Abstract
We report that quinoline derivative MC1626, first described as an inhibitor of the histone acetyltransferase (HAT) GCN5, is active against the protozoan parasite Toxoplasma gondii in vitro. However, MC1626 does not inhibit Toxoplasma GCN5 HATs or reduce HAT-mediated activity; rather, this quinoline may target the plastid organelle called the apicoplast.
Histone acetyltransferases (HATs) play a central role in modifying chromatin to create a favorable environment for DNA processes, including transcription. Many genetic studies have implicated HATs as important factors in disease (for reviews, see references 8, 13, and 18). The impact of HATs on cellular physiology and disease would benefit from the identification of specific pharmacological inhibitors, but very few have been described. Two natural products, anacardic acid and garcinol, inhibit p300/CBP (CREB-binding protein) and PCAF (p300/CBP-associating factor) in a 5- to 10-μM range in vitro (1, 2). In contrast, curcumin displays activity against p300/CBP, but not PCAF (3). Anacardic acid may be a broad-spectrum HAT inhibitor, as it also interferes with the MYST (named for members MOZ, Ybf2/Sas3, Sas2, and Tip60) HAT Tip60 (16). Isothiazolones were identified as inhibitors of PCAF and p300 (15), but like the aforementioned compounds, activity against GCN5 was not determined. Moreover, isothiazolones are strongly reactive with thiol groups and likely to have substantial nonspecific effects. Small-molecule inhibitors of GCN5 include a butyrolactone (5) and MC1626 (2-methyl-3-carbethoxyquinoline) (11).
We have established that histone acetylation correlates with differentiation of the protozoan parasite Toxoplasma gondii (phylum Apicomplexa) (14). Differentiation from the rapidly growing tachyzoite stage to the encysted bradyzoite stage is critical to pathogenesis (6). Serious opportunistic Toxoplasma infections occur in immunocompromised patients because the encysted bradyzoites cannot be cleared by immunity or drugs (17).
MC1626 inhibits Toxoplasma growth in vitro.
Given that histone acetylation accompanies changes in gene expression relevant to Toxoplasma differentiation, HATs may serve as attractive candidates for drug design. Therefore, we tested the impact of MC1626 (0 to 300 μM) on Toxoplasma grown in human foreskin fibroblasts by using standard [3H]uracil uptake and plaque assays (12). Relative to a vehicle control (dimethyl sulfoxide [DMSO]), MC1626 curtails parasite growth in a dose-dependent fashion, with 100 μM reducing growth by ∼50%. Host cells showed no obvious signs of damage until concentrations of MC1626 exceeded 300 μM.
MC1626 does not inhibit parasite HAT activities.
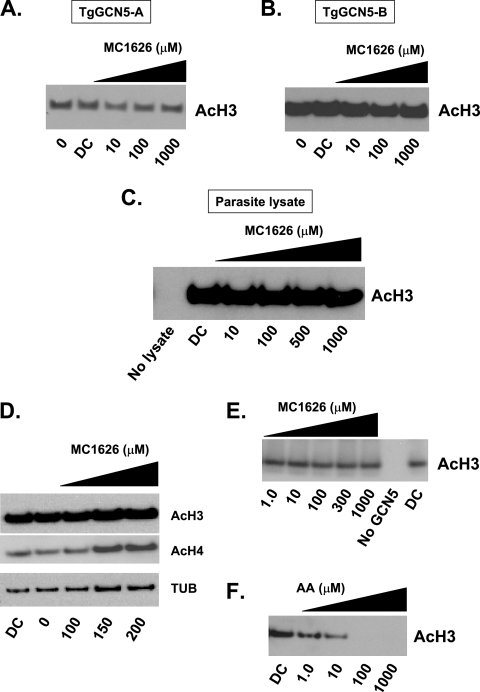
We examined if MC1626 diminished the HAT activity of Toxoplasma GCN5 on histone H3, its preferred substrate (4). As shown in Fig. 1A and B, recombinant TgGCN5-A and -B (4) exhibit robust HAT activity on H3 in a standard in vitro HAT assay (7). However, MC1626 does not appear to have any significant effect on the ability of recombinant TgGCN5-A or -B to acetylate H3. We considered that MC1626 may act as a prodrug that must be processed by parasite factors in order to become capable of inhibiting GCN5. Therefore, we examined the ability of MC1626 to ablate HAT activities from Toxoplasma lysates. Results indicate that Toxoplasma lysate acetylates the H3 substrate, but MC1626 has no detectable impact on its ability to do so (Fig. 1C). We also evaluated if MC1626 alters levels of acetylated histones in cultured Toxoplasma by two independent means. In an immunofluorescence assay, parasites at 24 to 72 h postinfection continued to exhibit intense staining for acetylated H3 despite the presence of 200 μM MC1626 (data not shown). Immunoblotting showed that lysates of tachyzoites cultured in increasing concentrations of MC1626 for 3 days also exhibited no decrease in overall acetylated H3 (Fig. 1D). We also examined levels of acetylated H4 and again detected no significant decrease in drug-treated parasites, suggesting that MC1626 does not inhibit MYST HAT activities either (Fig. 1D).
FIG. 1.
Effects of MC1626 on HAT activities. HAT assays were performed with recombinant TgGCN5-A (A) or TgGCN5-B (B) in the presence of increasing concentrations of MC1626 or a DMSO vehicle control (DC). (C) HAT assays with 0.5 μg of tachyzoite lysate as the enzyme source in the presence of increasing concentrations of MC1626. (D) Histone acetylation levels in lysates of parasites treated with MC1626. Tachyzoites were incubated in the presence of various concentrations of MC1626 or with DMSO for 3 days. After treatment, lysates were made to assess by Western blotting if acetylation of H3 or H4 was diminished relative to that of the untreated or vehicle-treated control. Tubulin (TUB) was used as a loading control. Note that the final two lanes are overloaded, which likely explains the apparent increase in histone acetylation seen in these samples. (E) MC1626 does not inhibit recombinant yeast GCN5 in vitro. Recombinant S. cerevisiae GCN5 (1.0 μg) was used as the enzyme source in HAT assays containing no drug or designated concentrations of MC1626. (F) Control experiment demonstrating that S. cerevisiae GCN5 (1.0 μg) is inhibited by anacardic acid (AA). AcH3 and AcH4, acetylated H3 and H4, respectively.
Recombinant yeast GCN5 is not inhibited by MC1626.
MC1626 was described as a GCN5 inhibitor on the basis of indirect observations; however, no direct test was performed (11). Figure 1E shows that the ability of recombinant yeast Gcn5 to acetylate H3 in the HAT assay is not inhibited by MC1626. A control experiment was performed with anacardic acid (Fig. 1F), a bona fide inhibitor of GCN5 family HATs (2). Considered along with the above studies, these data strongly suggest that the growth-inhibitory effects of MC1626 on yeast and Toxoplasma are not due to direct inhibition of Gcn5. We cannot dismiss the possibility that Saccharomyces cerevisiae processes MC1626 differently than Toxoplasma. Alternatively, MC1626 may perturb the ability of yeast Gcn5 to associate with cofactors required for its HAT activity in vivo. Further investigation is required to resolve the mechanism of action of MC1626 in S. cerevisiae.
Anacardic acid has been shown to inhibit PCAF, a member of the GCN5 family, as well as the MYST HAT Tip60 (2, 16). This is the first report formally showing that it also inhibits GCN5. The effects of anacardic acid on Toxoplasma growth and HAT activities are currently being investigated.
Apicoplast loss in Toxoplasma treated with MC1626 and quinoline.
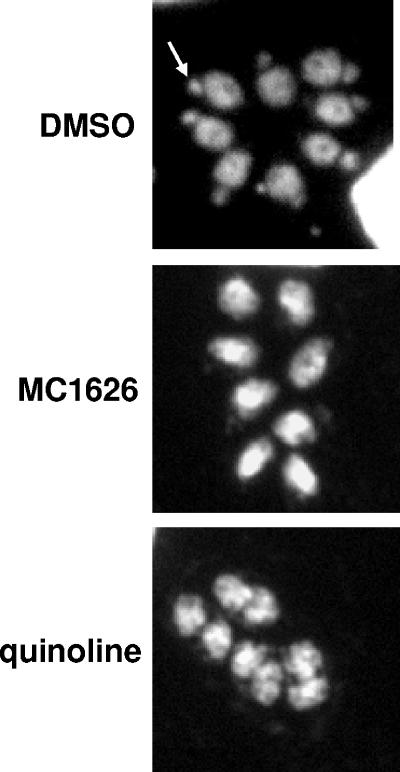
The chemical structure of MC1626 (11) is related to that of quinolones, which target the apicoplast (9), a plastid organelle in many apicomplexans that houses an extrachromosomal element acquired through secondary endosymbiosis of an alga (10). We tested if MC1626 treatment is linked to the disappearance of apicoplast DNA. DNA staining with 4′,6′-diamidino-2-phenylindole (DAPI) shows the apicoplast as a distinct organelle anterior to the parasite nucleus (Fig. 2). Parasites treated with fluoroquinolones such as ciprofloxacin no longer exhibit this staining pattern because of the loss of apicoplast DNA (9). To test if MC1626 functions similarly, we examined if Toxoplasma maintained in 200 μM MC1626 exhibited a decrease in the number of parasites containing apicoplasts. Figure 2 is a representative image of MC1626-treated parasites that have lost their apicoplasts. We also tested the effect of quinoline (Sigma), which inhibits Toxoplasma growth at similar concentrations (data not shown). The results obtained are identical and further suggest that this class of drug perturbs the apicoplast (Fig. 2). A count of 50 random vacuoles indicated that apicoplasts are present in only 52 to 57% of MC1626- and quinoline-treated parasites, respectively. This is the first demonstration that quinolines and quinoline derivatives have activity against Toxoplasma and share a mechanism of action similar to that of fluoroquinolones. While some quinolines, e.g., chloroquine, have no effect on Toxoplasma, derivatives such as MC1626 may prove useful in developing therapeutics.
FIG. 2.
Effects of quinolines on the parasite apicoplast organelle. Toxoplasma tachyzoites were cultured in the presence of DMSO (control), 200 μM MC1626, or 200 μM quinoline prior to processing for DAPI staining. DAPI stains DNA, including the extrachromosomal DNA of the apicoplast. An example apicoplast is indicated by the arrow as a distinct dot anterior to the parasite nucleus.
In summary, these studies are significant because they argue that MC1626 is not a direct inhibitor of GCN5, as previously reported, and they suggest that quinolines and quinoline derivatives (e.g., MC1626) may be novel leads for drug development because they selectively kill parasites by targeting their unique apicoplasts.
Acknowledgments
This work was supported by Public Health Service grant GM065051 from the National Institutes of Health.
We thank Arunasalam Naguleswaran (IUSM) for critical reading of the manuscript. We also acknowledge Rachael Herrell for technical assistance in the initial characterization of MC1626 activity against Toxoplasma.
Footnotes
Published ahead of print on 18 December 2006.
REFERENCES
- 1.Balasubramanyam, K., M. Altaf, R. A. Varier, V. Swaminathan, A. Ravindran, P. P. Sadhale, and T. K. Kundu. 2004. Polyisoprenylated benzophenone, garcinol, a natural histone acetyltransferase inhibitor, represses chromatin transcription and alters global gene expression. J. Biol. Chem. 279:33716-33726. [DOI] [PubMed] [Google Scholar]
- 2.Balasubramanyam, K., V. Swaminathan, A. Ranganathan, and T. K. Kundu. 2003. Small molecule modulators of histone acetyltransferase p300. J. Biol. Chem. 278:19134-19140. [DOI] [PubMed] [Google Scholar]
- 3.Balasubramanyam, K., R. A. Varier, M. Altaf, V. Swaminathan, N. B. Siddappa, U. Ranga, and T. K. Kundu. 2004. Curcumin, a novel p300/CREB-binding protein-specific inhibitor of acetyltransferase, represses the acetylation of histone/nonhistone proteins and histone acetyltransferase-dependent chromatin transcription. J. Biol. Chem. 279:51163-51171. [DOI] [PubMed] [Google Scholar]
- 4.Bhatti, M. M., M. Livingston, N. Mullapudi, and W. J. Sullivan, Jr. 2006. Pair of unusual GCN5 histone acetyltransferases and ADA2 homologues in the protozoan parasite Toxoplasma gondii. Eukaryot. Cell 5:62-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biel, M., A. Kretsovali, E. Karatzali, J. Papamatheakis, and A. Giannis. 2004. Design, synthesis, and biological evaluation of a small-molecule inhibitor of the histone acetyltransferase Gcn5. Angew. Chem. Int. Ed. Engl. 43:3974-3976. [DOI] [PubMed] [Google Scholar]
- 6.Black, M. W., and J. C. Boothroyd. 2000. Lytic cycle of Toxoplasma gondii. Microbiol. Mol. Biol. Rev. 64:607-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brownell, J. E., J. Zhou, T. Ranalli, R. Kobayashi, D. G. Edmondson, S. Y. Roth, and C. D. Allis. 1996. Tetrahymena histone acetyltransferase A: a homolog to yeast Gcn5p linking histone acetylation to gene activation. Cell 84:843-851. [DOI] [PubMed] [Google Scholar]
- 8.Davis, P. K., and R. K. Brackmann. 2003. Chromatin remodeling and cancer. Cancer Biol. Ther. 2:22-29. [DOI] [PubMed] [Google Scholar]
- 9.Fichera, M. E., and D. S. Roos. 1997. A plastid organelle as a drug target in apicomplexan parasites. Nature 390:407-409. [DOI] [PubMed] [Google Scholar]
- 10.Köhler, S., C. F. Delwiche, P. W. Denny, L. G. Tilney, P. Webster, R. J. Wilson, J. D. Palmer, and D. S. Roos. 1997. A plastid of probable green algal origin in apicomplexan parasites. Science 275:1485-1489. [DOI] [PubMed] [Google Scholar]
- 11.Ornaghi, P., D. Rotili, G. Sbardella, A. Mai, and P. Filetici. 2005. A novel Gcn5p inhibitor represses cell growth, gene transcription and histone acetylation in budding yeast. Biochem. Pharmacol. 70:911-917. [DOI] [PubMed] [Google Scholar]
- 12.Roos, D. S., R. G. Donald, N. S. Morrissette, and A. L. Moulton. 1994. Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. Methods Cell Biol. 45:27-63. [DOI] [PubMed] [Google Scholar]
- 13.Roth, S. Y., J. M. Denu, and C. D. Allis. 2001. Histone acetyltransferases. Annu. Rev. Biochem. 70:81-120. [DOI] [PubMed] [Google Scholar]
- 14.Saksouk, N., M. M. Bhatti, S. Kieffer, A. T. Smith, K. Musset, J. Garin, W. J. Sullivan, Jr., M. F. Cesbron-Delauw, and M. A. Hakimi. 2005. Histone-modifying complexes regulate gene expression pertinent to the differentiation of the protozoan parasite Toxoplasma gondii. Mol. Cell. Biol. 25:10301-10314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stimson, L., M. G. Rowlands, Y. M. Newbatt, N. F. Smith, F. I. Raynaud, P. Rogers, V. Bavetsias, S. Gorsuch, M. Jarman, A. Bannister, T. Kouzarides, E. McDonald, P. Workman, and G. W. Aherne. 2005. Isothiazolones as inhibitors of PCAF and p300 histone acetyltransferase activity. Mol. Cancer Ther. 4:1521-1532. [DOI] [PubMed] [Google Scholar]
- 16.Sun, Y., X. Jiang, S. Chen, and B. D. Price. 2006. Inhibition of histone acetyltransferase activity by anacardic acid sensitizes tumor cells to ionizing radiation. FEBS Lett. 580:4353-4356. [DOI] [PubMed] [Google Scholar]
- 17.Weiss, L. M., and K. Kim. 2000. The development and biology of bradyzoites of Toxoplasma gondii. Front. Biosci. 5:D391-D405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang, X. J. 2004. The diverse superfamily of lysine acetyltransferases and their roles in leukemia and other diseases. Nucleic Acids Res. 32:959-976. [DOI] [PMC free article] [PubMed] [Google Scholar]