Abstract
The in vitro activities of cloxyquin (5-chloroquinolin-8-ol) against 9 standard strains and 150 clinical isolates of Mycobacterium tuberculosis were studied. The MICs ranged from 0.062 to 0.25 μg/ml. The MIC50 and MIC90 were 0.125 and 0.25 μg/ml, respectively. These indicate that cloxyquin exhibited good antituberculosis activity, even for multidrug-resistant isolates.
Current first-line drugs for treatment of tuberculosis consist of only 5 agents, i.e., isoniazid (INH), rifampin (RIF), ethambutol (EMB), pyrazinamide (PZA), and streptomycin (STR). Resistance to the first-line drugs, especially RIF and INH, usually causes treatment failure and necessitates the use of the second-line drugs with a prolonged period of therapy. Even with that, treatment frequently fails. New antituberculous agents, especially the ones with novel mechanisms of action are urgently required.
Bihalogenated 8-hydroxyquinolines (quinolin-8-ols) are a group of known drugs with antiamebic activities and were widely used to treat intestinal infection. The commonly used ones include broxyquinoline, clioquinol chlorquinaldol, and iodoquinol (4, 6). They also exhibit antibacterial and antifungal activities (1, 14).
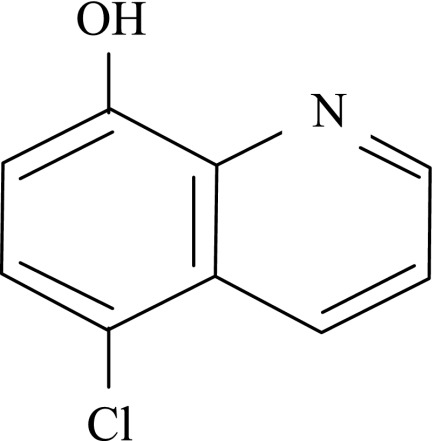
Herewith, we report the antituberculosis activities of a monohalogenated 8-hydroxyquinoline, cloxyquin (5-chloroquinolin-8-ol), against 150 clinical Mycobacterium tuberculosis isolates, including multidrug-resistant strains. Cloxyquin (Fig. 1) was known to possess activities against bacteria, fungi, and protozoa (3, 10, 11, 12), but the antimycobacterial activity has never been documented.
FIG. 1.
Chemical structure of cloxyquin.
A total of 159 strains of M. tuberculosis, including 9 reference strains (H37Rv ATCC 27294, H37Ra ATCC 25177, H37Rv-PAS-R ATCC 35821 [p-aminosalicylic acid resistant], H37Rv-CS-R ATCC 35826 [cycloserine resistant], H37Rv-KM-R ATCC 35827 [kanamycin resistant], H37Rv-PZA-R ATCC 35828 [pyrazinamide resistant], H37Rv-TAC-R ATCC 35829 [thiacetazone resistant], H37Rv-ETA-R ATCC 35830 [ethionamide resistant], and H37Rv-EMB-R ATCC 35837 [ethambutol resistant]) and 150 isolates from pulmonary and extrapulmonary patients in Ramathibodi Hospital, Bangkok, Thailand, including 100 sensitive strains, 20 drug-resistant strains (7, 3, 3, and 12 isolates resistant to INH, RIF, EMB, and STR, respectively), and 30 multidrug-resistant (MDR) strains (7 isolates resistant to INH and RIF, 3 isolates additionally resistant to EMB, 13 isolates additionally resistant to STR, and 7 isolates additionally resistant to EMB and STR), were investigated. The MICs of cloxyquin (Sigma Chemical Co., St. Louis, MO) for all M. tuberculosis isolates were determined duplicated by microplate Alamar blue assay (8), which has been showed to correlate well (>90%) with the BACTEC and proportional methods (2, 8, 16, 19). Briefly, cloxyquin was prepared in dimethyl sulfoxide (Sigma) and subsequently diluted twofold in 100 μl of Middlebrook 7H9GC in clear flat-bottom, 96-well microplates. A mycobacterial suspension was prepared in 0.04% Tween 80 and diluted with sterile distilled water to a turbidity of the McFarland no. 1. The suspension was then diluted 1:50 with 7H9GC, and 100 μl was added to the wells. The highest final concentration of dimethyl sulfoxide was 0.156% (vol/vol). The plates were incubated at 37°C for 7 days; 12.5 μl of 20% Tween 80 and 20 μl of Alamar blue (SeroTec Ltd., Oxford, United Kingdom) were added to all wells. Growth of the organisms was determined after reincubation at 37°C for 16 to 24 h by visual determination of a color change from blue to pink. The MIC was defined as the lowest concentration which prevented the color change. RIF and INH (Sigma) were included as controls.
The MICs of 8-hydroxyquinoline, cloxyquin, clioquinol, chlorquinaldol, and broxyquinoline against M. tuberculosis H37Ra were 0.125, 0.125, 6.25, 0.38, and 6.25 μg/ml, respectively. This suggested that 8-hydroxyquinoline and its derivatives are fairly active against M. tuberculosis. To elucidate more on their potentials, MICs of cloxyquin were further studied. The MICs of cloxyquin for the 9 reference strains ranged from 0.125 to 0.25 μg/ml. Similarly, the MICs of cloxyquin for 150 clinical isolates ranged from 0.062 to 0.25 μg/ml. The MIC50 and MIC90 were 0.125 and 0.25 μg/ml, respectively (Table 1). There were no statistically significant differences of MICs between drug-sensitive, drug-resistant, and MDR strains. Nor were there any observable differences in MICs of strains with different antibiotic resistance patterns. The MICs of RIF and INH against M. tuberculosis H37Rv were 0.031 and 0.062 μg/ml, respectively.
TABLE 1.
MICs of cloxyquin for clinical isolates of M. tuberculosisa
| M. tuberculosis strain type (n) | No. (%) of isolates for which MIC (μg/ml) is:
|
MIC90 (μg/ml) | ||
|---|---|---|---|---|
| 0.062 | 0.125 | 0.25 | ||
| Drug sensitive (100) | 15 (15) | 74 (74) | 11 (11) | 0.25 |
| Drug resistant (20) | 2 (10) | 13 (65) | 5 (25) | 0.25 |
| MDR (30) | 5 (16.7) | 24 (80) | 1 (3.3) | 0.125 |
| Total (150) | 22 (14.7) | 111 (74) | 17 (11.3) | 0.25 |
MIC50 are 0.125 μg/ml for all groups.
The fact that cloxyquin is equally active across various mono- and multidrug-resistant clinical isolates suggested that its mechanism of action is not shared by previously known drugs. The antimicrobial action of bihalogenated 8-hydroxyquinolines is likely to relate to their chelating activities. It is proposed that the iron chelation deprives the microbes of the essential nutrient. However, the mechanisms may actually be more complex. For example, bihalogenated 8-hydroxyquinolines were found to inhibit the RNA-dependent DNA polymerase of respiratory syncytial virus by chelation of copper (17) and to inhibit RNA synthesis by chelation of Mn2+, Mg2+, and Zn2+ (9). Moreover, the antibacterial action may be the property of the metal complexes but not the free compounds (13, 17). It had previously been proposed that iodinated 8-hydroxyquinolines worked through the release of free iodine in the intestinal lumen, but some bihalogenated 8-hydroxyquinolines have antimicrobial activities even without containing any iodine. It was proposed later that the iodine residue may play a role in delaying the absorption of the drugs and makes the drugs stay longer in the intestinal lumen (6). Precise mechanisms of action of halogenated 8-hydroxyquinolines remain to be investigated. There have been a few studies of the antituberculosis activity of quinolines. For example, clioquinol had good activity in guinea pigs but not in mice (14, 18). The N-sulfonic acid derivative of 5-hydroxyamino-8-hydroxyquinoline and 8-butoxyquinoline also had good antituberculous activity in guinea pigs. The MICs of 5-nitro-8-hydroxyquinoline and 8-hydroxyquinoline against Mycobacterium bovis BCG were found to be 1.9 and 0.3 μg/ml, respectively (15). Moreover, both showed moderate bactericidal activity in the in vitro model of dormant M. bovis BCG (15). The antituberculous effect of cloxyquin has never been reported. There is no clear information regarding the safety of cloxyquin either. However, clioquinol was reported as a possible cause of subacute myelooptic neuropathy, an uncommon neurological syndrome that occurred primarily in Japan (4, 7). The cause of the syndrome is, however, far from established, as environmental factors, such as B12 deficiency, are also likely to be important. Nevertheless, recently, the interest in clioquinol has been increased due to its favorable effects on Alzheimer's disease (5, 7). In conclusion, the excellent in vitro activity (even for MDR tuberculosis) of cloxyquin against M. tuberculosis deserves further investigation.
Acknowledgments
This study was supported by the National Center for Genetic Engineering and Biotechnology, Thailand.
Many standard strains were kindly provided by Scott G. Franzblau, University of Illinois at Chicago, IL.
Footnotes
Published ahead of print on 18 December 2006.
REFERENCES
- 1.Baker, J. W., I. Schumacher, and D. P. Roman. 1970. Antiseptics and disinfectants, p. 635. In A. Burger (ed.), Medicinal chemistry, 3rd ed. John Wiley & Sons, New York, NY.
- 2.Collins, L., and S. G. Franzblau. 1997. Microplate alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob. Agents Chemother. 41:1004-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cosgrove, R. F., and S. Baines. 1978. In vitro activity of chlorhydroxyquinoline against Mycoplasma species. Antimicrob. Agents Chemother. 13:540-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Croft, S. L. 1997. Antiprotozoal agents, p. 526-527. In F. O'Grady, H. P. Lambert, R. G. Finch, and D. Greenwood (ed.), Antibiotic and chemotherapy: anti-infective agents and their use in therapy, 7th ed. Churchill Livingstone, New York, NY.
- 5.Doraiswamy, P. M., and A. E. Finefrock. 2004. Metals in our minds: therapeutic implications for neurodegenerative disorders. Lancet Neurol. 3:431-434. [DOI] [PubMed] [Google Scholar]
- 6.Elslager, E. F. 1970. Antiamebic agents, p. 540-541. In A. Burger (ed.), Medicinal chemistry, 3rd ed. John Wiley & Sons, New York, NY.
- 7.Finefrock, A. E., A. I. Bush, and P. M. Doraiswamy. 2003. Current status of metals as therapeutic targets in Alzheimer's disease. J. Am. Geriatr. Soc. 51:1143-1148. [DOI] [PubMed] [Google Scholar]
- 8.Franzblau, S. G., R. S. Witzig, J. C. McLaughlin, P. Torres, G. Madico, A. Hernandez, M. T. Degnan, M. B. Cook, V. K. Quenzer, R. M. Ferguson, and R. H. Gilman. 1998. Rapid low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue assay. J. Clin. Microbiol. 36:362-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraser, R. S., and J. Creanor. 1975. The mechanism of inhibition of ribonucleic acid synthesis by 8-hydroxyquinoline and the antibiotic lomofungin. Biochem. J. 147:401-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gartner, R., and U. Kaben. 1977. Therapeutic experience with 5-chloro-8-hydroxyquinoline. Dermatol. Monatsschr. 163:711-714. [PubMed] [Google Scholar]
- 11.Gershon, H., M. Gershon, and D. D. Clarke. 2004. Synergistic mixtures of fungitoxic monochloro and dichloro-8-quinolinols against five fungi. Mycopathologia 158:131-135. [DOI] [PubMed] [Google Scholar]
- 12.Grunder, K., and D. Petzoldt. 1979. Effect of a corticosteroid additive on the result of local therapy of bacterial superinfection in tinea pedis intertriginosa. Hautarzt 30:392-395. [PubMed] [Google Scholar]
- 13.Leanderson, P., and C. Tagesson. 1996. Iron bound to the lipophilic iron chelator, 8-hydroxyquinoline, causes DNA strand breakage in cultured lung cells. Carcinogenesis 17:545-550. [DOI] [PubMed] [Google Scholar]
- 14.Lewis, A., and R. G. Shepherd. 1970. Antimycobacterial agents, p. 449. In A. Burger (ed.), Medicinal chemistry, 3rd ed. John Wiley & Sons, New York, NY.
- 15.Murugasu-Oei, B., and T. Dick. 2001. In vitro activity of the chelating agents nitroxoline and oxine against Mycobacterium bovis BCG. Int. J. Antimicrob. Agents. 18:579-582. [DOI] [PubMed] [Google Scholar]
- 16.Palomino, J. C., and F. Portaels. 1999. Simple procedure for drug susceptibility testing of Mycobacterium tuberculosis using a commercial colorimetric assay. Eur. J. Clin. Microbiol. Infect. Dis. 18:380-383. [DOI] [PubMed] [Google Scholar]
- 17.Rohde, W., B. Cordell, R. Webster, and W. Levinson. 1977. Inhibition of amino acyl tRNA synthetase activity by copper complexes of two metal binding ligands N-methyl isatin beta-thiosemicarbazone and 8-hydroxyquinoline. Biochim. Biophys. Acta 477:102-111. [DOI] [PubMed] [Google Scholar]
- 18.Tison, F. 1952. The remarkable effect of a combination of iodochloroxyquinoline with a subactive dose of streptomycin on experimental tuberculosis in guinea pigs. Ann. Inst. Pasteur (Paris) 83:275-276. [PubMed] [Google Scholar]
- 19.Yajko, D. M., J. J. Madej, M. V. Lancaster, C. A. Sanders, V. L. Cawthon, B. Gee, A. Babst, and W. K. Hadley. 1995. Colorimetric method for determining MICs of antimicrobial agents for Mycobacterium tuberculosis. J. Clin. Microbiol. 33:2324-2327. [DOI] [PMC free article] [PubMed] [Google Scholar]