Abstract
Azithromycin (AZM) ameliorates lung function in cystic fibrosis (CF) patients. This macrolide has been suggested to have anti-inflammatory properties as well as other effects potentially relevant for therapy of CF. In this study, we utilized three CF (IB3-1, 16HBE14o- AS3, and 2CFSMEo-) and two isogenic non-CF (C38 and 16HBE14o- S1) airway epithelial cell lines to investigate whether AZM could reduce tumor necrosis factor alpha (TNF-α) mRNA and protein levels by real-time quantitative PCR analysis and an enzyme-linked immunosorbent assay (ELISA), respectively. We studied the effects on the DNA binding of NF-κB and specificity protein 1 (Sp1) by an ELISA. Non-CF cells express significantly lower TNF-α mRNA and protein levels than an isogenic CF cell line. In CF cells, AZM treatment causes a 30% reduction of TNF-α mRNA levels (P < 0.05) and a 45% decrease in TNF-α secretion (P < 0.05), reaching approximately the levels of the untreated isogenic non-CF cells. In CF cells, NF-κB and Sp1 DNA binding activities were also significantly decreased (about 45 and 60%, respectively; P < 0.05) after AZM treatment. Josamycin, a macrolide lacking clinically described anti-inflammatory effects, was ineffective. Finally, AZM did not alter the mRNA expression levels of interleukin-6, a proinflammatory molecule not differentially expressed in CF and isogenic non-CF cells. The results of our study support the anti-inflammatory activities of this macrolide, since we show that AZM reduced the levels of TNF-α and propose inhibitions of NF-κB and Sp1 DNA binding as possible mechanisms of this effect.
Cystic fibrosis (CF) is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene encoding a transmembrane chloride channel.
Neutrophil-dominated inflammation is a hallmark of the airway disease in CF. Uncontrolled release of neutrophilic cytotoxic contents leads to mucus hypersecretion and progressive lung damage, ultimately contributing to morbidity and mortality in CF patients.
It remains controversial as to whether there is an intrinsic hyperinflammatory state arising directly from a lack of a functional CFTR (10, 24). The hypothesis of primary inflammation preceding detectable infection is based first on clinical observations of inflammation in CF neonates and young children (25, 34) but also on experimental reports (12, 40). Other evidences suggest that CF airway inflammatory response to infectious agents is exaggerated and/or prolonged (33). CF patients have been shown to exhibit larger amounts of neutrophils and interleukin-8 (IL-8) in bronchoalveolar lavage fluid than non-CF subjects in response to similar levels of infection (28). Furthermore, CF airway epithelial cell lines produced larger quantities of IL-8 than CFTR-corrected cells in response to IL-1β and tumor necrosis factor alpha (TNF-α) (1, 43) and to bacterial stimulation (16, 43).
Clinical studies have shown that macrolides have beneficial effects on lung function in CF patients (18, 36). CF patients treated with azithromycin (AZM) experienced improvement in the viscoelasticity of the sputum (4), decreased content of mucoid Pseudomonas aeruginosa in sputum samples (19), a decline in the number of pulmonary exacerbations (30, 35), and significant increases in respiratory function parameters such as FEV1 (forced expiratory volume in 1 second) and FVC (forced vital capacity) (19, 30, 35).
High concentrations of TNF-α (8, 43), a proinflammatory cytokine able to induce the production of secondary mediators by epithelial cells, including cytokines (e.g., IL-8), have been observed in CF airway fluids.
The aim of this work was to evaluate whether AZM could reduce TNF-α levels in CF cells and whether CF cells express larger amounts of this cytokine than non-CF cells. Finally, we investigated the molecular mechanisms of AZM effects on these cells by studying the transcriptional activities of NF-κB and specificity protein 1 (Sp1) transcription factors before and after incubation with AZM, as both of these proteins are involved in the regulation of TNF-α gene expression.
MATERIALS AND METHODS
Cell cultures.
IB3-1 and isogenic C38 cells, with CF and non-CF phenotypes, respectively, obtained from P. Zeitlin (Johns Hopkins University, MD) (46), were grown in LHC-8 medium (Biosource, Rockville, MD) supplemented with 5% fetal bovine serum (FBS) (Cambrex Bio Science, Verviers, Belgium). IB3-1 cells were derived from the bronchial epithelium of a CF patient, and the isogenic rescued C38 cell lines express a plasmid-encoded functional CFTR (46).
16HBE14o- AS3 and the isogenic 16HBE14o- S1 cell lines, with CF and non-CF phenotypes, respectively (32), obtained from P. Davis (Case Western Reserve University, OH), were grown in Eagle's minimal essential medium (Cambrex Bio Science) supplemented with 10% FBS, 1% l-glutamine (Cambrex Bio Science), and 0.4% G418 sulfate (Calbiochem, CN Biosciences, La Jolla, CA). 16HBE14o- AS3 cells lack CFTR expression following transfection with an antisense CFTR sequence, while isogenic 16HBE14o- S1 cells transfected with a sense CFTR sequence express wild-type CFTR (32).
The CF cell line 2CFSMEo-, a kind gift of D. Gruenert (University of California) (13), was derived from submucosal tracheobronchial glands of a CF patient and grown in Eagle's minimal essential medium supplemented with 10% FBS and 1% l-glutamine.
Epithelial respiratory cell lines were cultured at 37°C in a humidified atmosphere with 5% CO2. Cells were seeded in a concentration of 1.5 × 105 cells/cm2, and after 24 h, they were exposed to up to 8 μg/ml AZM (Pfizer, Italy) and/or josamycin (JM; Yamanouchi Pharmaceutical, Japan) for 24 h. These concentrations, which are in the sub-MIC range for P. aeruginosa, are consistent with those observed in lungs of patients treated with AZM (17, 23).
In the presence of 8 μg/ml AZM for 24 h, cell viability was >95%, as determined by a trypan blue exclusion test, while at higher concentrations starting from 16 μg/ml, viability was <95%.
Serial dilutions (0, 0.125, 0.5, 2, and 8 μg/ml) of the macrolides were utilized in dose-response experiments. We observed no statistically significant effects on TNF-α expression after treatment with AZM concentrations lower than 8 μg/ml (data not shown).
RNA isolation, quantification, and reverse transcription.
Cells were lysed. Total RNA was extracted with a total RNA isolation kit (Roche, Germany) and converted to cDNA using a high-capacity cDNA archive kit (Applied Biosystems). The reaction mixture was then incubated at 25°C for 10 min and at 37°C for 2 h.
RNA quantification.
Relative quantification of gene expression was performed by real-time quantitative PCR analysis as described by the manufacturer (Applied Biosystems User Bulletin 2). The cDNA (2 μl) was amplified using Platinum SYBR green quantitative PCR supermix-UDG (Invitrogen, Grand Island, NY) in the ABI Prism 5700 sequence detection system. Primer Express Software (Applied Biosystems) was used to select the specific primers. The primer sets (Sigma-Genosys, St. Louis, MO) are shown in Table 1. The PCR thermal protocol consisted of 2 min at 50°C, a denaturation step at 95°C for 2 min, followed by 50 cycles of a 15-s 95°C denaturation step, and a 30-s annealing/extension step at 60°C. The real-time PCRs were performed in duplicate for both target and normalizer genes. Changes in mRNA expression level were calculated following normalization to GAPDH (glyceraldehyde-3-phosphate). The degree of variation of the target gene/normalizer gene ratios in the experiments was less than 8% in each cell line. Results were expressed as means ± standard deviations (SD).
TABLE 1.
Primer sequences utilized in the quantitative PCR analysis
| Gene | Primera | Sequence (5′→3′) | Accession no. | Primer concn (nM) |
|---|---|---|---|---|
| TNF-α gene | FW | GGACCTCTCTCTAATCAGCCCTC | NM_000594 | 25 |
| RV | TCGAGAAGATGATCTGACTGCC | 25 | ||
| IL-6 gene | FW | CGGTACATCCTCGACGGC | NM_000600 | 25 |
| RV | CTTGTTACATGTCTCCTTTCTCAGG | 450 | ||
| GAPDH gene | FW | GTGGAGTCCACTGGCGTCTT | J04038 | 25 |
| RV | GCAAATGAGCCCAGCCTTC | 150 |
FW, forward; RW, reverse.
Protein quantification.
TNF-α secretion in supernatants from the cell cultures described above was determined by an enzyme-amplified sensitivity immunoassay using a TNF-α EASIA kit (Bender MedSystems, Austria) according to the manufacturer's instructions. The limit of detection was 3.83 pg/ml. Measurements were performed at least in duplicate. Values were normalized to 106 cells; results were expressed as means ± SD.
DNA binding activity studies.
DNA binding activities of NF-κB and Sp1 in nuclear extracts were measured using TransAM kits (Active Motif, Belgium) and a Mercury TransFactor profiling kit (Clontech), respectively, according to the manufacturers' instructions. Measurements were performed at least in duplicate; results were expressed as means ± SD.
Statistical analysis.
Statistical calculations and tests were performed using the Friedman test for comparison between non-CF and CF cells and the Mann-Whitney U test for the evaluation of treatments with macrolides of the CF cell line.
The limit of statistical significance was defined as a P value of ≤0.05. All data were expressed as means ± SD.
RESULTS
Regulation of TNF-α mRNA expression by AZM treatment.
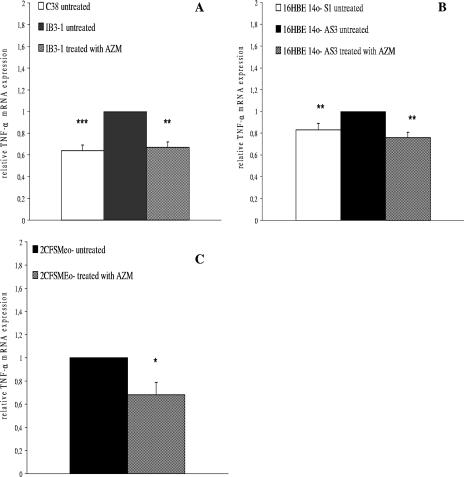
In this study, first of all, we measured the expression levels of the TNF-α gene. All cell lines constitutively expressed TNF-α mRNA; however, the level of basal expression in CF cells was significantly higher than in isogenic non-CF cells (Fig. 1). We confirmed this differential TNF-α expression by using cells at different passages as well as after 96 h from sedimentation (data not shown). Following exposure of CF cell lines to 8 μg of AZM for 24 h, we found that this macrolide reduced TNF-α mRNA levels of about 35% and 25% in IB3-1 and 16HBE14o- AS3 cells, respectively (n = 5; P < 0.01) (Fig. 1A and B), approximately to the levels of untreated isogenic non-CF cells C38 and 16HBE14o- S1, respectively. A 30% reduction of TNF-α mRNA levels was detected in 2CFSMEo- cells after AZM treatment (n = 5; P < 0.05) (Fig. 1C). The macrolide JM, known to lack clinical anti-inflammatory properties, had no significant effects on TNF-α mRNA expression in all cell lines (data not shown).
FIG. 1.
TNF-α mRNA expression. Expression of TNF-α mRNA in (A) non-CF C38 cells and an isogenic CF IB3-1 cell line, (B) non-CF 16HBE14o- S1 cells and an isogenic CF 16HBE14o- AS3 cell line, and (C) 2CFSMEo- cells at a constitutive level and after treatment with AZM. Total RNA was extracted and retrotranscribed. The values of TNF-α mRNA are based on real-time PCR analysis. The values represent the expression levels relative to those of untreated (A) IB3-1, (B) 16HBE14o- AS3, and (C) 2CFSMEo- cells (means ± SD). The experiment was repeated five times. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Effects of AZM treatment on IL-6 mRNA expression.
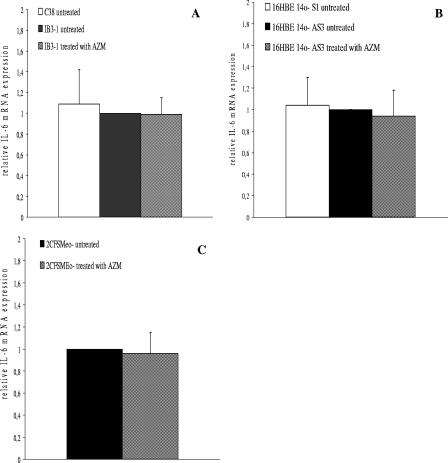
In terms of IL-6 mRNA expression, we found no statistically significant differences between CF cell lines and isogenic non-CF cells (Fig. 2). We then exposed CF cell lines to 8 μg of AZM for 24 h. AZM had no statistically significant effects on IL-6 mRNA expression in IB3-1, 16HBE14o- AS3, and 2CFSMEo- cells (Fig. 2A, B, and C, respectively).
FIG. 2.
IL-6 mRNA expression. Expression of IL-6 mRNA in (A) non-CF C38 cells and an isogenic CF IB3-1 cell line, (B) non-CF 16HBE14o- S1 cells and an isogenic CF 16HBE14o- AS3 cell line, and (C) 2CFSMEo- cells at a constitutive level and after treatment with AZM. Total RNA was extracted and retrotranscribed. The values of IL-6 mRNA are based on real-time PCR analysis. The values represent the expression levels relative to those of untreated (A) IB3-1, (B) 16HBE14o- AS3, and (C) 2CFSMEo- cells (means ± SD). The experiment was repeated five times.
Regulation of TNF-α protein levels by AZM treatment.
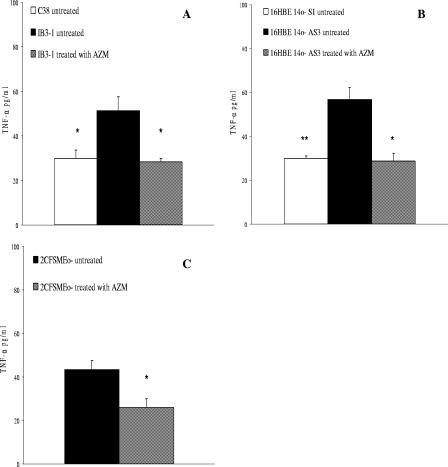
We confirmed the higher TNF-α expression in CF cells than in isogenic non-CF cells at the protein level (Fig. 3). Treatment with 8 μg of AZM was effective in reducing TNF-α protein levels of 45% and 50% in IB3-1 and 16HBE14o- AS3 cells treated for 24 h, respectively (n = 3; P < 0.05) (Fig. 3A and B), to the levels of untreated isogenic non-CF cells. Furthermore AZM reduced TNF-α protein expression of 40% in 2CFSMEo- cells (n = 3; P < 0.05) (Fig. 3C).
FIG. 3.
TNF-α protein release. Secretion of TNF-α in (A) non-CF C38 cells and an isogenic CF IB3-1 cell line, (B) non-CF 16HBE14o- S1 cells and an isogenic CF 16HBE14o- AS3 cell line, and (C) 2CFSMEo- cells at a constitutive level and after treatment with AZM and JM. TNF-α protein levels were measured by a commercial enzyme-linked immunosorbent assay kit. The values represent the secretion levels relative to those of untreated (A) IB3-1, (B) 16HBE14o- AS3, and (C) 2CFSMEo- cells (means ± SD). The experiment was repeated three times. *, P < 0.05; **, P < 0.01.
Effects of AZM on NF-κB DNA binding activity.
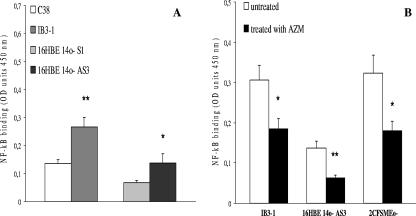
We found that IB3-1 and 16HBE14o- AS3 cells showed twofold-higher constitutive NF-κB DNA binding levels than isogenic non-CF cell lines C38 and 16HBE14o- S1 (n = 3; P < 0.01 and P < 0.05, respectively) (Fig. 4A). Following exposure of CF cell lines to 8 μg/ml AZM for 24 h, NF-κB DNA binding activity in IB3-1 and 16HBE14o- AS3 cells was reduced from 40% and 45%, respectively, nearly to the levels of untreated C38 and 16HBE14o- S1 cells. A 45% reduction of NF-κB DNA binding activity was detected in 2CFSMEo- cells after AZM treatment (n = 3; P < 0.05) (Fig. 4B). Furthermore, JM had no effects on NF-κB DNA binding activity (data not shown).
FIG. 4.
DNA binding of NF-κB. (A) Constitutive binding to the DNA of NF-κB in non-CF C38 cells and an isogenic CF IB3-1 cell line and in non-CF 16HBE14o- S1 cells and an isogenic CF 16HBE14o- AS3 cell line. (B) Effect of the treatment with AZM on the DNA binding of NF-κB in CF cells (IB3-1, 16HBE14o- AS3, and 2CFSMEo-). NF-κB DNA binding activity was analyzed using a commercial kit following the manufacturer's instructions. The experiment was repeated three times (means ± SD). *, P < 0.05; **, P < 0.01. OD, optical density.
Effects of AZM on Sp1 DNA binding activity.
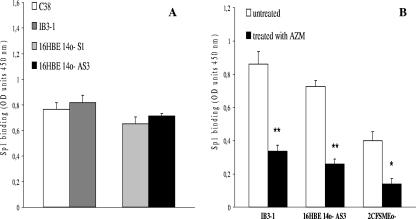
We decided to evaluate whether AZM could affect the levels of Sp1 DNA binding. We did not detect statistically significant differences in the constitutive Sp1 DNA binding levels in IB3-1 and 16HBE14o- AS3 cells versus those of isogenic non-CF cell lines C38 and 16HBE14o- S1, respectively, as shown in Fig. 5A. After 24 h of treatment with 8 μg of AZM, 60%, 64%, and 65% Sp1 DNA binding activity reductions were detected in IB3-1, 16HBE14o- AS3, and 2CFSMEo- cells, respectively (n = 3; P < 0.01, P < 0.01, and P < 0.05, respectively) (Fig. 5B). JM was also not effective on Sp1 DNA binding activity (data not shown).
FIG. 5.
DNA binding of Sp1. (A) Constitutive binding to the DNA of Sp1 in non-CF C38 cells and an isogenic CF IB3-1 cell line and in non-CF 16HBE14o- S1 cells and an isogenic CF 16HBE14o- AS3 cell line. (B) Effect of the treatment with AZM on the DNA binding of Sp1 in CF cells (IB3-1, 16HBE14o- AS3, and 2CFSMEo-). Sp1 DNA binding activity was analyzed using a commercial kit following the manufacturer's instructions. The experiment was repeated three times (means ± SD). *, P < 0.05; **, P < 0.01. OD, optical density.
DISCUSSION
Defective expression or function of the CFTR channel in airway epithelial cells leads to persistent and overwhelming infection and inflammation. Several studies indicate that inflammation occurs very early in the lungs of CF patients and often seems to precede clear signs of infection (25, 34, 40). Moreover, CF airway inflammation may manifest as disproportionately increased or prolonged in relation to the level of stimuli (1, 16, 28, 43). However, whether a dysregulation of inflammation exists in CF patients is debated (2, 15).
Data from literature regarding AZM effects on TNF-α expression are scarce and contradictory. Although AZM has been reported to inhibit TNF-α expression both in vivo in animal models and in vitro (20, 21), in healthy human subjects serum TNF-α protein concentration was unaffected by a 24-h-long treatment with AZM (14). In this regard, the experimental model seems to be critical and, in particular, differences between CF and non-CF models are relevant.
In this work, we first aimed to establish whether differential expression of a relevant inflammatory marker such as TNF-α could be detected in our CF cell models since it plays a relevant role in the pathogenesis of CF. We showed not only that its constitutive expression was significantly higher in CF cells than in isogenic non-CF cells at both mRNA and protein levels but also that this finding was not dependent on cells' passage or period from sedimentation. Moreover, we found no differential IL-6 mRNA expression between CF and non-CF cell lines. We determined that exposition to lipopolysaccharide derived from P. aeruginosa for 24 h induced higher expression of TNF-α mRNA in CF cells than in non-CF cells in our models (data not shown), confirming an exaggerated inflammatory response in CF cells in the presence as well in the absence of stimuli.
The association between increased inflammatory markers and CFTR mutations is, however, controversial. Aldallal et al. (1) found higher IL-8 expression in CF cells than in non-CF cells in cell line models but not in primary cultures, revealing a considerable variability in airway epithelial cell inflammation among different individuals and cell models. Becker et al. (5) found no differential IL-8 and IL-6 expression between CF and non-CF cell lines and primary cultures, respectively. These contradictory results could be due not only to the choice of the cell model and its origin but also to different culture conditions. Our experimental model consists of several human CF cell lines derived from different airway cell types, and two of them have been compared to their isogenic non-CF cell lines. This experimental model was appropriate for reproducing the anti-inflammatory effects of AZM described in vivo (41, 45).
Macrolide antibiotics are receiving increasing attention for their possible therapeutic benefits in the treatment of CF. AZM was chosen over other macrolides because of its ease of administration and its accumulation in sputum and tissues. Its plasma half-life is considerably longer than those of other macrolides. It also accumulates in alveolar macrophages, which could represent a delivery vehicle to transport it to affected sites. Finally, the results of several clinical trials (19, 30, 35) encourage clinicians to subject CF patients to long-term treatment with AZM, although certain heterogeneity in the response has been reported. However, the mechanisms of its efficacy are still unclear. Clinical beneficial effects of AZM might derive from the synergism of different effects, including inhibition of P. aeruginosa bacterial growth (29, 44), decreased expression of bacterial virulence factors (39, 44), modulation of the inflammatory response (41), ion transport (31), and tight junctions (3).
We found that AZM reduced TNF-α expression at both transcript and protein levels in all of our CF cell lines, bringing it to the levels of untreated isogenic non-CF cells. Conversely, IL-6 mRNA expression was not significantly affected by AZM treatment. As we found higher expression of TNF-α, but not of IL-6, in CF cells than in non-CF cells, we can speculate that AZM may be effective towards those proinflammatory molecules induced in the constitutive inflammation. The specificity of the results is warranted by the observation that JM, a macrolide known to lack clinical anti-inflammatory properties (38), was ineffective.
The possibility that AZM may act at the transcriptional level was tested by measuring the DNA binding activities of two transcription factors relevant in the regulation of the TNF-α gene, NF-κB (27) and Sp1 (42).
We found that in the presence of AZM, NF-κB DNA binding activity in CF cells was reduced approximately to the levels detected in isogenic non-CF cells. Also, Sp1 DNA binding was reduced following treatment with AZM, while the activities of this transcription factor were not significantly different in CF and non-CF cells. Once again, the inhibitory effect was peculiar to AZM, as JM had no effect on NF-κB and Sp1 DNA binding activities. Assays of transcription factors binding to DNA do not rule out an effect on their activation. This approach has been utilized in order to establish whether NF-κB and Sp1 could be considered targets of AZM potentially involved in the regulation of TNF-α transcription by this macrolide. We are focusing on the ability of AZM to affect the activation of NF-κB, Sp1, and AP-1 at different levels (11, 12).
Increased NF-κB activation in CF versus isogenic non-CF specimens was observed in several studies, both in the absence and in the presence of stimulation (9, 16, 22, 43) in different experimental models. Furthermore, our results are consistent with previous studies describing higher NF-κB activation in CF cells than in non-CF cells in a cellular model utilized in this study (16, 43).
Furthermore, it is of note that therapeutic inhibition of NF-κB has been proposed for the treatment of inflammatory and immune diseases (7, 37). Decreased levels of TNF-α and IL-8, two NF-κB-regulated genes, could reduce the recruitment of neutrophils which are considered responsible for epithelial damage in CF airways (41).
Sp1 can functionally cooperate with NF-κB to elicit maximal promoter activation of inflammatory genes (26). Investigating the effects of AZM on Sp1 was considered relevant, as this transcription factor has been described to regulate several inflammatory genes, including the chemokine macrophage inflammatory protein-2, heparanase, and TNF-α (42), and therefore, its inhibition could influence inflammatory responses. This approach was considered appropriate for investigating a possible mechanism of regulation of TNF-α by AZM. Inhibition of Sp1 activity by AZM seems to be a novel effect of this macrolide.
At present, no satisfactory anti-inflammatory treatments are available for clinical use in CF because of limited efficacy and/or undesired effects (6, 24). Therefore, the identification of novel therapeutic targets is required to develop novel strategies for the treatment of lung inflammation in CF. Our results indicate that the antibiotic AZM has the features of an anti-inflammatory drug and that NF-κB and Sp1 transcription factors are relevant targets whose inhibition might contribute to ameliorate the excessive inflammatory response in CF.
Acknowledgments
We thank Federica Quiri (Azienda Ospedaliera di Verona, Verona, Italy) for excellent technical assistance with cell cultures. We are grateful to Anna Bossi (University of Milan, Milan, Italy) for her kind support in statistical analysis.
This work was supported by the Italian Cystic Fibrosis Research Foundation (grants FFC no. 03/2002 and FFC no. 10/05 by Istituti Scolastici Veronesi), Comitato di Vicenza dell'Associazione Veneta per la lotta contro la Fibrosi Cistica, and Legge 548/93 Finanziamento Ricerca Fibrosi Cistica 2004.
Footnotes
Published ahead of print on 8 January 2007.
REFERENCES
- 1.Aldallal, N., E. E. McNaughton, L. J. Manzel, A. M. Richards, J. Zabner, T. W. Ferkol, and D. C. Look. 2002. Inflammatory response in airway epithelial cells isolated from patients with cystic fibrosis. Am. J. Respir. Crit. Care Med. 166:1248-1256. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong, D. S., K. Grimwood, J. B. Carlin, R. Carzino, J. P. Gutierrez, J. Hull, A. Olinsky, E. M. Phelan, C. F. Robertson, and P. D. Phelan. 1997. Lower airway inflammation in infants and young children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 156:1197-1204. [DOI] [PubMed] [Google Scholar]
- 3.Asgrimsson, V., T. Gudjonsson, G. H. Gudmundsson, and O. Baldursson. 2006. Novel effects of azithromycin on tight junction proteins in human airway epithelia. Antimicrob. Agents Chemother. 50:1805-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumann, U., M. King, E. M. App, S. Tai, A. Konig, J. J. Fischer, T. Zimmermann, W. Sextro, and H. von der Hardt. 2004. Long term azithromycin therapy in cystic fibrosis patients: a study on drug levels and sputum properties. Can. Respir. J. 11:151-155. [DOI] [PubMed] [Google Scholar]
- 5.Becker, M. N., M. S. Sauer, M. S. Muhlebach, A. J. Hirsh, Q. Wu, M. W. Verghese, and S. H. Randell. 2004. Cytokine secretion by cystic fibrosis airway epithelial cells. Am. J. Respir. Crit. Care Med. 169:645-653. [DOI] [PubMed] [Google Scholar]
- 6.Birke, F. W., C. J. Meade, and R. Anderskewitz. 2001. In vitro and in vivo pharmacological characterization of BIIL 284, a novel and potent leukotriene B (4) receptor antagonist. J. Pharmacol. Exp. Ther. 297:458-466. [PubMed] [Google Scholar]
- 7.Blease, K., A. Lewis, and H. K. Raymon. 2003. Emerging treatments for asthma. Expert Opin. Emerg. Drugs 8:71-81. [DOI] [PubMed] [Google Scholar]
- 8.Bonfield, T. L., J. R. Panuska, M. W. Konstan, K. A. Hilliard, J. B. Hilliard, H. Ghnaim, and M. Berger. 1995. Inflammatory cytokines in cystic fibrosis lungs. Am. J. Respir. Crit. Care Med. 152:2111-2118. [DOI] [PubMed] [Google Scholar]
- 9.Carrabino, S., D. Carpani, A. Livraghi, M. Di Cicco, D. Costantini, E. Copreni, C. Colombo, and M. Conese. 2006. Dysregulated interleukin-8 secretion and NF-kappaB activity in human cystic fibrosis nasal epithelial cells. J. Cyst. Fibros. 5:113-119. [DOI] [PubMed] [Google Scholar]
- 10.Chmiel, J. F., and P. B. Davis. 2003. State of the art: why do the lungs of patients with cystic fibrosis become infected and why can't they clear the infection? Respir. Res. 4:8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cigana, C., E. Nicolis, M. Pasetto, B. M. Assael, and P. Melotti. 2005. Regulation of pro-inflammatory transcription factors by azithromycin in cystic fibrosis epithelial airway epithelial cells. Pediatr. Pulmonol. Suppl. 28:271. [Google Scholar]
- 12.Cigana, C., E. Nicolis, M. Pasetto, B. M. Assael, and P. Melotti. 2006. Anti-inflammatory effects of azithromycin in cystic fibrosis airway epithelial cells. Biochem. Biophys. Res. Commun. 350:977-982. [DOI] [PubMed] [Google Scholar]
- 13.Cozens, A. L., M. J. Yezzi, L. Chin, E. M. Simon, W. E. Finkbeiner, J. A. Wagner, and D. C. Gruenert. 1992. Characterization of immortal cystic fibrosis tracheobronchial gland epithelial cells. Proc. Natl. Acad. Sci. USA 89:5171-5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Culic, O., V. Erakovic, I. Cepelak, K. Barisic, K. Brajsa, Z. Ferencic, R. Galovic, I. Glojnaric, Z. Manojlovic, V. Munic, R. Novak-Mircetic, V. Pavicic-Beljak, M. Sucic, M. Veljaca, T. Zanic-Grubisic, and M. J. Parnham. 2002. Azithromycin modulates neutrophil function and circulating inflammatory mediators in healthy human subjects. Eur. J. Pharmacol. 450:277-289. [DOI] [PubMed] [Google Scholar]
- 15.Dakin, C. J., A. H. Numa, H. Wang, J. R. Morton, C. C. Vertzyas, and R. L. Henry. 2002. Inflammation, infection, and pulmonary function in infants and young children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 165:904-910. [DOI] [PubMed] [Google Scholar]
- 16.DiMango, E., A. J. Ratner, R. Bryan, S. Tabibi, and A. Prince. 1998. Activation of NF-kappaB by adherent Pseudomonas aeruginosa in normal and cystic fibrosis respiratory epithelial cells. J. Clin. Investig. 101:2598-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Paolo, A., C. Barbara, A. Chella, C. A. Angeletti, and M. Del Tacca. 2002. Pharmacokinetics of azithromycin in lung tissue, bronchial washing, and plasma in patients given multiple oral doses of 500 and 1000 mg daily. Pharmacol. Res. 46:545-550. [DOI] [PubMed] [Google Scholar]
- 18.Ferrara, G., M. Losi, F. Franco, L. Corbetta, L. M. Fabbri, and L. Richeldi. 2005. Macrolides in the treatment of asthma and cystic fibrosis. Respir. Med. 99:1-10. [DOI] [PubMed] [Google Scholar]
- 19.Hansen, C. R., T. Pressler, C. Koch, and N. Hoiby. 2005. Long-term azitromycin [sic] treatment of cystic fibrosis patients with chronic Pseudomonas aeruginosa infection; an observational cohort study. J. Cyst. Fibros. 4:35-40. [DOI] [PubMed] [Google Scholar]
- 20.Ianaro, A., A. Ialenti, P. Maffia, L. Sautebin, L. Rombola, R. Carnuccio, T. Iuvone, F. D'Acquisto, and M. Di Rosa. 2000. Anti-inflammatory activity of macrolide antibiotics. J. Pharmacol. Exp. Ther. 292:156-163. [PubMed] [Google Scholar]
- 21.Ivetic Tkalcevic, V., B. Bosnjak, B. Hrvacic, M. Bosnar, N. Marjanovic, Z. Ferencic, K. Situm, O. Culic, M. J. Parnham, and V. Erakovic. 2006. Anti-inflammatory activity of azithromycin attenuates the effects of lipopolysaccharide administration in mice. Eur. J. Pharmacol. 539:131-138. [DOI] [PubMed] [Google Scholar]
- 22.Joseph, T., D. Look, and T. Ferkol. 2005. NF-kappaB activation and sustained IL-8 gene expression in primary cultures of cystic fibrosis airway epithelial cells stimulated with Pseudomonas aeruginosa. Am. J. Physiol. Lung Cell. Mol. Physiol. 288:L471-L479. [DOI] [PubMed] [Google Scholar]
- 23.Kawamura-Sato, K., Y. Iinuma, T. Hasegawa, T. Horii, T. Yamashino, and M. Ohta. 2000. Effect of subinhibitory concentrations of macrolides on expression of flagellin in Pseudomonas aeruginosa and Proteus mirabilis. Antimicrob. Agents Chemother. 44:2869-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koehler, D. R., G. P. Downey, N. B. Sweezey, A. K. Tanswell, and J. Hu. 2004. Lung inflammation as a therapeutic target in cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 31:377-381. [DOI] [PubMed] [Google Scholar]
- 25.Konstan, M. W., and M. Berger. 1997. Current understanding of the inflammatory process in cystic fibrosis: onset and etiology. Pediatr. Pulmonol. 24:137-142. [DOI] [PubMed] [Google Scholar]
- 26.Lee, K. W., Y. Lee, H. J. Kwon, and D. S. Kim. 2005. Sp1-associated activation of macrophage inflammatory protein-2 promoter by CpG-oligodeoxynucleotide and lipopolysaccharide. Cell. Mol. Life Sci. 62:188-198. [DOI] [PubMed] [Google Scholar]
- 27.Liu, H., P. Sidiropoulos, G. Song, L. J. Pagliari, M. J. Birrer, B. Stein, J. Anrather, and R. M. Pope. 2000. TNF-alpha gene expression in macrophages: regulation by NF-kappa B is independent of c-Jun or C/EBP beta. J. Immunol. 164:4277-4285. [DOI] [PubMed] [Google Scholar]
- 28.Muhlebach, M. S., and T. L. Noah. 2002. Endotoxin activity and inflammatory markers in the airways of young patients with cystic fibrosis. Am. J. Respir. Crit. Care Med. 165:911-915. [DOI] [PubMed] [Google Scholar]
- 29.Nalca, Y., L. Jansch, F. Bredenbruch, R. Geffers, J. Buer, and S. Haussler. 2006. Quorum-sensing antagonistic activities of azithromycin in Pseudomonas aeruginosa PAO1: a global approach. Antimicrob. Agents Chemother. 50:1680-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pirzada, O. M., J. McGaw, C. J. Taylor, and M. L. Everard. 2003. Improved lung function and body mass index associated with long-term use of macrolide antibiotics. J. Cyst. Fibros. 2:69-71. [DOI] [PubMed] [Google Scholar]
- 31.Pradal, U., A. Delmarco, M. Morganti, M. Cipolli, E. Mini, and G. Cazzola. 2005. Long-term azithromycin in cystic fibrosis: another possible mechanism of action? J. Chemother. 17:393-400. [DOI] [PubMed] [Google Scholar]
- 32.Rajan, S., G. Cacalano, R. Bryan, A. J. Ratner, C. U. Sontich, A. van Heerckeren, P. Davis, and A. Prince. 2000. Pseudomonas aeruginosa induction of apoptosis in respiratory epithelial cells: analysis of the effects of cystic fibrosis transmembrane conductance regulator dysfunction and bacterial virulence factors. Am. J. Respir. Cell Mol. Biol. 23:304-312. [DOI] [PubMed] [Google Scholar]
- 33.Ribeiro, C. M., A. M. Paradiso, U. Schwab, J. Perez-Vilar, L. Jones, W. O'Neal, and R. C. Boucher. 2005. Chronic airway infection/inflammation induces a Ca2+i-dependent hyperinflammatory response in human cystic fibrosis airway epithelia. J. Biol. Chem. 280:17798-17806. [DOI] [PubMed] [Google Scholar]
- 34.Rosenfeld, M., R. L. Gibson, S. McNamara, J. Emerson, J. L. Burns, R. Castile, P. Hiatt, K. McCoy, C. B. Wilson, A. Inglis, A. Smith, T. R. Martin, and B. W. Ramsey. 2001. Early pulmonary infection, inflammation, and clinical outcomes in infants with cystic fibrosis. Pediatr. Pulmonol. 32:356-366. [DOI] [PubMed] [Google Scholar]
- 35.Saiman, L., N. Mayer-Hamblett, P. Campbell, B. C. Marshall, and the Macrolide Study Group. 2005. Heterogeneity of treatment response to azithromycin in patients with cystic fibrosis. Am. J. Respir. Crit. Care Med. 172:1008-1012. [DOI] [PubMed] [Google Scholar]
- 36.Shinkai, M., and B. K. Rubin. 2005. Macrolides and airway inflammation in children. Paediatr. Respir. Rev. 6:227-235. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki, J., R. Morishita, J. Amano, Y. Kaneda, and M. Isobe. 2000. Decoy against nuclear factor-kappa B attenuates myocardial cell infiltration and arterial neointimal formation in murine cardiac allografts. Gene Ther. 7:1847-1852. [DOI] [PubMed] [Google Scholar]
- 38.Takizawa, H., M. Desaki, T. Ohtoshi, S. Kawasaki, T. Kohyama, M. Sato, M. Tanaka, T. Kasama, K. Kobayashi, J. Nakajima, and K. Ito. 1997. Erythromycin modulates IL-8 expression in normal and inflamed human bronchial epithelial cells. Am. J. Respir. Crit. Care Med. 156:266-271. [DOI] [PubMed] [Google Scholar]
- 39.Tateda, K., Y. Ishii, T. Matsumoto, T. Kobayashi, S. Miyazaki, and K. Yamaguchi. 2000. Potential of macrolide antibiotics to inhibit protein synthesis of Pseudomonas aeruginosa: suppression of virulence factors and stress response. J. Infect. Chemother. 6:1-7. [DOI] [PubMed] [Google Scholar]
- 40.Tirouvanziam, R., S. de Bentzmann, C. Hubeau, J. Hinnrasky, J. Jacquot, B. Péault, and E. Puchelle. 2000. Inflammation and infection in naive human cystic fibrosis airway grafts. Am. J. Respir. Cell Mol. Biol. 23:121-127. [DOI] [PubMed] [Google Scholar]
- 41.Tsai, W. C., M. L. Rodriguez, K. S. Young, J. C. Deng, V. J. Thannickal, K. Tateda, M. B. Hershenson, and T. J. Standiford. 2004. Azithromycin blocks neutrophil recruitment in Pseudomonas endobronchial infection. Am. J. Respir. Crit. Care Med. 170:1331-1339. [DOI] [PubMed] [Google Scholar]
- 42.Tsytsykova, A. V., and A. E. Goldfeld. 2002. Inducer-specific enhanceosome formation controls tumor necrosis factor alpha gene expression in T lymphocytes. Mol. Cell. Biol. 22:2620-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Venkatakrishnan, A., A. A. Stecenko, G. King, T. R. Blackwell, K. L. Brigham, J. W. Christman, and T. S. Blackwell. 2000. Exaggerated activation of nuclear factor-kappaB and altered IkappaB-beta processing in cystic fibrosis bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 23:396-403. [DOI] [PubMed] [Google Scholar]
- 44.Wagner, T., G. Soong, S. Sokol, L. Saiman, and A. Prince. 2005. Effects of azithromycin on clinical isolates of Pseudomonas aeruginosa from cystic fibrosis patients. Chest 128:912-919. [DOI] [PubMed] [Google Scholar]
- 45.Wolter, J., S. Seeney, S. Bell, S. Bowler, P. Masel, and J. McCormack. 2002. Effect of long term treatment with azithromycin on disease parameters in cystic fibrosis: a randomised trial. Thorax 57:212-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeitlin, P. L., L. Lu, J. Rhim, G. Cutting, G. Stetten, K. A. Kieffer, R. Craig, and W. B. Guggino. 1991. A cystic fibrosis bronchial epithelial cell line: immortalization by adeno-12-SV40 infection. Am. J. Respir. Cell Mol. Biol. 4:313-319. [DOI] [PubMed] [Google Scholar]