Abstract
Susceptibility (18 antimicrobial agents including high-dose tobramycin) and checkerboard synergy (23 combinations) studies were performed for 2,621 strains of Burkholderia cepacia complex isolated from 1,257 cystic fibrosis patients. Minocycline, meropenem, and ceftazidime were the most active, inhibiting 38%, 26%, and 23% of strains, respectively. Synergy was rarely noted (range, 1% to 15% of strains per antibiotic combination).
Since the late 1970s, Burkholderia cepacia complex bacteria have been recognized as particularly virulent pathogens in cystic fibrosis (CF) (24, 40). Infection with B. cepacia complex can be associated with a rapid decline in lung function and markedly shorter median survival (9, 26, 31, 40). Currently, B. cepacia complex bacteria are isolated from 3% to 4% of CF patients in the United States (10-17). B. cepacia complex bacteria are multidrug resistant due to innate and acquired mechanisms of resistance (1, 5, 6, 23, 30), and the susceptibility profiles of strains from CF patients may differ from those noted in strains from non-CF patients, presumably since CF patients receive multiple courses of oral, intravenous, and aerosolized antibiotics (20, 22, 29, 35, 41). However, there have been few recent surveys of the antimicrobial susceptibility patterns of these microorganisms in CF patients, particularly in an era when selective media for Burkholderia are widely used in clinical microbiology laboratories (1, 5, 33).
We report the in vitro activity of antimicrobial agents against B. cepacia complex strains from patients with CF and the potential synergistic activity of these agents in combination as studied at the CF Referral Center for Susceptibility and Synergy Studies (http://synergy.columbia.edu/) at Columbia University (36, 37, 39). The Institutional Review Board of Columbia University approved this study.
From 1996 to 2004, the CF Referral Center received an average of 291 isolates (range, 119 to 408) of B. cepacia complex per year. Overall, 2,621 B. cepacia complex strains from 1,257 patients (range, 1 to 17 strains per patient) from 150 CF care centers located in 46 states were processed. To assess the generalizability of this study's findings, the numbers of patients harboring B. cepacia complex that were reported to the U.S. CF Foundation Patient Registry annually (10-18) were compared to the numbers of patients whose B. cepacia complex isolates were processed at the CF Referral Center. We estimated that each year, isolates from 34% (range, 21% to 42% per year) of CF patients with B. cepacia complex were processed.
Strains referred as Burkholderia spp. were plated within 24 h of receipt on Biplate media (Remel, Lenexa, KS) for purity and oxidative-fermentative-polymyxin B-bacitracin-lactose media (OFPBL; Remel, Lenexa, KS) to verify species phenotype (38). Plates were incubated at 35°C for 18 to 24 h, and if needed, slowly growing strains were reincubated for an additional 18 to 24 h.
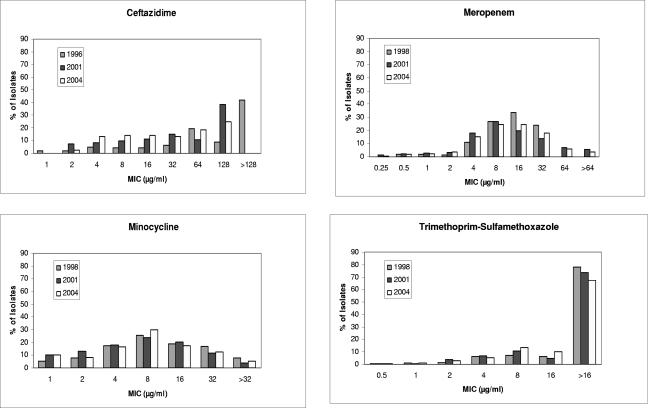
Throughout the study period, susceptibility to 18 antimicrobial agents including high-dose tobramycin was determined by broth microdilution assay using frozen commercially prepared microtiter plates (Microtech Medical Systems, Inc., Aurora, CO). This assay has been endorsed by the Clinical and Laboratory Standards Institute (CLSI) to determine the antimicrobial susceptibility of Pseudomonas aeruginosa isolated from patients with CF (32). Susceptibility panels were incubated at 35°C for 18 h, and slowly growing strains were incubated for an additional 18 to 24 h. Susceptibility and synergy plates were placed on a mirrored surface, and turbidity was visualized using the microtiter test reading mirror (DYNEX Technologies, Chantilly, VA). CLSI interpretive criteria for susceptibility breakpoints for non-Enterobacteriaceae were used when breakpoints for Burkholderia spp. were unavailable (7, 8). Minocycline, meropenem, and ceftazidime were the most active and inhibited 38%, 26%, and 23% of strains, respectively (Table 1). High concentrations of tobramycin (256 μg/ml), such as could be achieved by aerosolization, inhibited 45% of strains. Overall, 18% (473/2,621) of strains were resistant to all the agents tested. No changes in susceptibility trends for the commonly used agents ceftazidime, minocycline, meropenem, or trimethoprim-sulfamethoxazole were noted during the study period (Fig. 1).
TABLE 1.
Activity of selected antimicrobial agents against Burkholderia cepacia complex isolated from patients with cystic fibrosis, 1996 to 2004
| Antimicrobial agent | No. of strains tested | MIC (μg/ml)
|
Strains tested (%)
|
||||
|---|---|---|---|---|---|---|---|
| Range tested | 50% | 90% | Susceptiblea | Intermediate | Resistant | ||
| Ceftazidimeb | 2,621 | 1->128 | 32 | 128 | 23 | 12 | 65 |
| Chloramphenicolc | 2,405 | 2->64 | 64 | >64 | 5 | 10 | 85 |
| Doxycyclined | 1,903 | 1->32 | 16 | >32 | 22 | 17 | 61 |
| Meropenemc | 2,405 | 0.25->64 | 8 | 32 | 26 | 26 | 48 |
| Minocyclinec | 2,405 | 1->32 | 8 | 32 | 38 | 25 | 37 |
| Piperacillinb | 2,621 | 4->256 | 256 | >256 | 11 | 9 | 80 |
| Piperacillin-tazobactamc | 2,405 | 4->256 | 256 | >256 | 16 | 9 | 75 |
| Trimethoprim-sulfamethoxazolec | 2,405 | 0.5->16 | >16 | >16 | 5 | N/Ae | 95 |
Fewer than 10% of strains were inhibited by cefepime (8%), imipenem (7%), ciprofloxacin (5%), amikacin (4%), ticarcillin-clavulanic acid (3%), ampicillin-sulbactam (2%), aztreonam (2%), ticarcillin (2%), or rifampin (1%).
Used from January 1996 to December 2004.
Used from August 1997 to December 2004.
Used from October 1999 to December 2004.
N/A, not applicable.
FIG. 1.
The distributions of MICs for Burkholderia cepacia complex bacteria isolated from patients with CF in 1996 (n = 187), 1998 (n = 239), 2001 (n = 382), and 2004 (n = 302) are shown for the selected agents ceftazidime, meropenem, minocycline, and trimethoprim-sulfamethoxazole. The highest concentrations of antibiotics tested varied during the study period for some agents. For ceftazidime, the highest concentration tested in 1996 was 128 μg/ml and in 2001 and 2004 the highest concentration tested was 64 μg/ml. Strains not inhibited by 128 μg/ml are depicted as an MIC of >128 μg/ml. For meropenem, the highest concentration tested in 1998 was 16 μg/ml and in 2001 and 2004 the highest concentration tested was 64 μg/ml. Strains that were not inhibited by 64 μg/ml are depicted as an MIC of >64 μg/ml.
Checkerboard synergy testing of 23 pairs of antimicrobial agents was performed on frozen commercially prepared microtiter plates (Microtech Medical Systems, Inc., Aurora, CO) using the same inoculum as the susceptibility studies (Table 2). The fractional inhibitory concentration (FIC) was calculated as previously described (37). FIC of ≤0.5 was considered synergistic, FIC of >0.5 to ≤4.0 was considered nonsynergistic, and FIC of >4.0 was considered antagonistic (19, 25, 34). Clinically achievable concentrations were generally used in the synergy panels. As a result, the range of concentrations tested in these panels was lower than the range of concentrations tested in the susceptibility panels. Thus, the precise FIC was ‘indeterminate’ for a given two agents if growth occurred in all the combination wells. Synergistic activity was rare (range, 1% to 15% of isolates per antibiotic combination) as was antagonistic activity (range, 0% to 9% of isolates per antibiotic combination). In contrast, nonsynergistic or indeterminate activity was observed among 37% (range, 8% to 88%) and 55% (range, 2% to 87%) of isolates per combination, respectively.
TABLE 2.
Synergistic activity of pairs of antimicrobial agents against Burkholderia cepacia complex isolated from patients with cystic fibrosis, 1996 to 2004
| Antimicrobial pair | No. of strains tested | Ranges tested (μg/ml) | Strains tested (%)
|
|||
|---|---|---|---|---|---|---|
| Synergistic | Nonsynergistic | Antagonistic | Indeterminate | |||
| Aztreonam + tobramycina | 216 | 4-32/1-8 | 6 | 10 | 2 | 82 |
| Cefepime + chloramphenicolb | 1,903 | 2-16/2-16 | 4 | 24 | 0 | 72 |
| Ceftazidime + chloramphenicolc | 2,405 | 2-16/2-16 | 10 | 48 | 1 | 41 |
| Ceftazidime + trimethoprim-sulfamethoxazolec | 2,405 | 2-16/0.25-2 | 6 | 38 | 2 | 54 |
| Ceftazidime + amikacina | 216 | 4-128/4-32 | 15 | 45 | 7 | 33 |
| Ceftazidime + tobramycina | 216 | 4-128/1-8 | 12 | 46 | 9 | 33 |
| Chloramphenicol + ciprofloxacinb | 1,903 | 2-16/0.25-2 | 1 | 17 | 0 | 82 |
| Chloramphenicol + trimethoprim-sulfamethoxazoled | 502 | 4-32/1-32 | 1 | 44 | 1 | 54 |
| Ciprofloxacin + trimethoprim-sulfamethoxazoled | 502 | 0.5-16/1-8 | 1 | 27 | 1 | 71 |
| Doxycycline + chloramphenicolb | 1,903 | 2-16/1-8 | 7 | 45 | 0 | 48 |
| Doxycycline + trimethoprim-sulfamethoxazoleb | 1,903 | 1-8/0.5-4 | 1 | 43 | 0 | 56 |
| Imipenem + ciprofloxacine | 718 | 0.5-16/0.5-4 | 5 | 29 | 1 | 65 |
| Imipenem + tobramycina | 216 | 0.5-16/1-8 | 4 | 17 | 3 | 76 |
| Imipenem + amikacina | 216 | 0.5-16/4-32 | 3 | 16 | 3 | 78 |
| Meropenem + chloramphenicolb | 1,903 | 1-8/2-16 | 4 | 54 | 2 | 40 |
| Meropenem + ciprofloxacinc | 2,405 | 1-8/0.5-2 | 4 | 57 | 2 | 37 |
| Meropenem + doxycyclineb | 1,903 | 1-8/1-8 | 7 | 64 | 2 | 27 |
| Meropenem + tobramycinb | 1,903 | 1-8/1-8 | 3 | 51 | 2 | 44 |
| Meropenem + trimethoprim-sulfamethoxazoleb | 1,903 | 1-8/0.25-2 | 4 | 44 | 2 | 50 |
| Minocycline + chloramphenicold | 502 | 1-32/4-32 | 9 | 88 | 1 | 2 |
| Piperacillin + ciprofloxacinf | 2,621 | 8-64/0.25-2 | 10 | 27 | 1 | 62 |
| Piperacillin + tobramycina | 216 | 8-64/1-8 | 9 | 19 | 0 | 72 |
| Ticarcillin-clavulanate + tobramycina | 216 | 8-64/1-8 | 5 | 8 | 0 | 87 |
Used from January 1996 to August 1997.
Used from October 1999 to December 2004.
Used from August 1997 to December 2004.
Used from August 1997 to October 1999.
Used from January 1996 to October 1999.
Used from January 1996 to December 2004.
This is the largest survey of antibiotic susceptibility of B. cepacia complex published to date. Although the CF Referral Center solicits multidrug-resistant organisms, these results may be broadly generalizable to the CF patient population in the United States, as approximately one-third of CF patients harboring Burkholderia spp. were studied.
Treatment options for B. cepacia complex remain limited. The CLSI recommends testing susceptibility to ticarcillin plus clavulanic acid, ceftazidime, meropenem, minocycline, levofloxacin, chloramphenicol, and trimethoprim-sulfamethoxazole (8). Similarly, treatment recommendations for B. cepacia complex include meropenem, ciprofloxacin, minocycline, trimethoprim-sulfamethoxazole, and chloramphenicol (21, 27). However, in this study of isolates from CF patients, only 3% to 38% of strains were susceptible to these agents. Somewhat surprisingly, the relative proportion of strains resistant to the commonly used agents ceftazidime, meropenem, minocycline, and trimethoprim-sulfamethoxazole did not change during the 9 years of the study. This observation may reflect the solicitation of multidrug-resistant strains by the CF Referral Center coupled with the extensive use of these agents in the CF population throughout the study period.
Aaron et al. have published in vitro synergy studies for B. cepacia complex isolated from CF patients using the multiple combination bactericidal test (MCBT) (1). In contrast to the synergy methods described in our study, the MCBT methodology tests the activity of peak serum concentrations of antimicrobial agents. In the MCBT assays, meropenem combined with minocycline, amikacin, or ceftazidime was bactericidal against 76%, 73%, and 73% of isolates, respectively. In addition, triple antibiotic combinations such as tobramycin, meropenem, plus another agent were bactericidal against 81% to 93% of isolates. Unlike the MCBT method, the checkerboard assay used in our study demonstrated few synergistic combinations. These observations may reflect differences in the methods used, including the concentrations of antibiotics tested and/or the use of inhibitory versus bactericidal activity. Differences in the isolates studied, the patient populations, or agents tested may also have contributed to the differing results.
Although two agents are generally recommended for treatment of a CF pulmonary exacerbation (20), there are relatively few published reports describing treatment of B. cepacia complex in CF patients. Blumer et al. studied the safety and efficacy of meropenem and tobramycin in 14 subjects with B. cepacia complex (4). A reduction in bacterial density (−1.8 log10, P = 0.02) was noted, but improvement in lung function was not reported in this subgroup. Aaron et al. explored the efficacy of treatment guided by MCBT versus treatment guided by conventional susceptibility testing in 132 patients including 54 infected with B. cepacia complex (2). While subjects in both groups clinically improved, no differences in response to treatment, defined as the time to the next pulmonary exacerbation, or improvement in lung function were noted.
There were some limitations to our study. We studied multidrug-resistant organisms, which could overestimate the relative proportion of resistant B. cepacia complex strains in CF patients. We do not know the genomovars of the isolates, although to date, there have been limited studies comparing the antimicrobial susceptibilities of different genomovars of Burkholderia (3, 33, 42). It is possible that some strains were misidentified as B. cepacia complex (28). Many of the synergy results were indeterminate; this may reflect the limitations of the panel design as these multidrug-resistant strains were not inhibited by the clinically achievable concentrations tested in the combination wells. Finally, the clinical relevance of synergy studies utilizing the FIC methodology described in this report has not been assessed in patients with CF.
In conclusion, the management of multidrug-resistant B. cepacia complex remains challenging in the CF population. Agents used in non-CF patients have limited activity against CF strains, and it is likely that checkerboard synergy studies have limited utility against B. cepacia complex strains. Newer treatment options are needed for B. cepacia complex.
Acknowledgments
This study was funded by the United States Cystic Fibrosis Foundation.
Footnotes
Published ahead of print on 11 December 2006.
REFERENCES
- 1.Aaron, S. D., W. Ferris, D. A. Henry, D. P. Speert, and N. E. MacDonald. 2000. Multiple combination bactericidal antibiotic testing for patients with cystic fibrosis infected with Burkholderia cepacia. Am. J. Respir. Crit. Care Med. 161:1206-1212. [DOI] [PubMed] [Google Scholar]
- 2.Aaron, S. D., K. L. Vandemheen, W. Ferris, D. Fergusson, E. Tullis, D. Haase, Y. Berthiaume, N. Brown, P. Wilcox, V. Yozghatlian, P. Bye, S. Bell, F. Chan, B. Rose, A. Jeanneret, A. Stephenson, M. Noseworthy, A. Freitag, N. Paterson, S. Doucette, C. Harbour, M. Ruel, and N. MacDonald. 2005. Combination antibiotic susceptibility testing to treat exacerbations of cystic fibrosis associated with multiresistant bacteria: a randomised, double-blind, controlled clinical trial. Lancet 366:463-471. [DOI] [PubMed] [Google Scholar]
- 3.Bevivino, A., C. Dalmastri, S. Tabacchioni, L. Chiarini, M. L. Belli, S. Piana, A. Materazzo, P. Vandamme, and G. Manno. 2002. Burkholderia cepacia complex bacteria from clinical and environmental sources in Italy: genomovar status and distribution of traits related to virulence and transmissibility. J. Clin. Microbiol. 40:846-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blumer, J. L., L. Saiman, M. W. Konstan, and D. Melnick. 2005. The efficacy and safety of meropenem and tobramycin vs ceftazidime and tobramycin in the treatment of acute pulmonary exacerbations in patients with cystic fibrosis. Chest 128:2336-2346. [DOI] [PubMed] [Google Scholar]
- 5.Burns, J. L., and L. Saiman. 1999. Burkholderia cepacia infections in cystic fibrosis. Pediatr. Infect. Dis. J. 18:155-156. [DOI] [PubMed] [Google Scholar]
- 6.Burns, J. L., C. D. Wadsworth, J. J. Barry, and C. P. Goodall. 1996. Nucleotide sequence analysis of a gene from Burkholderia (Pseudomonas) cepacia encoding an outer membrane lipoprotein involved in multiple antibiotic resistance. Antimicrob Agents Chemother. 40:307-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standards Institute. 2005. Performance standards for antimicrobial susceptibility testing; 15th informational supplement, vol. 25. Clinical and Laboratory Standards Institute, Wayne, PA.
- 8.Clinical and Laboratory Standards Institute. 2006. Performance standards for antimicrobial susceptibility testing; 16th informational supplement, vol. 26. Clinical and Laboratory Standards Institute, Wayne, PA.
- 9.Corey, M., and V. Farewell. 1996. Determinants of mortality from cystic fibrosis in Canada, 1970-1989. Am. J. Epidemiol. 143:1007-1017. [DOI] [PubMed] [Google Scholar]
- 10.Cystic Fibrosis Foundation. 1997. Patient registry 1996 annual report. Cystic Fibrosis Foundation, Bethesda, MD.
- 11.Cystic Fibrosis Foundation. 1998. Patient registry 1997 annual report. Cystic Fibrosis Foundation, Bethesda, MD.
- 12.Cystic Fibrosis Foundation. 1999. Patient registry 1998 annual report. Cystic Fibrosis Foundation, Bethesda, MD.
- 13.Cystic Fibrosis Foundation. 2000. Patient registry 1999 annual report. Cystic Fibrosis Foundation, Bethesda, MD.
- 14.Cystic Fibrosis Foundation. 2001. Patient registry 2000 annual report. Cystic Fibrosis Foundation, Bethesda, MD.
- 15.Cystic Fibrosis Foundation. 2002. Patient registry 2001 annual report. Cystic Fibrosis Foundation, Bethesda, MD.
- 16.Cystic Fibrosis Foundation. 2003. Patient registry 2002 annual report. Cystic Fibrosis Foundation, Bethesda, MD.
- 17.Cystic Fibrosis Foundation. 2004. Patient registry 2003 annual report. Cystic Fibrosis Foundation, Bethesda, MD.
- 18.Cystic Fibrosis Foundation. 2005. Patient registry 2004 annual report. Cystic Fibrosis Foundation, Bethesda, MD.
- 19.Eliopoulos, G. M., and R. C. Moellering. 1991. Antibiotic combinations, p. 432-492. In V. Lorian (ed.), Antibiotics in laboratory medicine, 3rd ed. Williams & Wilkins Co., Baltimore, MD.
- 20.Gibson, R. L., J. L. Burns, and B. W. Ramsey. 2003. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am. J. Respir. Crit. Care Med. 168:918-951. [DOI] [PubMed] [Google Scholar]
- 21.Gilbert, D., R. Moellering, G. Eliopoulos, and M. Sande (ed.). 2005. The Sanford Guide to Antimicrobial Therapy, 35th ed. Antimicrobial Therapy, Inc., Hyde Park, VT.
- 22.Govan, J. R., and V. Deretic. 1996. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol. Rev. 60:539-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hancock, R. E. 1998. Resistance mechanisms in Pseudomonas aeruginosa and other nonfermentative gram-negative bacteria. Clin. Infect. Dis. 27(Suppl. 1):S93-S99. [DOI] [PubMed] [Google Scholar]
- 24.Isles, A., I. Maclusky, M. Corey, R. Gold, C. Prober, P. Fleming, and H. Levison. 1984. Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J. Pediatr. 104:206-210. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs, D. S., W. R. DeMott, and D. K. Oxley. 2001. Jacobs & DeMott laboratory test handbook: with key word index, 5th ed. Lexi Comp, Hudson, OH.
- 26.Liou, T. G., F. R. Adler, S. C. Fitzsimmons, B. C. Cahill, J. R. Hibbs, and B. C. Marshall. 2001. Predictive 5-year survivorship model of cystic fibrosis. Am. J. Epidemiol. 153:345-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mandell, G. L., J. E. Bennett, and R. Dolin. 2005. Principles and practice of infectious diseases, 6th ed. Elsevier Inc., Philadelphia, PA.
- 28.McMenamin, J. D., T. M. Zaccone, T. Coenye, P. Vandamme, and J. J. LiPuma. 2000. Misidentification of Burkholderia cepacia in US cystic fibrosis treatment centers: an analysis of 1,051 recent sputum isolates. Chest 117:1661-1665. [DOI] [PubMed] [Google Scholar]
- 29.Moss, R. B. 1995. Cystic fibrosis: pathogenesis, pulmonary infection, and treatment. Clin. Infect. Dis. 21:839-849. [DOI] [PubMed] [Google Scholar]
- 30.Nair, B. M., K. J. Cheung, Jr., A. Griffith, and J. L. Burns. 2004. Salicylate induces an antibiotic efflux pump in Burkholderia cepacia complex genomovar III (B. cenocepacia). J. Clin. Investig. 113:464-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Navarro, J., M. Rainisio, H. K. Harms, M. E. Hodson, C. Koch, G. Mastella, B. Strandvik, and S. G. McKenzie. 2001. Factors associated with poor pulmonary function: cross-sectional analysis of data from the ERCF. European Epidemiologic Registry of Cystic Fibrosis. Eur. Respir. J. 18:298-305. [DOI] [PubMed] [Google Scholar]
- 32.NCCLS. 2001. Performance standards for antimicrobial susceptibility testing; 11th informational supplement, vol. 21, M100-S11. NCCLS, Wayne, PA.
- 33.Nzula, S., P. Vandamme, and J. R. Govan. 2002. Influence of taxonomic status on the in vitro antimicrobial susceptibility of the Burkholderia cepacia complex. J. Antimicrob Chemother. 50:265-269. [DOI] [PubMed] [Google Scholar]
- 34.Odds, F. C. 2003. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 52:1. [DOI] [PubMed] [Google Scholar]
- 35.Ramsey, B. W. 1996. Management of pulmonary disease in patients with cystic fibrosis. N. Engl. J. Med. 335:179-188. [DOI] [PubMed] [Google Scholar]
- 36.Saiman, L., Y. Chen, S. Tabibi, P. San Gabriel, J. Zhou, Z. Liu, L. Lai, and S. Whittier. 2001. Identification and antimicrobial susceptibility of Alcaligenes xylosoxidans isolated from patients with cystic fibrosis. J. Clin. Microbiol. 39:3942-3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saiman, L., F. Mehar, W. W. Niu, H. C. Neu, K. J. Shaw, G. Miller, and A. Prince. 1996. Antibiotic susceptibility of multiply resistant Pseudomonas aeruginosa isolated from patients with cystic fibrosis, including candidates for transplantation. Clin. Infect. Dis. 23:532-537. [DOI] [PubMed] [Google Scholar]
- 38.Saiman, L., and J. Siegel. 2003. Infection control recommendations for patients with cystic fibrosis: microbiology, important pathogens, and infection control practices to prevent patient-to-patient transmission. Infect. Control Hosp. Epidemiol. 24:S6-S52. [DOI] [PubMed] [Google Scholar]
- 39.San Gabriel, P., J. Zhou, S. Tabibi, Y. Chen, M. Trauzzi, and L. Saiman. 2004. Antimicrobial susceptibility and synergy studies of Stenotrophomonas maltophilia isolates from patients with cystic fibrosis. Antimicrob. Agents Chemother. 48:168-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tablan, O. C., W. J. Martone, C. F. Doershuk, R. C. Stern, M. J. Thomassen, J. D. Klinger, J. W. White, L. A. Carson, and W. R. Jarvis. 1987. Colonization of the respiratory tract with Pseudomonas cepacia in cystic fibrosis. Risk factors and outcomes. Chest 91:527-532. [DOI] [PubMed] [Google Scholar]
- 41.Taylor, R. F., H. Gaya, and M. E. Hodson. 1993. Pseudomonas cepacia: pulmonary infection in patients with cystic fibrosis. Respir. Med. 87:187-192. [DOI] [PubMed] [Google Scholar]
- 42.Vermis, K., P. A. Vandamme, and H. J. Nelis. 2003. Burkholderia cepacia complex genomovars: utilization of carbon sources, susceptibility to antimicrobial agents and growth on selective media. J. Appl. Microbiol. 95:1191-1199. [DOI] [PubMed] [Google Scholar]