Abstract
Posaconazole is an orally bioavailable triazole antifungal agent for the treatment and prophylaxis of invasive fungal infection. We evaluated plasma posaconazole concentration data from juvenile (younger than 18 years; n = 12) and adult (18 to 64 years; n = 194) patients who participated in a multicenter, phase 3, open-label study that assessed the efficacy and safety of posaconazole treatment for persons who were intolerant of or had invasive fungal infection refractory to standard antifungal therapies. With the exception of one juvenile patient who received 400 mg/day as a divided dose on the day of sample collection, all patients received posaconazole at 800 mg/day as an oral suspension in divided doses. Plasma samples were analyzed through a validated liquid chromatographic-tandem mass spectrometric method with a lower limit of quantitation of 1 ng/ml. Because plasma posaconazole concentrations are relatively constant at steady state, the average of all plasma concentrations (Cav) for each patient was calculated to provide a single steady-state plasma posaconazole concentration. A blinded data review committee reviewed all treatment outcomes. Variable posaconazole plasma concentrations were observed within both the juvenile and adult populations. Mean (median [range]) Cav values for juvenile and adult patients were 776 ng/ml (579 ng/ml [85.3 to 2,891 ng/ml]) and 817 ng/ml (626 ng/ml [0 to 3,710 ng/ml]), respectively. Overall success rates and adverse event profiles were comparable. In conclusion, posaconazole concentrations in plasma were similar for juvenile and adult patients, suggesting that clinical outcomes are expected to be similar in adults and children with refractory invasive fungal infection.
Posaconazole is an extended-spectrum triazole antifungal agent used for the treatment and prophylaxis of refractory invasive fungal infection (13). In vitro studies have shown that posaconazole is active against many common yeasts and molds, including Candida and Aspergillus, as well as against Fusarium, Coccidioides, zygomycetes, and other filamentous and dimorphic fungi (2, 8, 9, 11, 13, 18-21, 27). In addition, posaconazole has been shown to be clinically effective for adult patients who have invasive fungal infections, including aspergillosis, zygomycosis, and candidiasis, and who are intolerant of or have disease refractory to other antifungal agents (28).
The pharmacokinetics of posaconazole in healthy adults have been well characterized. After rising single or multiple oral doses, concentrations in plasma increase in a dose-proportional manner up to a dose of 800 mg/day (5), and steady-state concentrations are achieved after 7 to 10 days of dosing (400 mg twice daily or 200 mg four times daily) (5, 10). The median time to peak posaconazole plasma concentration is approximately 3 to 5 h (data on file; Schering-Plough Research Institute, Kenilworth, NJ). Optimal exposure is achieved when 800 mg posaconazole is administered as an oral suspension in divided doses with food (6, 10). Posaconazole is eliminated primarily as a parent compound in feces, and the primary metabolic pathway is glucuronidation (14). Oxidative metabolism by cytochrome P450 (CYP) isoforms represents only a minor route of elimination. Posaconazole inhibits hepatic CYP3A4 activity but has no significant effect on CYP1A2, CYP2C8/9, CYP2D6, or CYP2E1 (30). Posaconazole is slowly eliminated, with a terminal-phase half-life of approximately 35 h at steady state (data on file; Schering-Plough Research Institute, Kenilworth, NJ). As a result of the long terminal-phase half-life, the steady-state plasma concentration-time profile of posaconazole after twice-daily dosing of 400 mg is relatively flat, with minimal differences in the observed maximum plasma concentration and observed minimum plasma concentration (5).
Salvage therapy for invasive fungal infections in children remains a therapeutic challenge that is often complicated by a delay in the conduct of definitive pediatric studies for new agents. Although investigation of the pharmacokinetic profile of a drug in children is often delayed during the drug development process, results have been published of studies examining the pharmacokinetics in pediatric patients of currently marketed azole antifungals, such as itraconazole (7, 12, 25), fluconazole (3, 15, 16, 26), and voriconazole (29). Pharmacokinetic profiles for itraconazole are inconsistent between pediatric patients and adults, and some pharmacokinetic parameters of fluconazole and voriconazole appear to differ between children and adults.
The primary objective of this analysis was to report available, albeit limited, plasma posaconazole concentration data for patients younger than 18 years of age and compare these data with those for adult patients. Secondary objectives were to analyze efficacy in the juvenile population and compare safety profiles of posaconazole for juvenile and adult patients.
MATERIALS AND METHODS
Patient population.
Patients were participants in a multicenter, phase 3, open-label study that assessed the efficacy and safety of posaconazole treatment for patients who were intolerant of or had invasive fungal infection refractory to standard antifungal therapies. The intent-to-treat (ITT) subset included all patients who received at least one dose of posaconazole, and the modified intent-to-treat (MITT) subset included only those patients from the ITT subset who had a proven or probable invasive fungal infection (based on criteria of the European Organization for Research and Treatment of Cancer/Mycoses Study Group) (1). Three hundred thirty patients were included in the ITT population; 238 were included in the MITT population. Within the ITT subset, sufficient pharmacokinetic data were available for a subset of 206 patients: 12 juvenile patients (younger than 18 years) and 194 adult patients (18 to 64 years).
This study was conducted in accordance with the Declaration of Helsinki and was approved by an accredited institutional review board. All patients provided written informed consent. For patients younger than 18 years, a parent or a legal guardian gave written informed consent before entry to the study.
Inclusion and exclusion criteria.
Patients with proven or probable invasive fungal infection according to criteria of the European Organization for Research and Treatment of Cancer/Mycoses Study Group (1) and evidence of intolerance of or disease refractory to other antifungal therapies were eligible for study participation. Patients were considered intolerant of available antifungal therapies if they had any of the following: (i) history of serious, severe, or life-threatening toxicity (i.e., organ toxicity greater than or equal to grade 3) while receiving antifungal therapy, nephrotoxicity (i.e., persistent serum creatinine levels of >2 times the upper limit of normal [ULN] or recurrent serum creatinine levels of >2 times the ULN after resumption of amphotericin B therapy); (ii) preexisting organ dysfunction, including renal insufficiency, that precluded the use of standard antifungal therapies; (iii) high risk for toxicity because of underlying disease or concomitant medications; or (iv) previous major idiosyncratic or hypersensitivity reaction to antifungal agents. Patients were considered to have disease refractory to standard therapies (e.g., amphotericin B or other azoles) if they experienced disease progression or if they showed no clinical improvement during antifungal therapy given for an adequate period of time (e.g., 10 to 14 days for cryptococcal meningitis, 7 days for infections caused by Aspergillus spp., 14 days for infections caused by non-Aspergillus molds, 30 days for histoplasmosis, and 180 days of azole therapy for coccidioidomycosis).
Patients were ineligible for study participation if they had concurrent progressive neurological disease (e.g., seizures, demyelinating syndromes, unstable multiple sclerosis, or peripheral neuropathy), required artificial ventilation and were unlikely to undergo extubation within 24 h of study entry, had a history of serious or severe hypersensitivity to azole antifungals, received medications that are known to interact with azoles and may lead to life-threatening adverse events (i.e., terfenadine, cisapride, or ebastine at entry or within 24 h before therapy or astemizole at entry or within 10 days of study entry), received drugs that lower azole serum concentrations (e.g., rifampin or phenytoin) within 7 days of study entry, had electrocardiography results showing a prolonged corrected QT interval (≥20% above normal) within 7 days of study entry, needed concomitant systemic antifungal agents during the study period, had significantly abnormal hepatic function test results (alanine aminotransferase or aspartate aminotransferase level of >10 times the ULN), or were expected to survive for less than 72 h. Women were excluded from participation if they were pregnant or lactating, were using inadequate contraception, or had not undergone surgical sterilization.
Treatment.
Patients received posaconazole as an oral suspension at doses of 200 mg four times daily or 400 mg twice daily while hospitalized and then at a dose of 400 mg twice daily after discharge from the hospital.
Pharmacokinetic analysis.
Blood samples (6 ml) for determining posaconazole concentrations in juvenile patients 13 years of age or older were to be collected before and after the first daily dose, at approximately 0, 2, 4, and 6 h at week 2 and approximately 30 min before the first daily dose and along with the adult patients at 2 to 4 h after that dose at week 16. However, the blood collection schedule for the juvenile population could not be strictly followed. On a given day, 2 to 6 samples were taken from 11 patients, and from 1 patient a single sample was collected.
Plasma was separated via centrifugation and stored frozen at −20°C until it could be analyzed. To determine posaconazole concentrations, plasma samples were analyzed using a positive-ion Turbo IonSpray (PE Applied Biosystems, Foster City, CA) liquid chromatographic-tandem mass spectrometric method. The calibration range was 1.00 to 4,000 ng/ml.
Between-run accuracy (percentage difference from actual value) values of the quality control (QC) for the lower limit of quantitation (1.00 ng/ml), low (3.00 ng/ml), medium (1,500 ng/ml), and high (3,000 ng/ml) QC samples were −7.59, −1.31, −0.504, and −3.71, respectively. Between-run precision (percentage coefficient of variation) values of the QC samples at the lower limit of quantitation, low, medium, and high concentrations were 15.7, 9.49, 7.07, and 8.54, respectively.
Because posaconazole is slowly eliminated and has a long terminal-phase half-life of 35 h, a twice-daily or four-times-daily dosing regimen provides plasma concentrations that are relatively flat and unchanged over time at steady state. Thus, the average of all the plasma concentrations (Cav) for a patient was calculated to provide a single steady-state plasma posaconazole concentration for each patient. Descriptive statistics (mean, median, standard deviation, minimum, and maximum) using each patient's Cav values were then calculated by age group. Juvenile pharmacokinetic data (composite plasma concentration-time plots and Cav) were compared with data for adult patients.
DRC.
A blinded data review committee (DRC) composed of 17 experts in the diagnosis and treatment (n = 15) or in the radiographic analysis (n = 2) of invasive fungal infections reviewed each case to ensure that all patients met predetermined eligibility criteria. Teams of two or three DRC members determined eligibility and outcomes using predefined criteria. The DRC reviewed treatment outcomes at month 1, month 3, and month 6 and at the end of therapy (or at 12 months if treatment went beyond that time).
Efficacy definitions.
A successful outcome was defined as the resolution of all attributable symptoms, signs, and radiographic abnormalities (complete response) or as clinically meaningful improvement in attributable symptoms, signs, and radiographic abnormalities (partial response). A nonsuccessful outcome was defined as no improvement in attributable symptoms, signs, and radiographic abnormalities (stable disease) or deterioration in attributable clinical or radiographic abnormalities that necessitated alternative antifungal therapy or resulted in death (treatment failure). If for any reason the response could not be assessed, the patient was considered to have had a nonsuccessful outcome.
Safety analysis.
Reported adverse events (spontaneously reported, solicited via generalized questioning, or identified during routine laboratory testing or physical examination), clinical laboratory test results, measurements of vital signs, and electrocardiograms were used to assess safety (24). Investigators determined whether each adverse event was unlikely to be related, possibly related, or probably related to posaconazole treatment. A treatment-related adverse event was defined as any event the investigator considered to be at least possibly related to posaconazole treatment or for which the investigator did not assign a relationship (i.e., any adverse event not specifically considered unlikely to be related to treatment).
RESULTS
Patient demographics.
Of the 12 juvenile patients evaluated, 7 were boys and 5 were girls. Baseline age and body weight ranged from 8 to 17 years and from 24 to 76 kg, respectively (Table 1). Most juvenile patients had hematologic malignancies; four also underwent bone marrow transplantation.
TABLE 1.
Demographics and baseline characteristics of juvenile patients
| Patient no. | Age (yr) | Weight (kg) | Gendera | Underlying diseaseb | Baseline species |
|---|---|---|---|---|---|
| 1 | 8 | 39.4 | M | Burkitt's lymphoma, ALL | Aspergillus spp. |
| 2 | 10 | 51.7 | M | CML, allogeneic BMT | Fusarium spp. |
| 3 | 12 | 24 | M | Chronic granulomatous disease | Aspergillus fumigatus |
| 4 | 12 | 36.7 | M | AML | Candida spp. |
| 5 | 14 | 34.5 | F | Cystic fibrosis | Aspergillus spp. |
| 6 | 15 | 54.5 | F | Osteosarcoma | Fusarium spp. |
| 7 | 15 | 76 | M | ALL | Candida krusei |
| 8 | 15 | 53.3 | M | AML, allogeneic BMT | Candida albicans |
| 9 | 16 | 40.6 | F | Inherited immunodeficiency | Candida albicans |
| 10 | 16 | 36 | F | ALL, allogeneic BMT | Aspergillus spp. |
| 11 | 16 | 59.9 | M | CML, allogeneic BMT | Scedosporium apiospermum, Aspergillus spp. |
| 12 | 17 | 45.4 | F | None | Coccidioides immitis |
M, male; F, female.
ALL, acute lymphoblastic leukemia; AML, acute myelogenous leukemia; BMT, bone marrow transplantation; CML, chronic myelogenous leukemia.
Pharmacokinetic data were available for 194 adult patients (age, 18 to 64 years) and were used for comparison with data for the juvenile population. Of the adult patients, 129 were men and 65 were women. The average age of the adult patient group was approximately 41 years. Most patients (102 of 194) were white; body weight in this group ranged from 29.5 to 119.6 kg.
Pharmacokinetics.
Eleven juvenile patients received a maintenance dose of 800 mg/day posaconazole oral suspension in divided doses. One patient (patient 1) received 400 mg/day posaconazole in divided doses on the day of sample collection and 800 mg/day in divided doses before the day of sample collection. Adult patients received posaconazole 800 mg/day in divided doses.
All infections.
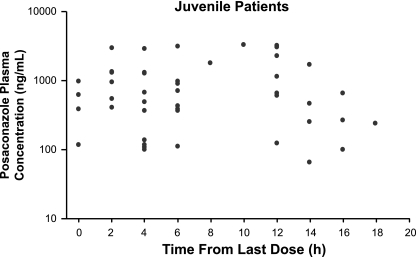
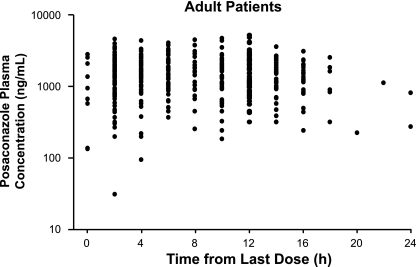
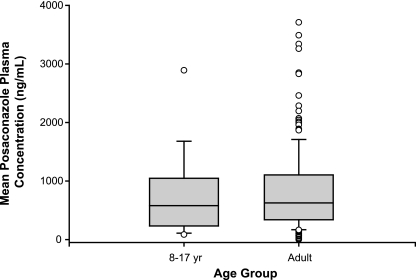
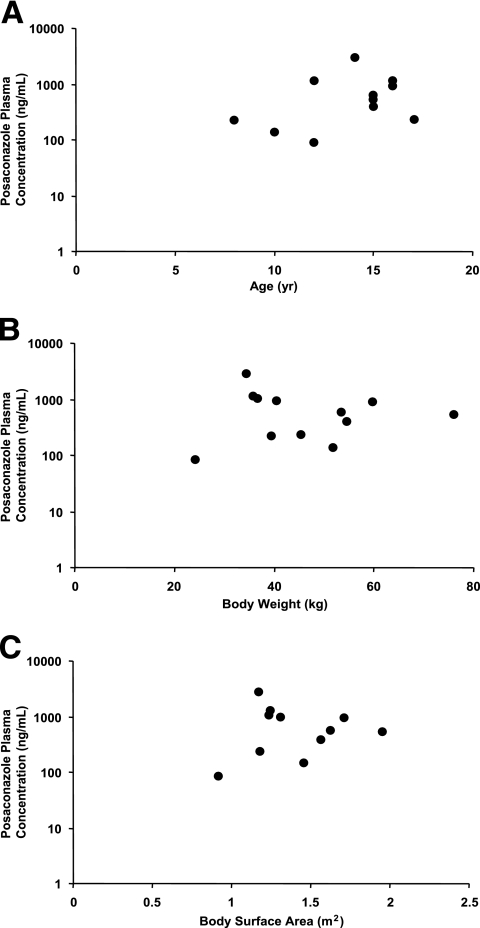
Posaconazole Cav values for juvenile and adult patients were comparable. Mean posaconazole Cav values were 776 ng/ml for juvenile patients and 817 ng/ml for adult patients (Table 2). Maximum posaconazole Cav values were 2,891 ng/ml and 3,710 ng/ml, respectively. Minimum posaconazole Cav values for juveniles and adults were 85.3 ng/ml and 0 ng/ml, respectively. Individual Cav values for juvenile patients are shown in Table 3. Composite plasma concentration-time profiles were relatively flat for both the juvenile and adult populations (Fig. 1 and 2). The range of plasma posaconazole concentrations showed considerable overlap between the adult and juvenile groups, indicating that the Cav values in these two age groups were similar (Fig. 3). However, because of the disparity between the juvenile and adult sample sizes, definitive conclusions cannot be made. Interpatient variability of the posaconazole plasma concentration has been noted in both patient populations. As shown in Fig. 4A to C, no association was apparent between age, body weight, or body surface area and posaconazole Cav (r2 = 0.06, 0.05, or 0.02, respectively).
TABLE 2.
Posaconazole concentrations in plasma (ng/ml) in adult and juvenile patients in the ITT populationa
| Patient group (nb) | Median concn | Mean (SD) concn | Minimum concn | Maximum concn |
|---|---|---|---|---|
| All infections | ||||
| Juvenile (12) | 579 | 776 (769) | 85.3 | 2,891 |
| Adult (194) | 626 | 817 (689) | 0 | 3,710 |
| Aspergillus infections | ||||
| Juvenile (5) | 927 | 1,058 (1,120) | 85.3 | 2,891 |
| Adult (77) | 530 | 684 (642) | 32.5 | 3,340 |
All plasma concentrations are expressed in ng/ml.
n, no. of patients.
TABLE 3.
Individual plasma concentrations, clinical response, and treatment-related adverse events
| Patient no. | Age (yr) | Weight (kg) | Posaconazole Cav (ng/ml) | Clinical responseb | Treatment-related adverse event(s) |
|---|---|---|---|---|---|
| 1a | 8 | 39.4 | 227 | PR | Vomiting; nausea |
| 2 | 10 | 51.7 | 140 | FL | |
| 3 | 12 | 24 | 85 | PR | Renal insufficiency |
| 4 | 12 | 36.7 | 1112 | SD | |
| 5 | 14 | 34.5 | 2891 | NAc | Renal failure |
| 6 | 15 | 54.5 | 396 | NAc | |
| 7 | 15 | 76 | 546 | CR | Vomiting |
| 8 | 15 | 53.3 | 613 | NAc | |
| 9 | 16 | 40.6 | 982 | NAc | |
| 10 | 16 | 36 | 1161 | UTD | Cardiorespiratory arrest; convulsions |
| 11 | 16 | 59.9 | 927 | CR | |
| 12 | 17 | 45.4 | 238 | PR |
Received 400 mg/day posaconazole in divided doses on the day of sample collection and 800 mg/day in divided doses before the day of sample collection.
CR indicates complete response; FL, failure; NA, not applicable; PR, partial response; SD, stable disease; UTD, unable to determine.
Only the MITT population was included in determining the clinical response; patients stopped treatment due to an adverse event.
FIG. 1.
Composite posaconazole plasma concentration-time profile showing observed concentrations in plasma for the juvenile population (younger than 18 years).
FIG. 2.
Composite posaconazole plasma concentration-time profile showing observed plasma posaconazole concentrations for the adult population (18 to 64 years).
FIG. 3.
Individual mean posaconazole Cav values for the juvenile (n = 12) and adult (n = 194) populations. The lower and upper limits of the box represent the 25th and 75th percentiles, respectively; the horizontal line within the box represents the median value, and the upper and lower whiskers represent the 10th and 90th percentiles; data points beyond the whiskers represent possible outliers.
FIG. 4.
Comparison of posaconazole Cav and (A) age, (B) body weight, and (C) body surface area.
Aspergillus infections.
Plasma posaconazole concentrations were also analyzed for the subset of patients who had Aspergillus infections (patients 1, 3, 5, 10, and 11), because these were the most common fungal infections observed in the present study and are the most frequently treated fungal infection in clinical practice. Although the pharmacokinetic data for the juvenile population were drawn from a small sample size (n = 5), the pharmacokinetic parameters were comparable to those for adults. Mean plasma posaconazole Cav values were 1,058 ng/ml for juvenile patients and 684 ng/ml for adult patients (Table 2). Maximum plasma posaconazole Cav values were 2,891 ng/ml and 3,340 ng/ml for juvenile and adult patients, respectively. Minimum posaconazole Cav values for juveniles and adults were 85.3 ng/ml and 32.5 ng/ml, respectively.
Clinical efficacy in the juvenile population.
Eight patients were included in the MITT group. Six patients had disease that was considered refractory to available antifungal therapy, one was considered intolerant to available antifungal therapy, and one was considered both refractory and intolerant.
Successful outcomes were observed for five of eight (62.5%) MITT patients who had infections caused by Aspergillus (patients 1 and 3), Candida (patient 7), Coccidioides (patient 12), or Scedosporium (patient 11) (Table 3). Nonsuccessful outcomes were observed for three (37.5%) patients who had infections caused by Candida, Aspergillus, or Fusarium.
Four patients were not included by the DRC in the MITT subset but were included in the pharmacokinetic analysis. One of these patients had a possible infection (Candida albicans [patient 9]), and three patients were not considered to have refractory disease or to be intolerant of standard therapy, though they had proven fungal infections (Fusarium species [patient 6], C. albicans [patient 8], and Aspergillus species [patient 5]). All four of these patients had successful outcomes with posaconazole.
Safety.
Of the 12 juvenile patients, 8 completed the study. Four patients discontinued prematurely because of adverse events. These adverse events were considered unlikely to be related to posaconazole treatment for three patients and possibly related (convulsions and cardiorespiratory arrest) for one of them.
Three deaths occurred in the juvenile population during the study. Two deaths (patients 2 and 7) were considered unlikely to be related to posaconazole therapy (one resulted from invasive fungal infection and the other from progression of acute lymphoblastic leukemia). The primary cause of death in the third patient (patient 10) was attributed to adverse events considered by the investigator to be possibly related to posaconazole treatment. The patient, a 16-year-old girl with a history of acute lymphoblastic leukemia, allogeneic bone marrow transplantation, and seizures, died on day 95 (1 day after the last dose of posaconazole) from cardiorespiratory failure after a prolonged seizure at home. Although the investigator could not eliminate posaconazole as a possible cause of the seizure, the event was most likely a recurrence of seizures noted before baseline. A fourth patient (patient 6), who had a complete response to posaconazole, died of progressive osteosarcoma 34 days after receiving the last posaconazole dose.
Posaconazole was generally safe and well tolerated for these juvenile patients, with only two patients reporting vomiting (one with concomitant nausea) possibly related to posaconazole (Table 3). In addition, two patients experienced renal insufficiency (possibly related to study treatment). In this small series of patients, there was no clear relationship between the duration of posaconazole exposure and the emergence of treatment-related adverse events.
Overall, the treatment-related adverse events were similar among juvenile and adult patients and consistent with a profoundly immunocompromised patient population with severe underlying disease. In the juvenile population, three of seven (43%) treatment-related adverse events were gastrointestinal. The most common (≥5%) treatment-related adverse events in the overall population (adult and juvenile) were nausea (9%), vomiting (6%), abdominal pain (5%), and headache (5%) (24).
Elevated liver function test results were reported for two juvenile patients: one patient (patient 4) had mild alanine aminotransferase elevation, and one patient (patient 7) had a moderate aspartate aminotransferase elevation. Both of these elevations were deemed unlikely to be related to posaconazole treatment.
DISCUSSION
This analysis compared posaconazole plasma Cav values for 12 juvenile patients with those for 194 adult patients. Patients enrolled in this phase 3 open-label clinical trial had invasive fungal infection and were intolerant of or had disease refractory to standard therapies. Our pharmacokinetic analysis showed that the range of posaconazole plasma Cav values was similar for juvenile and adult patients, with mean values of 776 ng/ml and 817 ng/ml, respectively. A secondary finding was that posaconazole was efficacious for five of eight (62.5%) juvenile patients, was generally safe and well tolerated, and had similar adverse event profiles for both juvenile and adult patients.
Results of studies examining the pediatric/juvenile pharmacokinetics of currently marketed azole antifungals, such as itraconazole (7, 12, 25), fluconazole (3, 15, 16, 26), and voriconazole (29), have been published. Two studies (12, 25) with itraconazole show similar peak and trough concentrations for pediatric and adult patients (17 patients, ages 2 to 18 years), and another (7) reports pediatric concentrations that were approximately one-third of those attained by adults (26 patients, ages 6 months to 12 years) (4, 7, 22, 23). The difference between pediatric and adult patients may result in part from greater itraconazole clearance in younger children (25). Fluconazole has an age-dependent volume of distribution that is greatest in neonates and decreases by young adulthood to values approaching those for adults (562 patients, ages 0 to 17) (3, 17). Compared with adults, fluconazole is eliminated much more slowly in neonates and premature infants and more rapidly in other pediatric patients (3, 17). Voriconazole is eliminated more rapidly in pediatric patients than it is in adult patients (35 patients, ages 2 to 11) (29).
In these studies, the safety profiles of itraconazole, fluconazole, and voriconazole were similar for adult and pediatric patients. For both adults and children, the adverse events most frequently reported with itraconazole (7, 12) and fluconazole (17) were gastrointestinal. Transient visual disturbances were the most common adverse events associated with voriconazole in children and in adults (29).
Our investigation focused on plasma posaconazole concentrations at an early stage of the drug development program and did not seek to provide exhaustive comparative data on the pharmacokinetics of posaconazole in juvenile and adult patients. In addition, the ITT population on which our analysis is based was limited to 12 juvenile patients, compared with 194 adults. Furthermore, the blood collection schedule for the juvenile population could not be strictly followed during the trial. However, limited plasma concentration data for juvenile patients did reveal a relatively flat steady-state profile consistent with a long terminal-phase half-life, as with adults. Although data are limited, a lack of association was apparent between the posaconazole plasma Cav and age, body weight, or body surface area. Posaconazole Cav values for the youngest (8-year-old) patient (227 ng/ml) and oldest (17-year-old) subject (238 ng/ml) were similar. It is noteworthy that some protocol deviations also occurred in juvenile studies with fluconazole; these deviations resulted from the clinical condition of the patients and from the priority given to their well-being rather than to the pharmacokinetic objectives of the studies (3).
Despite the limitations of the study and observed variability in concentrations within the juvenile and adult patient populations, the range of plasma posaconazole concentrations was similar for juvenile and adult patients with invasive fungal infection who were intolerant of or who had disease refractory to other antifungal therapy. There did not appear to be any association between Cav and treatment-related adverse events. A definitive relationship between plasma azole concentrations and clinical outcome has not been firmly established. Nevertheless, the similarity in concentrations suggests that clinical outcomes are expected to be similar for adults and children with refractory invasive fungal infection who receive posaconazole therapy. Our characterization of the plasma concentration-time profile of posaconazole in children supports the ongoing evaluation and management of this drug for juvenile patients with refractory invasive fungal infection.
Footnotes
Published ahead of print on 8 January 2007.
REFERENCES
- 1.Ascioglu, S., J. H. Rex, B. de Pauw, J. E. Bennett, J. Bille, F. Crokaert, D. W. Denning, J. P. Donnelly, J. E. Edwards, Z. Erjavec, D. Fiere, O. Lortholary, J. Maertens, J. F. Meis, T. F. Patterson, J. Ritter, D. Selleslag, P. M. Shah, D. A. Stevens, and T. J. Walsh. 2002. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin. Infect. Dis. 34:7-14. [DOI] [PubMed] [Google Scholar]
- 2.Barchiesi, F., D. Arzeni, A. W. Fothergill, L. F. Di Francesco, F. Caselli, M. G. Rinaldi, and G. Scalise. 2000. In vitro activities of the new antifungal triazole SCH 56592 against common and emerging yeast pathogens. Antimicrob. Agents Chemother. 44:226-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brammer, K. W., and P. E. Coates. 1994. Pharmacokinetics of fluconazole in pediatric patients. Eur. J. Clin. Microbiol. Infect. Dis. 13:325-329. [DOI] [PubMed] [Google Scholar]
- 4.Cartledge, J. D., J. Midgely, and B. G. Gazzard. 1997. Itraconazole solution: higher serum drug concentrations and better clinical response rates than the capsule formulation in acquired immunodeficiency syndrome patients with candidosis. J. Clin. Pathol. 50:477-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Courtney, R., S. Pai, M. Laughlin, J. Lim, and V. Batra. 2003. Pharmacokinetics, safety, and tolerability of oral posaconazole administered in single and multiple doses in healthy adults. Antimicrob. Agents Chemother. 47:2788-2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Courtney, R., D. Wexler, E. Radwanski, J. Lim, and M. Laughlin. 2004. Effect of food on the relative bioavailability of two oral formulations of posaconazole in healthy adults. Br. J. Clin. Pharmacol. 57:218-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Repentigny, L., J. Ratelle, J.-M. Leclerc, G. Cornu, E. M. Sokal, P. Jacqmin, and K. De Beule. 1998. Repeated-dose pharmacokinetics of an oral solution of itraconazole in infants and children. Antimicrob. Agents Chemother. 42:404-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diekema, D. J., S. A. Messer, R. J. Hollis, R. N. Jones, and M. A. Pfaller. 2003. Activities of caspofungin, itraconazole, posaconazole, ravuconazole, voriconazole, and amphotericin B against 448 recent clinical isolates of filamentous fungi. J. Clin. Microbiol. 41:3623-3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Espinel-Ingroff, A. 1998. Comparison of in vitro activities of the new triazole SCH56592 and the echinocandins MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J. Clin. Microbiol. 36:2950-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ezzet, F., D. Wexler, R. Courtney, G. Krishna, J. Lim, and M. Laughlin. 2005. Oral bioavailability of posaconazole in fasted healthy subjects: comparison between three regimens and basis for clinical dosage recommendations. Clin. Pharmacokinet. 44:211-220. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez, G. M., R. Tijerina, L. K. Najvar, R. Bocanegra, M. Rinaldi, D. Loebenberg, and J. R. Graybill. 2002. In vitro and in vivo activities of posaconazole against Coccidioides immitis. Antimicrob. Agents Chemother. 46:1352-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groll, A. H., L. Wood, M. Roden, D. Mickiene, C. C. Chiou, E. Townley, L. Dad, S. C. Piscitelli, and T. J. Walsh. 2002. Safety, pharmacokinetics, and pharmacodynamics of cyclodextrin itraconazole in pediatric patients with oropharyngeal candidiasis. Antimicrob. Agents Chemother. 46:2554-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herbrecht, R. 2004. Posaconazole: a potent, extended-spectrum triazole anti-fungal for the treatment of serious fungal infections. Int. J. Clin. Pract. 58:612-624. [DOI] [PubMed] [Google Scholar]
- 14.Krieter, P., B. Flannery, T. Musick, M. Gohdes, M. Martinho, and R. Courtney. 2004. Disposition of posaconazole following single-dose oral administration in healthy subjects. Antimicrob. Agents Chemother. 48:3543-3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee, J. W., N. L. Seibel, M. Amantea, P. Whitcomb, P. A. Pizzo, and T. J. Walsh. 1992. Safety and pharmacokinetics of fluconazole in children with neoplastic diseases. J. Pediatr. 120:987-993. [DOI] [PubMed] [Google Scholar]
- 16.Nahata, M. C., and M. T. Brady. 1995. Pharmacokinetics of fluconazole after oral administration in children with human immunodeficiency virus infection. Eur. J. Clin. Pharmacol. 48:291-293. [DOI] [PubMed] [Google Scholar]
- 17.Novelli, V., and H. Holzel. 1999. Safety and tolerability of fluconazole in children. Antimicrob. Agents Chemother. 43:1955-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ostrosky-Zeichner, L., J. H. Rex, P. G. Pappas, R. J. Hamill, R. A. Larsen, H. W. Horowitz, W. G. Powderly, N. Hyslop, C. A. Kauffman, J. Cleary, J. E. Mangino, and J. Lee. 2003. Antifungal susceptibility survey of 2,000 bloodstream Candida isolates in the United States. Antimicrob. Agents Chemother. 47:3149-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfaller, M. A., S. A. Messer, R. J. Hollis, and R. N. Jones. 2001. In vitro activities of posaconazole (SCH 56592) compared with those of itraconazole and fluconazole against 3,685 clinical isolates of Candida spp. and Cryptococcus neoformans. Antimicrob. Agents Chemother. 45:2862-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfaller, M. A., S. A. Messer, R. J. Hollis, R. N. Jones, and the SENTRY Participants Group. 2002. Antifungal activities of posaconazole, ravuconazole, and voriconazole compared to those of itraconazole and amphotericin B against 239 clinical isolates of Aspergillus spp. and other filamentous fungi: report from SENTRY Antimicrobial Surveillance Program, 2000. Antimicrob. Agents Chemother. 46:1032-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfaller, M. A., S. A. Messera, L. Boyken, R. J. Hollis, C. Rice, S. Tendolkar, and D. J. Diekema. 2004. In vitro activities of voriconazole, posaconazole, and fluconazole against 4,169 clinical isolates of Candida spp. and Cryptococcus neoformans collected during 2001 and 2002 in the ARTEMIS Global Antifungal Surveillance Program. Diagn. Microbiol. Infect. Dis. 48:201-205. [DOI] [PubMed] [Google Scholar]
- 22.Prentice, A. G., D. W. Warnock, S. A. Johnson, P. C. Taylor, and D. A. Oliver. 1995. Multiple dose pharmacokinetics of an oral solution of itraconazole in patients receiving chemotherapy for acute myeloid leukaemia. J. Antimicrob. Chemother. 36:657-663. [DOI] [PubMed] [Google Scholar]
- 23.Prentice, A. G., D. W. Warnock, S. A. N. Johnson, M. J. Phillips, and D. A. Oliver. 1994. Multiple dose pharmacokinetics of an oral solution of itraconazole in autologous bone marrow transplant recipients. J. Antimicrob. Chemother. 34:247-252. [DOI] [PubMed] [Google Scholar]
- 24.Raad, I. I., J. R. Graybill, A. B. Bustamante, O. A. Cornely, V. Gaona-flores, C. Afif, D. R. Graham, R. N. Greenberg, S. Hadley, A. Langston, R. Negroni, J. R. Perfect, P. Pitisuttithum, A. Restrepo, G. Schiller, L. Pedicone, and A. J. Ullmann. 2006. Safety of long-term oral posaconazole use in the treatment of refractory invasive fungal infections. Clin. Infect. Dis. 42:1726-1734. [DOI] [PubMed] [Google Scholar]
- 25.Schmitt, C., Y. Perel, J.-L. Harousseau, S. Lemerle, E. Chwetzoff, J.-P. le Moing, and J.-C. Levron. 2001. Pharmacokinetics of itraconazole oral solution in neutropenic children during long-term prophylaxis. Antimicrob. Agents Chemother. 45:1561-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seay, R. E., T. A. Larson, J. P. Toscano, B. C. Bostrom, M. C. O'Leary, and D. L. Uden. 1995. Pharmacokinetics of fluconazole in immune-compromised children with leukemia or other hematologic diseases. Pharmacotherapy 15:52-58. [PubMed] [Google Scholar]
- 27.Sun, Q. N., A. W. Fothergill, D. I. McCarthy, M. G. Rinaldi, and J. R. Graybill. 2002. In vitro activities of posaconazole, itraconazole, voriconazole, amphotericin B, and fluconazole against 37 clinical isolates of Zygomycetes. Antimicrob. Agents Chemother. 46:1581-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walsh, T., T. Patterson, A. Langston, J. A. van Burik, A. Louie, R. Herbrecht, S. Hadley, J. Perfect, C. Hsu, J. Gogate, C. Hardalo, H. Patino, L. Pedicone, G. Corcoran, I. Raad, and A. S. Wayne. 2003. Posaconazole for treatment of invasive aspergillosis in patients who are refractory to or intolerant of conventional therapy: an externally controlled blinded trial. Blood 102:195-196. [Google Scholar]
- 29.Walsh, T. J., M. O. Karlsson, T. Driscoll, A. G. Arguedas, P. Adamson, X. Saez-Llorens, A. J. Vora, A. C. Arrieta, J. Blumer, I. Lutsar, P. Milligan, and N. Wood. 2004. Pharmacokinetics and safety of intravenous voriconazole in children after single- or multiple-dose administration. Antimicrob. Agents Chemother. 48:2166-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wexler, D., R. Courtney, W. Richards, C. Banfield, J. Lim, and M. Laughlin. 2004. Effect of posaconazole on cytochrome P450 enzymes: a randomized, open-label, two-way crossover study. Eur. J. Pharm. Sci. 21:645-653. [DOI] [PubMed] [Google Scholar]