Abstract
We assessed the effect of voriconazole (VRC) on the expression and release of selected cytokines and chemokines in the THP-1 human monocytic cell line in response to Aspergillus fumigatus hyphal fragments (HF) by cDNA microarray analysis, reverse transcriptase (RT) PCR, and enzyme-linked immunosorbent assay. Stimulation of THP-1 cells by HF alone caused a significant up-regulation of CCL4 (MIP1B) and CCL16, while CCL2 (MCP1) was down-regulated. By comparison, in the presence of VRC, a large number of genes such as CCL3 (MIP1A), CCL4 (MIP1B), CCL5 (RANTES), CCL7 (MCP3), CCL11 (EOTAXIN), CCL15 (MIP1Δ), CXCL6, and CXCL13 were strongly up-regulated in THP-1 cells challenged by HF, whereas CCL20 (MIP3A) and CCL21 (MIP2) were down-regulated. Among five genes differentially expressed in THP-1 cells, IL12A, IL12B, and IL-16 were down-regulated whereas IL-11 and TGFB1 were significantly up-regulated in the presence of VRC. The inflammation-related genes IFNγ, IL1R1, and TNFA were also up-regulated in THP-1 cells exposed to HF only in the presence of VRC. RT-PCR of four selected genes validated the results of microarrays. The release of interleukin 1β (IL-1β) and IL-12 was significantly increased from monocytes stimulated either by HF alone (P < 0.05) or in the presence of VRC (P < 0.01 and P < 0.05, respectively). In contrast, tumor necrosis factor alpha release from monocytes was enhanced only in the presence of VRC (P < 0.01). The chemokines monocyte chemoattractant protein 1 and macrophage inflammatory protein 1β were decreased under both conditions (P < 0.01). These results demonstrate that in the presence of VRC, HF induces a more pronounced profile of gene expression in THP-1 cells than HF alone, potentially leading to more-efficient host resistance to A. fumigatus.
Invasive aspergillosis (IA) is an increasing cause of excessive morbidity and mortality in patients with hematological malignancies (16, 18, 47), hematopoietic stem cells (25), solid organ transplants (40), or immunodeficiency syndromes (2) or in those receiving immunosuppressive therapies (6, 21, 38). Epidemiological studies have shown that 5% to 10% of stem cell transplant recipients develop IA (26). The case fatality rate for IA may be as much as 95% (24). Aspergillus fumigatus is the most common cause of IA.
The innate immune response against A. fumigatus is contributed by mononuclear phagocytes constituting a first line of host defense and representing the precursor cell population of dendritic cells and tissue macrophages, which activate the adaptive immune system (36). Upon fungal pattern recognition and stimulation, these cells activate a cascade of molecular events that set off the expression and release of proinflammatory cytokines, chemokines, and immunoregulatory molecules, resulting in the recruitment of additional Th1 and Th2 cell populations (44). Several in vitro and in vivo studies have shown substantial evidence for the important contribution of cytokines to the host response to alive or killed conidia and hyphae of A. fumigatus (8, 9, 34, 35, 37, 46).
Voriconazole (VRC) is an antifungal triazole with activity against a number of pathogenic fungi and is considered the drug of choice for first-line single-drug therapy of IA (17). In vitro studies have shown that VRC either alone or combined with monocytes (MNCs) effectively inhibits the growth of A. fumigatus hyphae (23, 42).
The modulatory effects of antifungal therapy on the host response and in particular on the expression profiles of multiple genes mediating the innate immune response to A. fumigatus are not well understood. The aim of this study was to evaluate the transcriptional profiles of the genes involved in the immune response to A. fumigatus hyphae in the presence or absence of VRC using a pathway-specific DNA microarray of human immune response-related cytokines and chemokines. We also monitored the posttranscriptional expression and release of a selected number of cytokines, namely, interleukin 1β (IL-1β), tumor necrosis factor alpha (TNF-α), IL-12, monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein 1β (MIP-1β), and IL-10, by monocytes in response to A. fumigatus.
MATERIALS AND METHODS
Cell culture.
The THP-1 monocytic cell line (ATCC TIB202; American Type Culture Collection, Manassas, VA) was grown in a humidified CO2 incubator at 37°C in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (FCM; Gibco BRL, Life Technologies Ltd., Paisley, Scotland). The cell line has previously been used as a readily available source of physiologically robust monocytes/macrophages as evidenced by the expression of cytokines, differentiation, and phagocytosis (3, 20). We used the THP-1 cell line as a source of monocytes due to the need for a large number of cells for the mRNA studies. The cells were adjusted to 1 × 106 cells per ml and placed into 12-well culture plates. THP-1 cells were induced with 10 ng/ml phorbol myristate acetate at 37°C for 6 h (20). Cells were then washed once with Hanks' balanced salt solution without Ca2+ and Mg2+ and incubated with FCM at 37°C for 22 h prior to treatment with A. fumigatus hyphal fragments (HF) and VRC as described below.
Fungal growth conditions and isolation of hyphal fragments.
A well-characterized A. fumigatus isolate (strain AF 4215, deposited in the ATCC as ATCC MYA 1163) recovered from a cancer patient with invasive pulmonary aspergillosis was used in these studies. The isolate was preserved on potato dextrose agar (Merck Darmstadt, Germany) slants frozen at −24°C. A. fumigatus conidia were cultured on potato dextrose agar plates at 37°C for 2 days, harvested, and suspended in phosphate-buffered saline (PBS; Biochrom KG, Berlin, Germany) as described previously (34). They were kept at 4°C for no longer than 3 weeks. For hyphal growth, 1 × 106 conidia per ml were suspended in yeast nitrogen base broth (Scharlau Chemie SA, Spain) supplemented with 2% glucose and incubated at 37°C for 16 h.
Hyphae were washed two times in PBS and disrupted to form hyphal fragments in a 50 mM Tris-HCl (pH 7.5) buffer containing 50 mM EDTA using a UP50H sonicator (5 min in total, in 10-s bursts with 10-s intervals) (14). Hyphal inactivation was performed to avoid the overgrowth of hyphae during subsequent incubations with MNCs. The extent of hyphal disruption was tested microscopically, and the nonviability was checked by plating onto Sabouraud agar medium (Scharlau Chemie). The suspension was stored at −30°C.
Incubation of THP-1 monocytes with A. fumigatus HF and VRC.
VRC (Pfizer Inc., Groton, CT), a lyophilized powder, was reconstituted with sterile water at a concentration of 1 mg/ml and stored at −30°C. THP-1 monocytes (1 × 106 monocytes per ml) were incubated with A. fumigatus HF at an effector-target (E:T) ratio of 10:1 in the presence or absence of 0.1 μg/ml VRC at 37°C in a humidified CO2 incubator for 6 or 20 h. We selected this concentration as slightly subinhibitory since the MIC50 of VRC for various A. fumigatus clinical strains is 0.25 μg/ml (11). The MIC of VRC for the particular strain 4215 is 0.5 μg/ml as measured by the CLSI (formerly NCCLS) M-38A method of susceptibility testing (31a). This concentration is easily achievable in the sera and tissues of patients with IA receiving VRC (43). The cell viability of untreated and treated THP-1 cells was assessed by trypan blue exclusion.
In our previous studies, using a 20-h coincubation of 0.1 μg/ml VRC with monocytes and A. fumigatus hyphae at an E:T ratio of 10:1, we observed 69.7% ± 3.6% hyphal damage [measured by 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT; Sigma) assay], while the same concentration of VRC alone resulted in 49.9% ± 3.3% hyphal damage (39). For this reason, we initially performed two cDNA microarray experiments after incubation for 20 h but found no cytokine expression under any experimental condition (data not shown). When we lowered the time of coincubation of 0.1 μg/ml VRC with THP-1 cells and A. fumigatus (strain 4215) hyphae at an E:T ratio of 10:1 to 6 h, we observed 43.9% mean hyphal damage, while the same concentration of VRC alone resulted in 26.8% mean hyphal damage (Table 1). Due to these results and the findings of our previous study (9), for the subsequent experiments, the results of which are presented in this study, we selected a 6-h time of incubation and 0.1 μg/ml VRC as an effective concentration for induction of up to 50% hyphal damage of the clinical strain at hand.
TABLE 1.
Effect of VRC and THP-1 cells on hyphal damage of A. fumigatus as determined by XTT assaya
| Treatment | Hyphal damage (%) for expt:
|
|
|---|---|---|
| 1 | 2 | |
| MNC | 25.40 | 25.85 |
| VRC | 26.76 | 26.77 |
| MNC + VRC | 42.11 | 43.65 |
A. fumigatus hyphae were incubated with THP-1 alone (MNC) at an E:T ratio of 10:1, VRC at 0.1 μg/ml alone (VRC), or a combination (MNC + VRC) for 6 h. Data are presented as means of duplicate measurements.
Hyphal damage assay.
THP-1-induced hyphal damage was assessed by a method modified from the XTT assay (31). Hyphae were generated as described above. Once the hyphal network was established, yeast nitrogen base broth was then replaced by RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum. VRC at 0.1 μg/ml, THP-1 at a 10:1 E:T ratio, or a combination of the two was added to corresponding wells. After incubation at 37°C with 5% CO2 for 6 h, THP-1 cells were lysed by two washes in 200 μl of H2O. One hundred fifty microliters of PBS containing 0.25 mg/ml XTT and 40 μg/ml coenzyme Q0 (both from Sigma) was then added. After incubation at 37°C and 5% CO2 for 1 h, 100 μl of XTT was transferred onto a new plate, and the optical density was assessed spectrophotometrically at a wavelength of 450 nm using a 690-nm reference filter. Antihyphal activity was calculated according to the following formula: percent hyphal damage = (1 − X/C) × 100, where X is the optical density of experimental wells and C is the optical density of control wells with hyphae only.
RNA isolation and cDNA synthesis.
Total RNA was extracted from THP-1 cells under each experimental condition using an ArrayGrade total RNA isolation kit (Super Array Inc., Bethesda, MD). An aliquot of RNA (4 μg) with a purity ratio of 1.9 to 2.0 measured at 260- and 280-nm absorbances was reverse transcribed into biotin-labeled cDNA probes using a gene-specific primer mixture, deoxynucleoside triphosphate mix (5 mM each dATP, dCTP, and dGTP and 0.5 mM dTTP), 5 units of Moloney murine leukemia virus (M-MuLV) reverse transcriptase (RT) (Finnzymes Inc., Espoo, Finland), and 0.1 mM biotin-16-dUTP (Roche Diagnostics, Penzberg, Germany) in a total RT annealing/cocktail reaction mixture of 20 μl at 70°C for 3 min, at 42°C for 90 min, and at 94°C for 5 min according to the GEArray True Labeling-RT kit protocol (SuperArray).
cDNA array hybridization and data analysis.
The cDNA probe mixture was denatured and hybridized to 96 cDNA fragments corresponding to human inflammatory cytokines and receptors printed on a GEArray nylon membrane (GEArray Q series) in quadruplicate format per cDNA fragment. Detection was performed by chemiluminescence on X-ray film using alkaline phosphatase and CDP-Star chemiluminescent substrate solution according to the manufacturer's instructions (SuperArray). Array images produced on X-ray film were captured with a desktop scanner (scan resolution, 1,200 dots per inch) and imported into Adobe Photoshop as TIFF files. The images were analyzed by GEArray Expression Analysis Suite software (SuperArray Inc. [http://geasuite.superarray.com]). Data were normalized by subtraction of the background as the average intensity value of three spots containing pUC18 plasmid DNA. The average of two GAPDH (glyceraldehyde-3-phosphate dehydrogenase) spots was used as a baseline value with which the signal intensity of other spots was compared.
A greater-than-2.5-fold increase or decrease in signal intensity between untreated THP-1 cells (control) and THP-1 cells treated with A. fumigatus HF or between untreated THP-1 cells and THP-1 cells treated with A. fumigatus HF and VRC was considered significant induction or reduction of gene expression, respectively. Three independent array experiments were performed. The relative intensity of the target amplification product from treated samples over that of the untreated control after normalization to the internal control is expressed as the change (n-fold) with respect to the untreated control.
Reverse transcriptase PCR analysis.
Total RNA (0.5 μg) was reverse transcribed by M-MuLV reverse transcriptase (Finnzymes) and then amplified by 30 cycles using IL-1β primers 5′-GTG GCA ATG AGG ATG ACT T-3′ and 5′-TGG GCT TAT CAT CTT TCA A-3′, IL-1 receptor antagonist primers 5′-TCC GCA GTC ACC TAA TCA-3′ and 5′-CTG TCT GAG CGG ATG AAG-3′, MCP-1 primers 5′-CAA ACT GAA GCT CGC ACT-3′ and 5′-GTT TGG GTT TGC TTG TCC-3′, and MIP-1β primers 5′-GAA GCT CTG CGT GAC TGT-3′ and 5′-TGG ACC CAG GAT TCA CTG-3′ (TIB MOLBIOL, Dahlem, Germany). Amplification was performed in a final reaction mixture volume of 50 μl containing 0.5 μg total RNA, 200 μM each deoxynucleoside triphosphate, 2.0 mM MgCl2, 20 μM each sense and antisense primers, 2.5 U of M-MuLV, and 1 U of DyNazyme EXT DNA polymerase (Finnzymes). Reverse transcription was performed at 48°C for 30 min, followed by a 94°C denaturation step for 2 min and 30 PCR cycles with each cycle consisting of 94°C for 15 s, 53°C for 30 s, and 72°C for 1 min. A negative control containing RNA instead of cDNA was also included to rule out genomic contamination. All PCR products were separated on a 1.5% agarose gel. The DNA amounts on agarose gels were quantified using the UviDoc software program (DOC-008.TFT; UVItec, Cambridge, United Kingdom). Three independent RT-PCR experiments were performed in order to confirm the sensitivity of the microarray method.
Measurement of cytokine and chemokine release.
After a 6-h incubation of THP-1 monocytes with A. fumigatus HF (E:T ratio of 10:1) in the presence or absence of VRC (0.1 μg/ml) at 37°C, culture supernatants were processed with commercial enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN) in order to measure the amount of protein released for IL-1β, TNF-α, IL-12, MCP-1, MIP-1β, and IL-10 cytokines and chemokines. Supernatants were stored at −30°C until assayed. The protein concentration of the six cytokines was determined in duplicate by the quantitative sandwich ELISA method. Absorbance was read on a 2010 ELISA microplate reader (Anthos Labtech, Vienna, Austria) set at 450 nm with a reference wavelength of 540 nm. Five independent experiments were performed. The lower detection limits for IL-1β, TNF-α, IL-12, MCP-1, MIP-1β, and IL-10 were 3.9, 7.8, 7.8, 15.6, 15.6, and 0.5 pg/ml, respectively.
Statistical analysis.
The averages of the duplicate wells from each ELISA experiment were used in the data analysis to calculate the means ± standard errors of the means of all the experiments for each particular cytokine. The statistics program Instat (GraphPad, Inc., San Diego, CA) was used. Parametric analysis of variance with the Dunnett test for multiple comparisons was used for statistical comparisons of cytokine concentrations between treated and untreated THP-1 cells. A P value of <0.05 was considered statistically significant.
RESULTS
Table 2 shows chemokine-encoding genes differentially expressed in response to Aspergillus HF in the presence or absence of VRC as detected by cDNA microarrays. A greater number of chemotactic proteins, of both CC and CXC classes, exhibited differential expression patterns in response to HF plus VRC compared to those in response to HF alone. Stimulation of THP-1 cells by Aspergillus HF alone caused a significant up-regulation of only CCL4 (MIP1B) and CCL16, while CCL2 (MCP1) was down-regulated. By comparison, in the presence of Aspergillus HF, VRC strongly up-regulated genes encoding CCL3 (MIP1A), CCL4 (MIP1B), CCL5 (RANTES), CCL7 (MCP3) (reported to mediate oxidative stress-induced neutrophilic lung accumulation), CCL11 (EOTAXIN) (potent eosinophil chemoattractant), CCL15 (MIP1Δ), CXCL6 (closely related to interleukin 8 and chemotactic to neutrophils), and CXCL13 (associated with allergic airway disease and participating in the accumulation of neutrophils, eosinophils, and macrophages in the lung) in THP-1 cells. However, in the presence of HF, VRC significantly down-regulated CCL20 (MIP3A) and CCL21 (MIP2) genes. A slight change in expression was observed for genes encoding CXCL13 (1.3-fold expression), CCL11 (eotaxin) (1.9-fold expression), and CCL5 (RANTES) (1.5-fold expression) in response to Aspergillus HF.
TABLE 2.
Average change (n-fold) in genes encoding chemokines as detected by cDNA arraysa
| Gene | Description | Fold change in THP-1 cells treated with A. fumigatus hyphae
|
|
|---|---|---|---|
| In the absence of VRC | In the presence of VRC | ||
| CCL2 | Chemokine (C-C motif) ligand 2 | −2.8 | −2.4 |
| CCL3 | Chemokine (C-C motif) ligand 3 | 1.1 | 19.3 |
| CCL4 | Chemokine (C-C motif) ligand 4 | 2.5 | 5.2 |
| CCL5 | Chemokine (C-C motif) ligand 5 | 1.5 | 17.7 |
| CCL7 | Chemokine (C-C motif) ligand 7 | 0.7 | 11.3 |
| CCL11 | Chemokine (C-C motif) ligand 11 | 1.9 | 2.5 |
| CCL15 | Chemokine (C-C motif) ligand 15 | 0.7 | 10.1 |
| CCL16 | Chemokine (C-C motif) ligand 16 | 2.5 | 0.04 |
| CCL20 | Chemokine (C-C motif) ligand 20 | 0.04 | −2.5 |
| CCL21 | Chemokine (C-C motif) ligand 21 | 0.2 | −2.6 |
| CXCL6 | Chemokine (C-X-C motif) ligand 6 | 0.08 | 3.5 |
| CXCL13 | Chemokine (C-X-C motif) ligand 13 | 1.3 | 7.0 |
Genes with a ≥2.5-fold increase in expression are defined as being up-regulated; genes with a ≥2.5-fold decrease in expression are defined as being down-regulated (indicated with minus signs); genes with changes (n-fold) less than the boundary values in either direction are considered to be not significantly changed.
Table 3 shows the changes (n-fold) in genes encoding various immunomodulatory cytokines. Of the five genes differentially expressed in THP-1 cells, no significant change in gene expression was detected in THP-1 cells stimulated with Aspergillus HF alone. In contrast, all genes were changed by VRC in the presence of HF. Thus, while IL12A, IL12B, and IL-16 were down-regulated, IL-11 and TGFB1 were significantly up-regulated by VRC in the presence of Aspergillus HF.
TABLE 3.
Average change (n-fold) in genes encoding various immunomodulatory and inflammation-related cytokines
| Gene | Description | Fold change of THP1 cells treated with A. fumigatus hyphae
|
|
|---|---|---|---|
| In the absence of VRC | In the presence of VRC | ||
| Genes encoding various immunomodulatory cytokines | |||
| IL-11 | Interleukin 11 | 1.6 | 6.4 |
| TGFB1 | Transforming growth factor β1 | 1.3 | 5.9 |
| IL12A | Interleukin 12A | 1.4 | −3.3 |
| IL12B | Interleukin 12B | 0.6 | −6.0 |
| IL-16 | Interleukin 16 | 0.2 | −7.7 |
| Genes encoding inflammation-related molecules | |||
| IFNγ | Gamma interferon | 1.0 | 3.3 |
| IL1R1 | Interleukin 1 receptor, type I | 1.2 | 6.3 |
| TNFA | Tumor necrosis factor | 0.4 | 3.1 |
The inflammation-related genes IFNγ, IL1R1, and TNFA were all up-regulated after the incubation of THP-1 cells with VRC in the presence of Aspergillus HF (Table 3). The increase in expression of these genes in response to Aspergillus HF alone was not significant, ranging from 0.4- to 1.2-fold.
To validate the sensitivity of the cDNA array method, RT-PCR was performed for four selected genes, namely, IL1B, TNFA, CCL2 (MCP1), and CCL4 (MIP1B). As shown in Table 4, the results obtained with RT-PCR were comparable with the array data.
TABLE 4.
Signal intensity of cDNA array and mRNA quantified by RT-PCR
| Gene | Fold change in signal intensity of THP1 cells treated with A. fumigatus hyphaea
|
|||
|---|---|---|---|---|
| cDNA array
|
RT-PCR
|
|||
| Without VRC | With VRC | Without VRC | With VRC | |
| IL1B | ND | ND | ND | ND |
| TNFA | 0.4 | 3.1 | 1.0 | 2.6 |
| CCL2 | −2.8 | −2.4 | −2.0 | −3.0 |
| CCL4 | 2.5 | 5.2 | 3.1 | 4.0 |
ND, not detected.
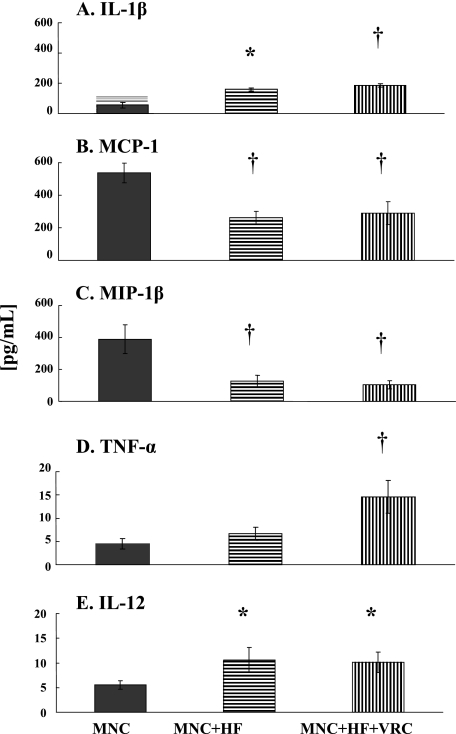
The release of IL-1β, TNF-α, IL-12, MCP-1, MIP-1β, and IL-10 by untreated THP-1 cells and cells exposed to Aspergillus HF in the absence or presence of VRC was also determined (Fig. 1). Significantly increased levels of IL-1β and IL-12 were released by monocytes stimulated with Aspergillus HF alone (Fig. 1A and E) (P < 0.05 for both cytokines) or in the presence of VRC (A and E) (P < 0.01 and P < 0.05, respectively). VRC significantly enhanced the release of TNF-α by THP-1 cells in the presence of Aspergillus HF (Fig. 1D) (P < 0.01). In contrast, comparable amounts of TNF-α were released both by untreated THP-1 cells (4.4 ± 1 pg/ml) and after stimulation with Aspergillus HF (6.7 ± 1.4 pg/ml). The chemokines MCP-1 and MIP-1β were decreased by both treatments (Fig. 1B and C) (P < 0.01). Under the experimental conditions and after the specific time of incubation that we employed in this study, we did not find gene expression and cytokine production of IL-10 in MNCs exposed or not exposed to Aspergillus HF for 6 h.
FIG. 1.
Profiles of IL-1β (A), MCP-1 (B), MIP-1β (C), TNF-α (D), and IL-12 (E) cytokine levels released after incubation of THP-1 cells (MNC) alone (dark columns), with A. fumigatus HF without VRC (MNC+HF) (horizontally striped columns), or in the presence of 0.1 μg/ml VRC (MNC+HF+VRC) (vertically striped columns) at 37°C for 6 h. Data are presented as means ± standard errors of the means derived from five donors/experiments. Comparisons between treated and control cells were performed using analysis of variance with Dunnett's test for multiple comparisons. Cells with P values of <0.05 are indicated by asterisks, and those with P values of <0.01 are indicated by daggers (†).
DISCUSSION
In this study, we have analyzed multiple genes associated with inflammation and cell recruitment. We have demonstrated that VRC induces a more pronounced profile of gene expression in human monocytes in the presence of Aspergillus HF than Aspergillus HF alone. Specifically, the up-regulated expression of several genes encoding chemokines and inflammation-related cytokines, including gamma interferon (IFN-γ), TNF-α, and transforming growth factor β (TGF-β), was more pronounced in the presence of both HF and VRC. Furthermore, the increased release of TNF-α in the presence of both HF and VRC strongly suggests that VRC plays an important role in cell recruitment and host resistance to A. fumigatus.
The results of this focused microarray study are consistent with our previous findings with whole-genome microarray analysis of the regulation of gene expression by human monocytes in response to A. fumigatus alone (9). By comparing the results of the two studies on the regulation of various chemokines and cytokines, we observed a qualitative consistency demonstrating similarity among immunoregulatory patterns (i.e., genes encoding IL-10 and TNF-α).
As VRC is currently widely used for the treatment of IA (17, 43), we sought to understand whether VRC could activate a genetic program in monocytes, which may lead to a more effective innate immune response against Aspergillus hyphae. In the present study, we focused our attention on gene expression profiles associated with inflammation and chemotactic activity in monocytes in order to evaluate probable immunomodulatory interactions of genes assigned to a defined biological pathway. We used pathway-specific cDNA array analysis and measured levels of cytokines released for selective mRNA species in order to identify inflammation-associated gene expression patterns in THP-1 cells exposed to Aspergillus HF in the presence or absence of VRC. In order to ensure optimal conditions and internal consistency, we selected a VRC concentration and times of incubation based on previous data showing that VRC and MNCs collaborate for an additive activity.
Our results showing that in the presence of Aspergillus HF, VRC induced a greater number of proinflammatory genes to be differentially expressed than did MNCs stimulated by A. fumigatus HF alone suggest that a key mechanism could be a direct effect of VRC on transcriptional and posttranscriptional pathways of MNCs. Since VRC acts on fungal hyphae by inhibiting the pathway of ergosterol biosynthesis in the metabolically active organism, we propose that VRC does not affect MNC function through an effect on disrupted nonviable hyphal fragments but that it facilitates the activation of a genetic program in monocytes, which may lead to a more effective innate immune response against Aspergillus hyphae. For example, a probable interaction of VRC with TLR2/TLR4 receptors on the surface of immune cells mediated by the activation of the NF-κB signal transduction pathway and facilitated by the presence of VRC could augment the fungicidal activity of host cells upon challenge with A. fumigatus hyphae by transcriptional induction of proinflammatory cytokines. A targeted analysis of key macrophage pathogen-associated molecular pattern receptors (i.e., TLR2, TLR4, Dectin-1, etc.) would provide greater insight into possible mechanisms of VRC function on the proinflammatory program of MNCs. Elucidation of this mechanism warrants further investigations on the molecular pathways by which VRC acts on recognition, signal transduction, or gene expression. In any case, monocyte and neutrophil recruitment to the site of infection via certain chemokines and the induction of IFN-γ and TNF-α by VRC in the presence of HF may contribute to the enhanced immune response of the host to A. fumigatus hyphae and to the clearance of pathogens from the host.
Several in vivo and in vitro studies have demonstrated the ability of both Aspergillus conidia and hyphae, alive or killed, to stimulate the expression of proinflammatory cytokines in leukocytes through a TLR2/TLR4-dependent signal transduction pathway (19, 32, 45). VRC may up- or down-regulate such molecules on the cell membrane or cytoplasm of host phagocytes. We confirmed the regulation of the expression of such inflammation-related genes, but we advise caution in the interpretation of the results of mRNA studies alone. Indeed, our protein measurement studies showed that posttranscriptional regulation or a delay of translation for certain mRNA species may exist, since mRNA and protein molecules of IL-1β, CCL4/MIP-1β, and IL-12 did not follow similar profiles of expression after 6 h of incubation. In contrast, mRNA and proteins of CCL2 (MCP-1), TNF-α, and IL-10 showed corresponding patterns of expression.
VRC has been shown to have antifungal activity against Aspergillus, Fusarium, and Scedosporium species (13). In vitro studies of drug antifungal activity have been extended to combination studies of VRC with host defense represented by phagocytes as a means of improved clearance of the fungus. Thus, Vora et al. showed that VRC additively collaborates with phagocytic cells in inhibiting hyphal growth of A. fumigatus (42). We have shown that MNC and VRC display significant antifungal activity against A. fumigatus hyphae at an E:T ratio of 10:1 after incubation for 6 h (Table 1) and 20 h (39). Other investigators recently showed that the addition of VRC to infected macrophages with non-Candida albicans Candida species reduces the number of viable intracellular organisms (4). We have reported similar combinational effects of VRC with another genus of difficult-to-treat filamentous fungi, Scedosporium spp. (15). Given the inherent limitations of in vitro studies, which may underestimate the complexity of the inflammatory and innate response to fungi in vivo in different clinical settings, the clinical relevance of the data presented here requires in vivo confirmation.
In our study, of the 12 chemokine-encoding genes that were differentially expressed, we observed a significant up-regulation of CCL4 (MIP1B) and CCL16 in THP-1 cells treated with Aspergillus HF. By comparison, in the presence of HF, VRC caused the up-regulation of CCL3 (MIP1A), CCL4 (MIP1B), CCL5 (RANTES), CCL7 (MCP3), CCL11 (EOTAXIN), CCL15 (MIP1Δ), CXCL6, and CXCL13. Among the CC chemokines, the MIP-1 family members initiate acute and chronic inflammatory responses at sites of infection by attracting proinflammatory cytokines (27). CCL3 (MIP-1α) and CCL4 (MIP-1β) have potent chemotactic activities for leukocytes and are important mediators of the host defense against IA in both immunocompetent and neutropenic hosts (28, 33).
Pylkkanen et al. previously observed significantly increased expression in TNF-α, MIP-1α, and MIP-1β mRNA with maximal induction at 6 h but very low IL-1β mRNA and no induction of any cytokines or chemokines at the protein level by exposure of mouse macrophages to A. fumigatus conidia (33). Our results demonstrate similar chemokine mRNA expression patterns but significantly increased production of IL-1β and TNF-α and decreased MIP-1β released by human THP-1 cells treated with Aspergillus HF in the presence or absence of VRC. Furthermore, the expression of MCP-1 was significantly down-regulated in THP-1 cells exposed either to Aspergillus HF alone or to Aspergillus HF and VRC, suggesting that MCP-1 production possesses different kinetics of expression.
Host resistance to A. fumigatus infection involves the induction of proinflammatory cytokines, including IFN-γ, IL-1β, IL-12, and TNF-α, while susceptibility to infection is associated with the production of IL-10 (7, 29, 30, 34). In our study, while IFNγ, IL1R1 (receptor for IL-1β), TNFA, and TGFB1 genes associated with inflammation were significantly up-regulated at 6 h in THP-1 cells exposed to Aspergillus HF in the presence of VRC, mRNA expression levels of these genes in cells exposed to hyphal fragments alone showed indifferent transcript levels compared to control cells. To our knowledge, only one other study has characterized innate inflammatory responses of macrophages after stimulation with killed 7-h-old A. fumigatus conidial germlings in the presence of VRC (19). This study showed increased TNF-α production by mouse alveolar macrophages after incubation with A. fumigatus in the presence of 0.5 μg/ml VRC for 18 h. In this current study, where human monocytes and not mouse macrophages were used, the inflammatory response for TNF-α could be initiated as early as 6 h in THP-1 cells exposed to VRC in the presence of Aspergillus HF.
VRC significantly augmented both the gene expression and the protein release of TNF-α by monocytes in response to A. fumigatus. The importance of TNF-α in the host defense against IA is well known with in vitro and in vivo studies (8, 29, 35). Moreover, patients receiving anti-TNF-α therapy with infliximab may be at increased risk to suffer from IA (1). While the molecular mechanism of this action of VRC is not well understood, the finding of the further up-regulation of TNF-α release due to VRC may be important for augmenting the innate host defense against IA.
IL-10, by itself, suppresses the antifungal activity of human MNCs challenged with Aspergillus hyphae (34). Under the conditions and after the specific time of incubation that we employed in this study, we found an absence of IL-10 expression and production. While we cannot exclude a role of IL-10 in the interaction between THP-1 monocytes and damaged hyphae, the increase of IL-1β and IL-12 release by stimulated THP-1 monocytes with or without VRC suggests that this cytokine profile augments initial innate antifungal immunity by increasing Th1 cytokine responses. Our findings are in agreement with murine studies of disseminated pulmonary aspergillosis in which A. fumigatus infection in IL-10-deficient mice showed inflammatory responses associated with the up-regulation of innate antifungal Th1 responses, such as the production of IL-12, TNF-α, and IFN-γ (8, 10). The down-regulation of the genes encoding IL12A and IL12B, the two subunits of IL-12, did not parallel IL-12 production levels, suggesting that gene transcription of IL-12 subunits and posttranslational subunit assembly may occur at later time intervals.
TGF-β is produced by several leukocyte populations controlling the differentiation and activation of immune cells. Peripheral blood monocytes become activated by TGF-β to promote phagocytosis and to increase the production of proinflammatory cytokines, while tissue macrophages are inhibited by TGF-β in order to reduce inflammatory tissue damage (22). IL-11 is another pleiotropic cytokine with immunoregulatory activity, which attenuates the inflammatory response by down-regulating proinflammatory cytokine release (5, 41). In this study, we observed a significant up-regulation of both TGFB1 and IL-11 expression in THP-1 cells challenged with Aspergillus HF in the presence of VRC, suggesting a balance between pro- and anti-inflammatory signaling pathways. The release of IL-16 by blood monocytes has been associated with apoptotic cell functions, since its release is paralleled by the activation of caspase-3 (12). In this study, the down-regulation of IL-16 expression in THP-1 cells under both experimental conditions constitutes confirmation for the integrity of the THP-1 cell functionality able to induce innate and T-cell-mediated immune responses.
A limitation of this study could be the fact that we used only two time points (6 and 20 h) after incubation of VRC and/or HF with the monocytes and only one VRC concentration. The complexity, however, of the various parts of the study and the difficulty to perform the various assays compelled us to select the most promising incubation times and VRC concentration to perform these experiments. Moreover, in previous studies, we and other investigators have shown that incubation for 6 h is appropriate to reveal cytokine up- or down-regulation (9, 33, 45, 46). On the other hand, we believe that the concentration of VRC selected for use is an appropriate subinhibitory concentration that is also very close to the MIC of the particular strain against conidia and at the same time damages about 50% of hyphae of the strain in combination with MNCs.
In conclusion, we have demonstrated that in the presence of Aspergillus HF, VRC induces a more pronounced profile of gene expression in human monocytes than Aspergillus HF alone. Future in vivo studies elucidating the role of these cytokines in combination with VRC and other antifungal drugs are warranted, as they may provide important information on the use of adjunctive immunotherapy against IA in immunocompromised patients.
Acknowledgments
This research was cofunded by the European Social Fund and National Resources-EPEAEK II-ARCHIMIDIS.
Footnotes
Published ahead of print on 18 December 2006.
REFERENCES
- 1.Alderson, J. W., T. G. Van Dinter, Jr., M. J. Opatowsky, and E. C. Burton. 2005. Disseminated aspergillosis following infliximab therapy in an immunosuppressed patient with Crohn's disease and chronic hepatitis C: a case study and review of the literature. Med. Gen. Med. 7:7. [PMC free article] [PubMed] [Google Scholar]
- 2.Almyroudis, N. G., S. M. Holland, and B. H. Segal. 2005. Invasive aspergillosis in primary immunodeficiencies. Med. Mycol. 43:S247-S259. [DOI] [PubMed] [Google Scholar]
- 3.Barker, K. S., T. Liu, and P. D. Rogers. 2005. Coculture of THP-1 human mononuclear cells with Candida albicans results in pronounced changes in host gene expression. J. Infect. Dis. 192:901-912. [DOI] [PubMed] [Google Scholar]
- 4.Bopp, L. H., A. L. Baltch, W. J. Ritz, P. B. Michelsen, and R. P. Smith. 2006. Antifungal effect of voriconazole on intracellular Candida glabrata, Candida krusei and Candida parapsilosis in human monocyte-derived macrophages. J. Med. Microbiol. 55:865-870. [DOI] [PubMed] [Google Scholar]
- 5.Bozza, M., J. L. Bliss, A. J. Dorner, and W. L. Trepicchio. 2001. Interleukin-11 modulates Th1/Th2 cytokine production from activated CD4+ T cells. J. Interferon Cytokine Res. 21:21-30. [DOI] [PubMed] [Google Scholar]
- 6.Brakhage, A. A. 2005. Systemic fungal infections caused by Aspergillus species: epidemiology, infection process and virulence determinants. Curr. Drug Targets 6:875-886. [DOI] [PubMed] [Google Scholar]
- 7.Brieland, J. K., C. Jackson, F. Menzel, D. Loebenberg, A. Cacciapuoti, J. Halpern, S. Hurst, T. Muchamuel, R. Debets, R. Kastelein, T. Churakova, J. Abrams, R. Hare, and A. O'Garra. 2001. Cytokine networking in lungs of immunocompetent mice in response to inhaled Aspergillus fumigatus. Infect. Immun. 69:1554-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cenci, E., A. Mencacci, C. Fe d'Ostiani, G. Del Sero, P. Mosci, C. Montagnoli, A. Bacci, and L. Romani. 1998. Cytokine- and T helper-dependent lung mucosal immunity in mice with invasive pulmonary aspergillosis. J. Infect. Dis. 178:1750-1760. [DOI] [PubMed] [Google Scholar]
- 9.Cortez, K. J., C. A. Lyman, S. Kottilil, H. S. Kim, E. Roilides, J. Yang, B. Fullmer, R. Lempicki, and T. J. Walsh. 2006. Functional genomics of innate host defense molecules in normal human monocytes in response to Aspergillus fumigatus. Infect. Immun. 74:2353-2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Del Sero, G., A. Mencacci, E. Cenci, C. F. d'Ostiani, C. Montagnoli, A. Bacci, P. Mosci, M. Kopf, and L. Romani. 1999. Antifungal type 1 responses are upregulated in IL-10-deficient mice. Microbes Infect. 1:1169-1180. [DOI] [PubMed] [Google Scholar]
- 11.Diekema, D. J., S. A. Messer, R. J. Hollis, R. N. Jones, and M. A. Pfaller. 2003. Activities of caspofungin, itraconazole, posaconazole, ravuconazole, voriconazole, and amphotericin B against 448 recent clinical isolates of filamentous fungi. J. Clin. Microbiol. 41:3623-3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elssner, A., A. I. Doseff, M. Duncan, M. Kotur, and M. D. Wewers. 2004. IL-16 is constitutively present in peripheral blood monocytes and spontaneously released during apoptosis. J. Immunol. 172:7721-7725. [DOI] [PubMed] [Google Scholar]
- 13.Espinel-Ingroff, A. 1998. In vitro activity of the new triazole voriconazole (UK-109,496) against opportunistic filamentous and dimorphic fungi and common and emerging yeast pathogens. J. Clin. Microbiol. 36:198-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fontaine, T., C. Simenel, G. Dubreucq, O. Adam, M. Delepierre, J. Lemoine, C. E. Vorgias, M. Diaquin, and J. P. Latge. 2000. Molecular organization of the alkali-insoluble fraction of Aspergillus fumigatus cell wall. J. Biol. Chem. 275:41528. [PubMed] [Google Scholar]
- 15.Gil-Lamaignere, C., E. Roilides, J. Mosquera, A. Maloukou, and T. J. Walsh. 2002. Antifungal triazoles and polymorphonuclear leukocytes synergize to cause increased hyphal damage to Scedosporium prolificans and Scedosporium apiospermum. Antimicrob. Agents Chemother. 46:2234-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groll, A. H., P. M. Shah, C. Mentzel, M. Schneider, G. Just-Nuebling, and K. Huebner. 1996. Trends in the postmortem epidemiology of invasive fungal infections at a university hospital. J. Infect. 33:23-32. [DOI] [PubMed] [Google Scholar]
- 17.Herbrecht, R., D. W. Denning, T. F. Patterson, J. E. Bennett, R. E. Greene, J. W. Oestmann, W. V. Kern, K. A. Marr, P. Ribaud, O. Lortholary, R. Sylvester, R. H. Rubin, J. R. Wingard, P. Stark, C. Durand, D. Caillot, E. Thiel, P. H. Chandrasekar, M. R. Hodges, H. T. Schlamm, P. F. Troke, and B. de Pauw. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 347:408-415. [DOI] [PubMed] [Google Scholar]
- 18.Herbrecht, R., A. Moghaddam, L. Mahmal, S. Natarajan-Ame, L. M. Fornecker, and V. Letscher-Bru. 2005. Invasive aspergillosis in the hematologic and immunologic patient: new findings and key questions in leukemia. Med. Mycol. 43(Suppl. 1):S239-S242. [DOI] [PubMed] [Google Scholar]
- 19.Hohl, T. M., H. L. Van Epps, A. Rivera, L. A. Morgan, P. L. Chen, M. Feldmesser, and E. G. Pamer. 2005. Aspergillus fumigatus triggers inflammatory responses by stage-specific beta-glucan display. PLoS Pathogens 1:e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lagoumintzis, G., M. Christofidou, G. Dimitracopoulos, and F. Paliogianni. 2003. Pseudomonas aeruginosa slime glycolipoprotein is a potent stimulant of tumor necrosis factor alpha gene expression and activation of transcription activators nuclear factor κB and activator protein 1 in human monocytes. Infect. Immun. 71:4614-4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Latge, J. P. 1999. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12:310-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Letterio, J. J., and A. B. Roberts. 1998. Regulation of immune responses by TGF-beta. Annu. Rev. Immunol. 16:137-161. [DOI] [PubMed] [Google Scholar]
- 23.Lewis, R. E., N. P. Wiederhold, and M. E. Klepser. 2005. In vitro pharmacodynamics of amphotericin B, itraconazole, and voriconazole against Aspergillus, Fusarium, and Scedosporium spp. Antimicrob. Agents Chemother. 49:945-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin, S. J., J. Schranz, and S. M. Teutsch. 2001. Aspergillosis case-fatality rate: systematic review of the literature. Clin. Infect. Dis. 32:358-366. [DOI] [PubMed] [Google Scholar]
- 25.Marr, K. A., R. A. Carter, M. Boeckh, P. Martin, and L. Corey. 2002. Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood 100:4358-4366. [DOI] [PubMed] [Google Scholar]
- 26.Marr, K. A., R. A. Carter, F. Crippa, A. Wald, and L. Corey. 2002. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 34:909-917. [DOI] [PubMed] [Google Scholar]
- 27.Maurer, M., and E. von Stebut. 2004. Macrophage inflammatory protein-1. Int. J. Biochem. Cell Biol. 36:1882-1886. [DOI] [PubMed] [Google Scholar]
- 28.Mehrad, B., T. A. Moore, and T. J. Standiford. 2000. Macrophage inflammatory protein-1 alpha is a critical mediator of host defense against invasive pulmonary aspergillosis in neutropenic hosts. J. Immunol. 165:962-968. [DOI] [PubMed] [Google Scholar]
- 29.Mehrad, B., R. M. Strieter, and T. J. Standiford. 1999. Role of TNF-alpha in pulmonary host defense in murine invasive aspergillosis. J. Immunol. 162:1633-1640. [PubMed] [Google Scholar]
- 30.Mencacci, A., E. Cenci, A. Bacci, C. Montagnoli, F. Bistoni, and L. Romani. 2000. Cytokines in candidiasis and aspergillosis. Curr. Pharm. Biotechnol. 1:235-251. [DOI] [PubMed] [Google Scholar]
- 31.Meshulam, T., S. M. Levitz, L. Christin, and R. D. Diamond. 1995. A simplified new assay for assessment of fungal cell damage with the tetrazolium dye, (2,3)-bis-(2-methoxy-4-nitro-5-sulphenyl)-(2H)-tetrazolium-5-carboxanil ide (XTT). J. Infect. Dis. 172:1153-1156. [DOI] [PubMed] [Google Scholar]
- 31a.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi. Approved standard. Document M-38A. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 32.Netea, M. G., A. Warris, J. W. Van der Meer, M. J. Fenton, T. J. Verver-Janssen, L. E. Jacobs, T. Andresen, P. E. Verweij, and B. J. Kullberg. 2003. Aspergillus fumigatus evades immune recognition during germination through loss of Toll-like receptor-4-mediated signal transduction. J. Infect. Dis. 188:320-326. [DOI] [PubMed] [Google Scholar]
- 33.Pylkkanen, L., H. Gullsten, M. L. Majuri, U. Andersson, E. Vanhala, J. Maatta, T. Meklin, M. R. Hirvonen, H. Alenius, and K. Savolainen. 2004. Exposure to Aspergillus fumigatus spores induces chemokine expression in mouse macrophages. Toxicology 200:255-263. [DOI] [PubMed] [Google Scholar]
- 34.Roilides, E., A. Dimitriadou, I. Kadiltsoglou, T. Sein, J. Karpouzas, P. A. Pizzo, and T. J. Walsh. 1997. IL-10 exerts suppressive and enhancing effects on antifungal activity of mononuclear phagocytes against Aspergillus fumigatus. J. Immunol. 158:322-329. [PubMed] [Google Scholar]
- 35.Roilides, E., A. Dimitriadou-Georgiadou, T. Sein, I. Kadiltsoglou, and T. J. Walsh. 1998. Tumor necrosis factor alpha enhances antifungal activities of polymorphonuclear and mononuclear phagocytes against Aspergillus fumigatus. Infect. Immun. 66:5999-6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romani, L. 2004. Immunity to fungal infections. Nat. Rev. Immunol. 4:1-23. [DOI] [PubMed] [Google Scholar]
- 37.Schelenz, S., D. A. Smith, and G. J. Bancroft. 1999. Cytokine and chemokine responses following pulmonary challenge with Aspergillus fumigatus: obligatory role of TNF-alpha and GM-CSF in neutrophil recruitment. Med. Mycol. 37:183-194. [DOI] [PubMed] [Google Scholar]
- 38.Silveira, F., and D. L. Paterson. 2005. Pulmonary fungal infections. Curr. Opin. Pulm. Med. 11:242-246. [DOI] [PubMed] [Google Scholar]
- 39.Simitsopoulou, M., M. Dalakiouridou, T. Konstantinou, C. Likartsis, J. Ioannidis, A. Orfanou, T. J. Walsh, and E. Roilides. 2005. Discordant combined antifungal effects of voriconazole and caspofungin with human monocytes against Aspergillus fumigatus. Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-1017, p. 435.
- 40.Singh, N. 2005. Invasive aspergillosis in organ transplant recipients: new issues in epidemiologic characteristics, diagnosis, and management. Med. Mycol. 43(Suppl. 1):S267-S270. [DOI] [PubMed] [Google Scholar]
- 41.Trepicchio, W. L., M. Bozza, G. Pedneault, and A. J. Dorner. 1996. Recombinant human IL-11 attenuates the inflammatory response through down-regulation of proinflammatory cytokine release and nitric oxide production. J. Immunol. 157:3627-3634. [PubMed] [Google Scholar]
- 42.Vora, S., S. Chauhan, E. Brummer, and D. A. Stevens. 1998. Activity of voriconazole combined with neutrophils or monocytes against Aspergillus fumigatus: effects of granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor. Antimicrob. Agents Chemother. 42:2299-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walsh, T. J., M. O. Karlsson, T. Driscoll, A. G. Arguedas, P. Adamson, X. Saez-Llorens, A. J. Vora, A. C. Arrieta, J. Blumer, I. Lutsar, P. Milligan, and N. Wood. 2004. Pharmacokinetics and safety of intravenous voriconazole in children after single- or multiple-dose administration. Antimicrob. Agents Chemother. 48:2166-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walsh, T. J., E. Roilides, K. Cortez, S. Kottilil, J. Bailey, and C. A. Lyman. 2005. Control, immunoregulation, and expression of innate pulmonary host defenses against Aspergillus fumigatus. Med. Mycol. 43(Suppl. 1):S165-S172. [DOI] [PubMed] [Google Scholar]
- 45.Wang, J. E., A. Warris, E. A. Ellingsen, P. F. Jorgensen, T. H. Flo, T. Espevik, R. Solberg, P. E. Verweij, and A. O. Aasen. 2001. Involvement of CD14 and Toll-like receptors in activation of human monocytes by Aspergillus fumigatus hyphae. Infect. Immun. 69:2402-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Warris, A., M. G. Netea, P. E. Verweij, P. Gaustad, B. J. Kullberg, C. M. Weemaes, and T. G. Abrahamsen. 2005. Cytokine responses and regulation of interferon-gamma release by human mononuclear cells to Aspergillus fumigatus and other filamentous fungi. Med. Mycol. 43:613-621. [DOI] [PubMed] [Google Scholar]
- 47.Wiederhold, N. P., R. E. Lewis, and D. P. Kontoyiannis. 2003. Invasive aspergillosis in patients with hematologic malignancies. Pharmacotherapy 23:1592-1610. [DOI] [PubMed] [Google Scholar]