Abstract
Simulated therapeutic vancomycin exposures were evaluated against agr wild-type and knockout Staphylococcus aureus groups I, II, III, and IV using an in vitro pharmacodynamic model. All agr groups developed intermediate resistance to vancomycin after subtherapeutic exposure. The free unbound fraction of the area under the concentration-time curve (fAUC/MIC) required to suppress resistance was fourfold higher (P < 0.001) in agr dysfunctional strains (112 to 169) than that in parent wild-type strains (28).
The accessory gene regulator is a quorum-sensing operon which coordinates the expression of secreted and cell-associated virulence factors and controls several metabolic pathways in Staphylococcus aureus in a growth phase-related fashion (4, 5, 7, 8). S. aureus strains which exhibit a dysfunction accessory gene regulator (agr) locus may possess an intrinsic survival advantage under vancomycin-selective pressure (11-14). However, the correlation between vancomycin exposure using the area under the concentration-time curve (AUC/MIC) necessary to suppress the development of vancomycin-intermediate resistance and agr function or group has not been investigated. We examined the relationship between vancomycin exposure and the development of intermediate-level resistance in agr groups I, II, III, and IV S. aureus using an in vitro pharmacodynamic model.
RN6390, RN6607, RN3984, and RN4850 represent agr-positive S. aureus prototype strains carrying agr groups I, II, III, and IV, respectively, and were obtained from the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA), supported under NIAID/NIH contract N01-AI-95359, and Richard P. Novick. RN6911, RN9120, and RN9121 are the agr-null derivates of groups I, II, and IV (NARSA), respectively. RN3984-M is the null derivate of RN3984, which demonstrated a loss of agr function by a lack of production of delta-hemolysin.
Vancomycin analytical-grade powder was commercially purchased (Sigma, St. Louis, MO). Stock solutions were freshly prepared at the beginning of each week and kept frozen daily at −4°C. Mueller-Hinton broth (Difco, Detroit, MI) supplemented with 25 μg/ml calcium and 12.5 μg/ml magnesium (SMHB) was used for in vitro pharmacodynamic models and susceptibility testing involving vancomycin. Colony counts were determined using tryptic soy agar (TSA; Difco, Detroit, MI) plates. MICs were determined by broth microdilution in SMHB according to CLSI guidelines (2). The function of the agr operon was qualitatively assessed in all isolates using S. aureus RN4420 (NARSA) for delta-hemolysin expression, as previously described (13).
An in vitro pharmacodynamic model was utilized as previously described for the collection of bacterial and antimicrobial dosing and sampling (1). All model simulations were conducted over 72 h and were performed in triplicate to ensure reproducibility. Vancomycin regimens of 62.5, 125, 250, 500, 750, and 1,000 mg every 12 h (free unbound fraction of the AUC [fAUC/MIC/24] exposures of 14 to 225 μg/ml/h; half-life 6 h) were simulated according to the manufacturer's recommendations for a patient with normal renal function. A protein binding level of 55% was utilized for all model simulations (9). Antibiotic concentrations were determined from each model at 0 to 72 h for pharmacokinetic analysis. The AUC from 0 to 24 h was calculated using the linear trapezoid method. Vancomycin concentrations were determined by fluorescence polarization immunoassay (TDx; Abbott Diagnostics) as previously described (16). Differences between regimens in log10 CFU/ml at 72 h were determined using analysis of variance with Tukey's test for multiple comparisons. All statistical analyses were performed using SPSS statistical software (Release 12.0; SPSS, Inc., Chicago, Illinois).
The development of resistance was evaluated at multiple time points throughout the simulation at 0, 24, 48, and 72 h for all model simulations. Resistance was determined by the detection of growth on TSA plates containing 3 and 6 μg/ml and by changes in MIC via Etest of all model samples.
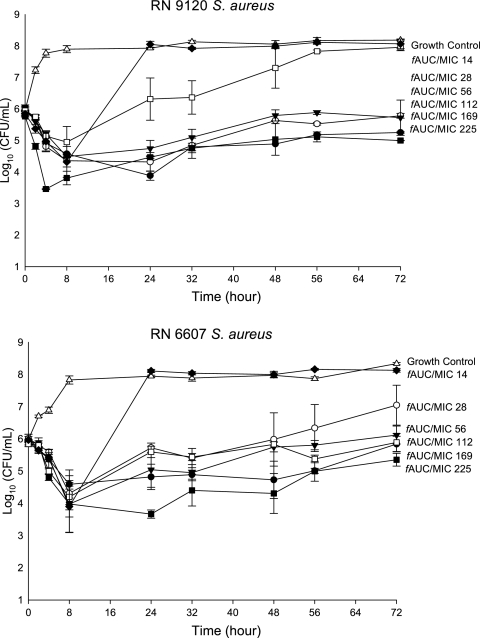
All of the characterized S. aureus strains evaluated were susceptible to vancomycin MICs of 1.0 μg/ml. Observed pharmacokinetic parameters for all tested therapeutic regimens are shown in Table 1. Quantitative changes in log10 CFU/ml over 72 h are graphically displayed in Fig. 1. Changes in MIC secondary to 72 h vancomycin exposure are displayed in Table 2. Against all agr-null strains (agr group I, II, III, and IV), the exposure of an ƒAUC/MIC of 14 resulted in a subsequent increase in MIC at 72 h. In agr-null group II, S. aureus increases to a MIC of 8 μg/ml were noted secondary to exposure as high as an ƒAUC/MIC of 112. Parent wild-type strains (agr groups I, II, III, and IV [RN6390, RN6607, RN3984, and RN8450, respectively]) also resulted in the development of intermediate resistance after exposure to an ƒAUC/MIC of 14, although the magnitude of MIC increases were lower compared with that in agr-null isolates: MIC increases of 3 to 4 μg/ml were noted in agr-positive isolates relative to increases of 6 to 8 μg/ml in agr-null isolates. The vancomycin exposure necessary to suppress the development of resistance in S. aureus agr knockout isolates was fourfold greater with fAUC/MICs of 112 to 169 versus an ƒAUC/MIC of 28 for wild-type, agr-positive isolates (P < 0.001).
TABLE 1.
Pharmacokinetic and pharmacodynamic parameters obtained with in vitro modelsa
| Targeted ƒAUC/MICb | Simulated regimenc | Achieved ƒAUC/MIC | ƒCmax/MIC | ƒCmin/MIC | Half-life (h) |
|---|---|---|---|---|---|
| 225 | 1,000 | 240.3 ± 8.8 | 18.8 ± 2.0 | 5.1 ± 0.8 | 6.1 ± 0.3 |
| 169 | 750 | 175.9 ± 10.8 | 13.7 ± 0.6 | 3.4 ± 0.5 | 5.8 ± 0.6 |
| 112 | 500 | 118.8 ± 6.6 | 9.6 ± 0.6 | 2.3 ± 0.3 | 6.5 ± 0.8 |
| 56 | 250 | 51.4 ± 1.6 | 4.3 ± 0.3 | 1.0 ± 0.2 | 6.5 ± 0.2 |
| 28 | 125 | 32.2 ± 6.5 | 2.3 ± 0.3 | 0.6 ± 0.1 | 5.9 ± 0.2 |
| 14 | 62.5 | 16.8 ± 2.3 | 1.2 ± 0.2 | 0.4 ± 0.1 | 6.8 ± 0.5 |
Cmax, maximum concentration of drug in serum; Cmin, minimum concentration of drug in serum. Values (μg/ml) are shown as means ± standard deviations unless indicated otherwise.
Values are expressed as micrograms per milliter.
Values are expressed as milligrams per every 12 h.
FIG. 1.
Representative activity of vancomycin at simulated regimens over 72 h against agr group II (RN6607 agr II wild type and the null derivate RN9120). Error bars indicate standard deviations.
TABLE 2.
Vancomycin MIC over 72 h secondary to varying ƒAUC/MIC exposures
| agr group | Isolate | ƒAUC/MIC | MIC (μg/ml) at:
|
|||
|---|---|---|---|---|---|---|
| 0 h | 24 h | 48 h | 72 h | |||
| I | RN6390 (agr+) | 14 | 1 | 2 | 2 | 4 |
| RN6911 (agr null) | 14 | 1 | 2 | 4 | 6 | |
| 28 | 1 | 2 | 2 | 3 | ||
| 56 | 1 | 2 | 2 | 3 | ||
| II | RN6607 (agr+) | 14 | 1 | 2 | 2 | 4 |
| RN9120 (agr null) | 14 | 1 | 6 | 6 | 8 | |
| 28 | 1 | 4 | 6 | 6 | ||
| 56 | 1 | 2 | 2 | 3 | ||
| 112 | 1 | 2 | 2 | 3 | ||
| III | RN3984 (agr+) | 14 | 1 | 2 | 2 | 3 |
| RN3984-M (agr null) | 14 | 1 | 2 | 2 | 6 | |
| 28 | 1 | 2 | 2 | 4 | ||
| 56 | 1 | 2 | 2 | 3 | ||
| IV | RN4850 (agr+) | 14 | 1 | 2 | 2 | 3 |
| RN9121 (agr null) | 14 | 1 | 3 | 3 | 6 | |
| 28 | 1 | 2 | 3 | 3 | ||
| 56 | 1 | 2 | 2 | 3 | ||
Recently, prolonged administration of vancomycin has been linked with vancomycin tolerance and down-regulation of the agr locus. In addition, S. aureus exposure to trough vancomycin concentrations of <10 μg/ml has been associated with an increase in the MIC and development of glycopeptide-intermediate S. aureus-like characteristics (15a, 17). In the present investigation, we sought to determine whether a relationship exists between the development of heterogeneous resistance among all wild-type and knockout agr isolates using an in vitro pharmacodynamic model simulating vancomcyin therapeutic dosing. S. aureus isolates that were dysfunctional in agr required vancomycin doses and fAUC/MICs more than fourfold higher than that necessary for wild-type isolates to prevent the development of resistance. Interestingly, although we observed MIC increases of up to 3 to 4 μg/ml (which may be currently classified as reduced susceptibility) in wild-type, agr-positive isolates, the alterations in MIC in agr dysfunctional isolates were typically at least onefold higher at 6 to 8 μg/ml at the same vancomycin exposure. Therefore, in all agr dysfunctional isolates, the development of intermediate resistance was evident after subtherapeutic vancomycin exposure using both the previous (≤4 μg/ml) and newer breakpoints of susceptibility (≤2 μg/ml) (2). Additionally, although we found the development of resistance in all agr dysfunctional groups, the magnitude of increase in vancomycin MIC was greatest in the agr-null group II isolate. This is in agreement with previous reports that this genotype may possess an intrinsic survival advantage (6, 15), as this isolate demonstrated the largest increase in MIC (8 μg/ml), and the greatest exposure of vancomycin was necessary to suppress the development of resistance.
We conclude that the development of vancomycin-intermediate resistance may be driven by suboptimal vancomycin exposure in the setting of dysfunction in the agr locus in S. aureus. These findings suggest the use of more aggressive vancomycin-dosing strategies to maintain optimal exposures. These findings highlight the potential problems associated with suboptimal vancomycin exposures, which ultimately impact the susceptibility of this antibiotic with possible consequences to other classes of antimicrobials (3, 10, 11, 17). More attention to optimal dosing of vancomycin may be important in managing methicillin-resistant Staphylococcus aureus infections.
Footnotes
Published ahead of print on 11 December 2006.
REFERENCES
- 1.Allen, G. P., R. Cha, and M. J. Rybak. 2002. In vitro activities of quinupristin-dalfopristin and cefepime, alone and in combination with various antimicrobials, against multidrug-resistant staphylococci and enterococci in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 46:2606-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clinical and Laboratory Standards Institute. 2005. Performance standards for antimicrobial susceptibility testing; 15th informational supplement. CLSI M100-S15. Clinical and Laboratory Standards Institute, Wayne, PA.
- 3.Cui, L., E. Tominaga, H. M. Neoh, and K. Hiramatsu. 2006. Correlation between reduced daptomycin susceptibility and vancomycin resistance in vancomycin-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 50:1079-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ji, G., R. Beavis, and R. P. Novick. 1997. Bacterial interference caused by autoinducing peptide variants. Science 276:2027-2030. [DOI] [PubMed] [Google Scholar]
- 5.Lyon, G. J., J. S. Wright, T. W. Muir, and R. P. Novick. 2002. Key determinants of receptor activation in the agr autoinducing peptides of Staphylococcus aureus. Biochemistry 41:10095-10104. [DOI] [PubMed] [Google Scholar]
- 6.Moise-Broder, P. A., G. Sakoulas, G. M. Eliopoulos, J. J. Schentag, A. Forrest, and R. C. Moellering, Jr. 2004. Accessory gene regulator group II polymorphism in methicillin-resistant Staphylococcus aureus is predictive of failure of vancomycin therapy. Clin. Infect. Dis. 38:1700-1705. [DOI] [PubMed] [Google Scholar]
- 7.Novick, R. P. 2003. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol. Microbiol. 48:1429-1449. [DOI] [PubMed] [Google Scholar]
- 8.Novick, R. P. 2000. Pathogenicity factors and their regulation, p. 392-407. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, and D. A. Portnoy (ed.), Gram-positive pathogens. American Society for Microbiology, Washington, DC.
- 9.Rybak, M. J. 2006. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin. Infect. Dis. 42(Suppl. 1):S35-S39. [DOI] [PubMed] [Google Scholar]
- 10.Sakoulas, G., J. Alder, C. Thauvin-Eliopoulos, R. C. Moellering, Jr., and G. M. Eliopoulos. 2006. Induction of daptomycin heterogeneous susceptibility in Staphylococcus aureus by exposure to vancomycin. Antimicrob. Agents Chemother. 50:1581-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakoulas, G., G. M. Eliopoulos, V. G. Fowler, Jr., R. C. Moellering, Jr., R. P. Novick, N. Lucindo, M. R. Yeaman, and A. S. Bayer. 2005. Reduced susceptibility of Staphylococcus aureus to vancomycin and platelet microbicidal protein correlates with defective autolysis and loss of accessory gene regulator (agr) function. Antimicrob. Agents Chemother. 49:2687-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakoulas, G., G. M. Eliopoulos, R. C. Moellering, Jr., R. P. Novick, L. Venkataraman, C. Wennersten, P. C. DeGirolami, M. J. Schwaber, and H. S. Gold. 2003. Staphylococcus aureus accessory gene regulator (agr) group II: is there a relationship to the development of intermediate-level glycopeptide resistance? J. Infect. Dis. 187:929-938. [DOI] [PubMed] [Google Scholar]
- 13.Sakoulas, G., G. M. Eliopoulos, R. C. Moellering, Jr., C. Wennersten, L. Venkataraman, R. P. Novick, and H. S. Gold. 2002. Accessory gene regulator (agr) locus in geographically diverse Staphylococcus aureus isolates with reduced susceptibility to vancomycin. Antimicrob. Agents Chemother. 46:1492-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakoulas, G., R. C. Moellering, Jr., and G. M. Eliopoulos. 2006. Adaptation of methicillin-resistant Staphylococcus aureus in the face of vancomycin therapy. Clin. Infect. Dis. 42(Suppl. 1):S40-S50. [DOI] [PubMed] [Google Scholar]
- 15.Sakoulas, G., P. A. Moise-Broder, J. Schentag, A. Forrest, R. C. Moellering, Jr., and G. M. Eliopoulos. 2004. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J. Clin. Microbiol. 42:2398-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15a.Sakoulas, G., H. S. Gold, R. A. Cohen, L. Venkataraman, R. C. Moellering, and G. M. Eliopoulos. 2006. Effects of prolonged vancomycin administration on methicillin-resistant Staphylococcus aureus (MRSA) in a patient with recurrent bacteraemia. J. Antimicrob. Chemother. 57:699-704. [DOI] [PubMed] [Google Scholar]
- 16.Tsuji, B. T., and M. J. Rybak. 2005. Short-course gentamicin in combination with daptomycin or vancomycin against Staphylococcus aureus in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 49:2735-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woods, C. W., A. C. Cheng, V. G. Fowler, Jr., M. Moorefield, J. Frederick, G. Sakoulas, V. G. Meka, F. C. Tenover, P. Zwadyk, and K. H. Wilson. 2004. Endocarditis caused by Staphylococcus aureus with reduced susceptibility to vancomycin. Clin. Infect. Dis. 38:1188-1191. [DOI] [PubMed] [Google Scholar]