Chemother. 47:2565-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galimand, M., S. Sabtcheva, P. Courvalin, and T. Lambert. 2005. Worldwide disseminated armA aminoglycoside resistance methylase gene is borne by composite transposon Tn1548. Antimicrob. Agents Chemother. 49:2949-2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez-Zorn, B., A. Catalan, J. A. Escudero, L. Dominguez, T. Teshager, C. Porrero, and M. A. Moreno. 2005. Genetic basis for dissemination of armA. J. Antimicrob. Chemother. 56:583-585. [DOI] [PubMed] [Google Scholar]
- 10.Kado, C. I., and S. T. Liu. 1981. Rapid procedure for detection and isolation of large and small plasmids. J. Bacteriol. 145:1365-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee, H., D. Yong, J. H. Yum, K. H. Roh, K. Lee, K. Yamane, Y. Arakawa, and Y. Chong. 2006. Dissemination of 16S rRNA methylase-mediated highly amikacin-resistant isolates of Klebsiella pneumoniae and Acinetobacter baumannii in Korea. Diagn. Microbiol. Infect. Dis. 56:305-312. [DOI] [PubMed] [Google Scholar]
- 12.Liou, G. F., S. Yoshizawa, P. Courvalin, and M. Galimand. 2006. Aminoglycoside resistance by ArmA-mediated ribosomal 16S methylation in human bacterial pathogens. J. Mol. Biol. 359:358-364. [DOI] [PubMed] [Google Scholar]
- 13.Micek, S. T., A. E. Lloyd, D. J. Ritchie, R. M. Reichley, V. J. Fraser, and M. H. Kollef. 2005. Pseudomonas aeruginosa bloodstream infection: importance of appropriate initial antimicrobial treatment. Antimicrob. Agents Chemother. 49:1306-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paterson, D. L. 2006. The epidemiological profile of infections with multidrug-resistant Pseudomonas aeruginosa and Acinetobacter species. Clin. Infect. Dis. 43(Suppl. 2):S43-S48. [DOI] [PubMed] [Google Scholar]
- 15.Takaya, A., M. Watanabe, and T. Yamamoto. 2006. Organization of Tn2610 containing two transposition modules. Antimicrob. Agents Chemother. 50:1143-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tavakoli, N., A. Comanducci, H. M. Dodd, M. C. Lett, B. Albiger, and P. Bennett. 2000. IS1294, a DNA element that transposes by RC transposition. Plasmid 44:66-84. [DOI] [PubMed] [Google Scholar]
- 17.Toleman, M. A., P. M. Bennett, and T. R. Walsh. 2006. Common regions e.g. orf513 and antibiotic resistance: IS91-like elements evolving complex class 1 integrons. J. Antimicrob. Chemother. 58:1-6. [DOI] [PubMed] [Google Scholar]
- 18.Toleman, M. A., P. M. Bennett, and T. R. Walsh. 2006. ISCR elements: novel gene-capturing systems of the 21st century? Microbiol. Mol. Biol Rev. 70:296-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toleman, M. A., K. Rolston, R. N. Jones, and T. R. Walsh. 2003. Molecular and biochemical characterization of OXA-45, an extended-spectrum class 2d′ β-lactamase in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 47:2859-2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trevors, J. T. 1986. Plasmid curing in bacteria. FEMS Microbiol. Rev. 32:149-157. [Google Scholar]
- 21.Wachino, J., K. Yamane, K. Kimura, N. Shibata, S. Suzuki, Y. Ike, and Y. Arakawa. 2006. Mode of transposition and expression of 16S rRNA methyltransferase gene rmtC accompanied by ISEcp1. Antimicrob. Agents Chemother. 50:3212-3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wachino, J., K. Yamane, K. Shibayama, H. Kurokawa, N. Shibata, S. Suzuki, Y. Doi, K. Kimura, Y. Ike, and Y. Arakawa. 2006. Novel plasmid-mediated 16S rRNA methylase, RmtC, found in a Proteus mirabilis isolate demonstrating extraordinary high-level resistance against various aminoglycosides. Antimicrob. Agents Chemother. 50:178-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamane, K., Y. Doi, K. Yokoyama, T. Yagi, H. Kurokawa, N. Shibata, K. Shibayama, H. Kato, and Y. Arakawa. 2004. Genetic environments of the rmtA gene in Pseudomonas aeruginosa clinical isolates. Antimicrob. Agents Chemother. 48:2069-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamane, K., J. Wachino, Y. Doi, H. Kurokawa, and Y. Arakawa. 2005. Global spread of multiple aminoglycoside resistance genes. Emerg. Infect. Dis. 11:951-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan, J. J., J. J. Wu, W. C. Ko, S. H. Tsai, C. L. Chuang, H. M. Wu, Y. J. Lu, and J. D. Li. 2004. Plasmid-mediated 16S rRNA methylases conferring high-level aminoglycoside resistance in Escherichia coli and Klebsiella pneumoniae isolates from two Taiwanese hospitals. J. Antimicrob. Chemother. 54:1007-1012. [DOI] [PubMed] [Google Scholar]
- 26.Yokoyama, K., Y. Doi, K. Yamane, H. Kurokawa, N. Shibata, K. Shibayama, T. Yagi, H. Kato, and Y. Arakawa. 2003. Acquisition of 16S rRNA methylase gene in Pseudomonas aeruginosa. Lancet 362:1888-1893. [DOI] [PubMed] [Google Scholar]
- 27.Zavascki, A. P., P. B. Gaspareto, A. F. Martins, A. L. Goncalves, and A. L. Barth. 2005. Outbreak of carbapenem-resistant Pseudomonas aeruginosa producing SPM-1 metallo-β-lactamase in a teaching hospital in southern Brazil. J. Antimicrob. Chemother. 56:1148-1151. [DOI] [PubMed] [Google Scholar]