Abstract
Drug resistance by overexpression of ATP-binding cassette (ABC) transporters is an impediment in the treatment of leishmaniasis. Flavonoids are known to reverse multidrug resistance (MDR) in Leishmania and mammalian cancers by inhibiting ABC transporters. Here, we found that synthetic flavonoid dimers with three (compound 9c) or four (compound 9d) ethylene glycol units exhibited a significantly higher reversing activity than other shorter or longer ethylene glycol-ligated dimers, with ∼3-fold sensitization of pentamidine and sodium stibogluconate (SSG) resistance in Leishmania, respectively. This modulatory effect was dosage dependent and not observed in apigenin monomers with the linker, suggesting that the modulatory effect is due to its bivalent nature. The mechanism of reversal activity was due to increased intracellular accumulation of pentamidine and total antimony in Leishmania. Compared to other MDR modulators such as verapamil, reserpine, quinine, quinacrine, and quinidine, compounds 9c and 9d were the only agents that can reverse SSG resistance. In terms of reversing pentamidine resistance, 9c and 9d have activities comparable to those of reserpine and quinacrine. Modulators 9c and 9d exhibited reversal activity on pentamidine resistance among LeMDR1−/−, LeMDR1+/+, and LeMDR1-overexpressed mutants, suggesting that these modulators are specific to a non-LeMDR1 pentamidine transporter. The LeMDR1 copy number is inversely related to pentamidine resistance, suggesting that it might be involved in importing pentamidine into the mitochondria. In summary, bivalency could be a useful strategy for the development of more potent ABC transporter modulators and flavonoid dimers represent a promising reversal agent for overcoming pentamidine and SSG resistance in parasite Leishmania.
Leishmaniasis, one of the six major parasitic diseases targeted by the World Health Organization (WHO), is endemic in 88 countries around the world. Most leishmaniasis occurs in northern Africa, Asia, Latin America, and the Middle East. There are 350 million people at risk of infection, with 2 million cases annually. About a quarter of these cases are visceral leishmaniasis, which is the lethal form if left untreated (1). The primary treatment of leishmaniasis is by the administration of pentavalent antimonials (Pentostam and Glucantime). Secondary treatment includes pentamidine and amphotericin B. These treatments have many side effects, and their efficacies are further impeded by the emergence of clinical resistance to some of these antileishmanials (5). It has been reported that more than 50% of the visceral leishmaniasis cases in India are resistant to the antimonials (43). The WHO has stated that the resistance to pentavalent antimonials in Leishmania is one of its top priorities (6). Newer treatments such as miltefosine, a hexadecylphosphocholine, has also shown tremendous promise. However, due to the long half-life in blood, treatment with miltefosine can easily lead to drug resistance.
ATP-binding cassette (ABC) transporters are characterized by the presence of the highly conserved ATP-binding domains. ABC transporters were first described in multidrug-resistant (MDR) cancer cells where P-glycoprotein (P-gp), a gene product of MDR1 (ABCB1), functioned as an ATP-dependent drug efflux pump to extrude a variety of hydrophobic drugs from the cancer cells, hence reducing the intracellular drug accumulation (26). Later on, the multidrug resistance-associated protein (MRP1 encoded by ABCC1) was found to be another ABC transporter that can also mediate the efflux of drugs and causes MDR (47). Both P-gp and MRP consist of two homologous halves, each composed of a transmembrane domain (TMD), involved in drug binding and efflux, and a cytosolic nucleotide-binding domain (NBD), with characteristic Walker A and B motifs involved in ATP binding and hydrolysis (45). Hydrolysis of ATP is tightly coupled to drug efflux. Recent evidence has shown that some P-gp (9, 11, 21, 23) and MRP (34, 35) transporters are involved in drug resistance in the protozoan parasite Leishmania (38). Resistance to pentavalent antimonials such as sodium stibogluconate (SSG) in Leishmania tarentolae is due to an MRP member (LtPGPA). LtPGPA may confer resistance to antimonials in promastigote cells by sequestration of the metal-thiol conjugates in an intracellular organelle located close to the flagellar pocket (30). Pentamidine is a second-line antileishmanial whose mode of action and resistance is not well understood. It has been reported that pentamidine resistance may be due to the exclusion of pentamidine from its target, mitochondria (4). Recently, a pentamidine resistance gene (PENr), encoding a protein termed pentamidine resistance protein 1 (PRP1), has been described (12). It is also an ABC transporter and exhibited a high similarity to members of the MRP-like family (ca. 30 to 40%) (12). Resistance to miltefosine has also been suggested to be due to increased drug efflux mediated by L. tropica MDR1 (37).
Flavonoids constitute a group of interesting polyphenolic compounds with a wide distribution in fruits and vegetables (27, 28) and have been shown to exert a wide range of beneficial effects on human health, including protection against cardiovascular diseases and different forms of cancers (18). In the past decade, some flavonoids have been implicated in the modulation of P-gp-type MDR in cancers and shown to inhibit a variety of ATP-binding proteins such as plasma membrane ATPase (24, 44), cyclic AMP-dependent protein kinase (25), and protein kinase C (17). It is thought that the modulating activity of the flavonoids arises from competitive binding to the NBDs of P-gp through their ability to mimic the adenine moiety of ATP. On the other hand, it has been suggested that some alkyl substituted flavonoids with increased hydrophobic interactions may inhibit MDR through binding with both the steroid-interacting region and the drug binding site of TMDs in P-gp. In addition, flavonoids have also been demonstrated to inhibit daunomycin efflux and resensitize L. tropica to daunomycin by binding to the NBD of the P-gp-like transporter (36). Therefore, flavonoids that are consumed daily and without any detrimental side effects are attractive targets for development of novel modulators of MDR to treat both protozoan parasite Leishmania and cancers.
Recently, an attempt to modulate the activity of P-gp through the use of polyvalent interaction has been reported (42). Functional derivatives of stipiamide were linked via ethylene glycol chains of various lengths. It was found that polyvalency could be a useful strategy to develop more potent P-gp modulators. Using a similar strategy, we recently reported the synthesis of a series of novel bivalent flavonoid dimers based on apigenin linked by various number of ethylene glycol units (Fig. 1) (8). Apigenin was used because it is a moderate modulator of MDR in breast cancer cells (48) and has displayed a moderate affinity for the NBD2 (14). We hypothesized that a dimer will cooperatively increase the efficacy of apigenin in binding to NBD, thereby inactivating P-gp. However, without the crystal structure of the P-gp, the distance between the two NBD is unknown, even though a model has been constructed with the two NBD at a distance of about 600 nm apart (31). On the other hand, it is known that the two NBD sites move closer upon binding with ligands (41). We therefore synthesized a whole series of flavonoid dimers with various linker lengths for screening purpose. These synthetic flavonoid dimers showed a linker length-dependent inhibition of the P-gp activity in a MDR breast cancer cell line and in a resistant leukemia cell line (8). We found that compound 9d was the most potent in reversing paclitaxel resistance in a breast cancer cell line (LCC6MDR) (8).
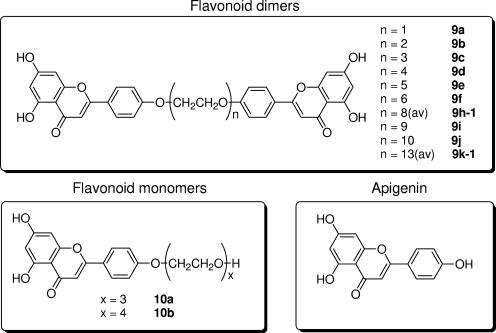
FIG. 1.
Structures of flavonoid dimers, flavonoid monomers with ethylene glycol linker and apigenin. The “n” is the number of ethylene glycol units present in the linker of the flavonoid dimers (9a to 9k-1). The “av” is the average number of ethylene glycol units used during flavonoid dimer synthesis. The “x” in 10a and 10b is the number of ethylene glycol units added to the flavonoid monomers (10a and 10b). 10a and 10b were used as controls. The synthesis of these compounds have been reported previously (8).
In view of the association between P-gp expression and SSG and pentamidine resistance in Leishmania reported by others, we hypothesize that our synthetic apigenin dimers will also have similar modulating effect on the SSG and pentamidine resistance in Leishmania. In this report, we will demonstrate that the flavonoid dimers also have a length-dependent MDR-modulating activity in three Leishmania cells that are resistant to pentamidine and SSG.
MATERIALS AND METHODS
Cell lines and cell culture.
Promastigotes of Leishmania enriettii (LePentR50, Le wild type, LeMDR1−/−, and LeMDR1-overexpressed LeV160 mutants) and Leishmania donovani (LdAG83, Ld2001, and Ld39) were used in the present study. The former is a natural infective strain of guinea pig, and the latter is a clinical strain, which may cause visceral leishmaniasis in humans. Both strains were cultured in Schneider's Drosophila medium (pH 6.9; Invitrogen) supplemented with 10% (vol/vol) heat-inactivated fetal calf serum (HyClone) with 4 mM glutamine (Sigma) and 25-μg/ml gentamicin solution (Invitrogen) at 27°C for 4 days (11).
Promastigotes of LePentR50 (pentamidine resistant, 50% inhibitory concentration [IC50] of pentamidine = 117 μg/ml), Ld2001 (SSG resistant, IC50 of SSG = 4.1 mg/ml), and Ld39 (SSG resistant, IC50 of SSG = 6.4 mg/ml) were cultured in the presence of 50 μg of pentamidine (Sigma)/ml and 3.5 mg of SSG (a gift from Glaxo SmithKline)/ml, respectively. No SSG was added to the L. donovani wild type (LdAG83, IC50 of SSG = 2.4 mg/ml). Promastigotes of LeV160 were cultured in the presence of 160 μg of vinblastine/ml. No pentamidine and vinblastine (Sigma) was added to the Le wild type and the LeMDR1−/− mutant.
Amastigotes of L. donovani were prepared by spinning down 50 ml of 4-day-old promastigotes (late log phase) and transferring them to an axenic medium containing M199 medium (Gibco), 0.5% Trypto casein soya, 3 mM l-cysteine, 15 mM d-glucose, 5 mM l-glutamine, 4 mM NaHCO3, 25 mM HEPES, 0.01 mM bathocuproine-disulfonic acid, and 0.023 mM hemin. Cells were then incubated at 37°C for 24 h. Amastigotes became ovoid in shape and were ready for drug accumulation assay.
Cell viability assay.
The viability of promastigotes was determined by the Cell Titer 96 aqueous assay (Promega), which uses a novel tetrazolium compound (MTS) and electron coupling reagent, phenazine methosulfate (PMS). Promastigotes were seeded into 96-well flat bottom microtiter plate at 105 cells per well in a final volume of 100 μl of medium. For determining the cytotoxic effect of flavonoid dimers to the parasites, various concentrations of flavonoid dimers were added to the promastigotes. For determining the reversal effect of flavonoid dimers with different spacer lengths, various concentrations of antileishmanial drugs, either pentamidine or SSG, vinblastine, and puromycin was added to the wells with or without flavonoid dimers. The parasites were incubated at 27°C for 72 h. Each concentration of antileishmanials with or without synthetic modulators was tested in triplicates in each experiment. We mixed 2 mg of MTS/ml and 0.92 mg of PMS/ml in an MTS/PMS ratio of 20:1. After 72 h of incubation, 10 μl of MTS-PMS mixture was added into each well of microtiter plate. The plate was then incubated at 27°C for 4 h for color development. After 4 h of incubation, the optical density values were determined at 490 nm by using an automatic microtiter plate reader (Bio-Rad). The results were presented as a percentage of the survivors (the optical density value of each well with a test compound is divided by the value for an untreated control well).
Pentamidine accumulation assay by HPLC.
The effect of flavonoid dimers on accumulation of pentamidine was investigated. Portions (1 ml) of 4-day-old promastigotes (late log phase with a cell density of about 2 × 108 cells/ml) were incubated with 0.84 mM pentamidine and various concentrations of flavonoid dimer (9d), including 0, 15, 30, and 60 μM, at 27°C for 3 h in the dark. Each concentration of 9d was tested in triplicates, and this was repeated twice in separate experiments. After 3 h of incubation, the parasites were washed thrice with cold phosphate-buffered saline (PBS; pH 7.4). The cell pellet was then dissolved in 350 μl of 75% acetonitrile and lysed by repeated freeze-thaw cycles. After lysing, the lysed cell suspension was centrifuged at 14,000 × g at 4°C for 10 min. The supernatant was collected and ready for determining the pentamidine concentration by using high-pressure liquid chromatography (HPLC; Agilent 1100 Series) (4). The pentamidine pools were analyzed on Zorbax ODS C18 column (4.6 mm by 25 cm, 5 μm) kept at 40°C. The mobile phase consisted of water (10 mM tetramethylammonium chloride [TMAC], 10 mM sodium heptanesulfonate [SHS], 4.2 mM phosphoric acid [PA]) for pump A and 75% acetonitrile (ACN) in water (10 mM TMAC, 10 mM SHS, 4.2 mM PA) for pump B. The column was equilibrated at 40°C overnight before analyses. Using a flow rate of 1.0 ml/min and signal at 265 nm, analyses were made at 58% pump A and 42% pump B. The retention time of pentamidine is 3.2 min. Compound 9d would not be eluted out under these conditions. To generate a standard curve, a 200 μM stock solution of pentamidine isethionate salt were prepared by dissolving 2.5 mg of pentamidine isethionate salt in 21 ml of 75% ACN (10 mM TMAC, 10 mM SHS, 4.2 mM PA). Concentrations of 100, 50, 25, and 13 μM were then made by serial dilution, allowing the generation of standard curve.
Total antimony [Sb(III) and Sb(V)] accumulation assay using ICP-MS.
The effect of flavonoid dimers on accumulation of antimony SSG was investigated. Amastigotes are more susceptible to SSG and therefore accumulate more SSG compared to promastigotes. We therefore chose to use amastigotes to study the Sb accumulation assay. A 1-ml portion of 4-day-old amastigotes (2 × 108 cells/ml) was incubated with 0.05 mM SSG and various concentrations of flavonoid dimer (9d), including 0, 30, and 60 μM, at 37°C for 3 h. Each concentration of 9d was tested in triplicates, and this was repeated twice in separate experiments. After 3 h of incubation, the parasites were washed three times with cold PBS (pH 7.4). The cell pellet was dissolved in 200 μl of concentrated nitric acid for 24 h at room temperature. The sample was diluted to 3 ml with distilled water, resulting in a final concentration of about 5 ppb of total Sb solution. It was then injected to inductively coupled plasma mass spectrometry (ICP-MS; Perkin-Elmer) for quantitation. Antimony was measured at its m/z ratios of 121 and 123 with indium (In, m/z = 115) as an internal standard. All chemicals used for the pretreatment of the samples were of at least analytical grade, and the distilled water was used directly as received without further purification (6).
RESULTS
Pentamidine-resistant L. enriettii (LePentR50) and SSG-resistant L. donovani (Ld39 and Ld2001).
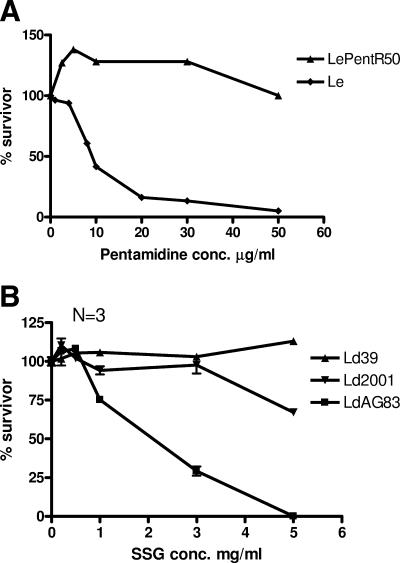
We used here three drug-resistant Leishmania cell lines, namely, LePentR50(pentamidine-resistant L. enriettii), Ld39, and Ld2001 (SSG-resistant L. donovani) to study the drug resistance-modulating activity of the synthetic flavonoid dimers. LePentR50 is a pentamidine-resistant L. enriettii cell line obtained by stepwise selection in our laboratory (unpublished data). It is maintained in the presence of 50 μg of pentamidine/ml and has an IC50 of about 117 μg/ml, whereas the wild-type L. enriettii (Le) has an IC50 of about 8.7 μg/ml (Fig. 2A). Ld39 and Ld2001 are two L. donovani cell lines that are resistant to the pentavalent antimonial SSG (2). Ld39 and Ld2001 are maintained in the presence of 3.5 mg SSG/ml and have IC50s of 6.1 and 4.1 mg/ml, respectively, whereas the wild-type L. donovani (LdAG83) has an IC50 of about 2.4 mg/ml (Fig. 2B).
FIG. 2.
Drug-resistant Leishmania used in the present study: pentamidine-resistant L. enriettii (LePentR50) and SSG-resistant L. donovani (Ld39 and Ld2001). (A) LePentR50 was a pentamidine-resistant promastigote cell line selected from wild-type L. enriettii (Le) by gradually increasing the pentamidine concentration in the culture medium to 50 μg/ml. (B) Ld39 and Ld2001 were L. donovani clinical isolates known to be resistant to SSG. Wild type L. enriettii (Le) and L. donovani (LdAG83) were included for comparison. Percentage survivor was determined by MTS essay.
In vitro cytotoxicity of synthetic flavonoid dimers to Leishmania parasites.
The structure of the synthetic flavonoid dimers is shown in Fig. 1. The synthesis, structural characterization, and numbering scheme of these flavonoid dimers have been reported elsewhere (8). Briefly, these flavonoid dimers are made up of two apigenin monomers linked by a biocompatible ethylene glycol linker with a different number of units (denoted by “n”). Compounds 9a to 9k-1 have n values equal to 1 to 13. We have previously suggested that each apigenin moiety of these flavonoid dimers will bind to P-gp, thereby inhibiting the pump activity (8). Compounds 10a and 10b are apigenin monomers with 3 and 4 units of ethylene glycol only.
The cytotoxicity of synthetic flavonoid dimers in each Leishmania cell line was measured by the MTS-based cell proliferation method. Table 1 summarizes the IC50 value of each synthetic modulator for LePentR50, LdAG83, and L39. Pentamidine-resistant LePentR50 was relatively resistant to synthetic flavonoid dimers (9a to 9f and 10a and 10b), with IC50 values ranging from 40 μM to greater than 200 μM. The sensitivity of L. donovani LdAG83 and Ld39 to synthetic flavonoid dimers was comparable to that of L. enriettii except for compounds 9c and 9d. It was found that both LdAG83 (IC50 of 9c = 8 ± 0.3 μM and IC50 of 9d = 7 ± 0.4 μM) and Ld39 (IC50 of 9c = 11 ± 0.7 μM and IC50 of 9d = 10 ± 0.9 μM) were more susceptible to 9c and 9d than was LePentR50. The species difference between L. enriettii and L. donovani was limited to the apigenin dimers 9c and 9d only. These two species were equally sensitive to apigenin monomer and apigenin with three (10a) or four (10b) ethylene glycol units (Table 1). The hypersensitivity of L. donovani, both LdAG83 and Ld39, to compounds 9c and 9d may mean that these two apigenin dimers may be useful as an anti-L. donovani agent. Indeed, we have previously demonstrated that 5 μM concentrations of 9c and 9d were nontoxic to mammalian cancer cells in vitro (8). In the studies described below, we used 6 μM concentrations of synthetic flavonoid dimers to test their modulating effect on the drug resistance in LePentR50, Ld39, and Ld2001.
TABLE 1.
IC50 of synthetic flavonoids for Leishmania parasites
| Compound | Mean IC50 (μM) ± SDa
|
||
|---|---|---|---|
| LePentR50 | LdAG83 | Ld39 | |
| 9a | >200 | 95 ± 3.2 | 117 ± 10 |
| 9b | >200 | >200 | >200 |
| 9c | >200 | 8 ± 0.3 | 11 ± 0.7 |
| 9d | >200 | 7 ± 0.4 | 10 ± 0.9 |
| 9e | 70 ± 3.0 | 30 ± 1.2 | 42 ± 2.3 |
| 9f | 40 ± 5.3 | 11 ± 2.0 | 13 ± 0.6 |
| 9h-1 | ND | 12 ± 0.2 | 14 ± 0.1 |
| 9i | ND | 10 ± 0.3 | 14 ± 0.1 |
| 9j | ND | >200 | >200 |
| 9k-1 | ND | 50 ± 7 | 60 ± 3 |
| 10a | >200 | >200 | >200 |
| 10b | >200 | >200 | >200 |
| Apigenin | 55 ± 2.6 | 32 ± 4.1 | 43 ± 5.9 |
The IC50 values of each synthetic flavone were determined by an MTS-based proliferation assay. Each IC50 value was derived from at least two independent experiments with triplicates in each experiment. A value of “>200” indicates that an IC50 value could not be determined because these modulators did not have any cytotoxic effect at the highest concentration tested (200 μM). ND, IC50 values were not determined for these modulators but no cytotoxic effect was observed at 12 μM, which was twice the concentration used to study drug resistance modulating activity.
Effect of synthetic flavonoid dimers on modulating pentamidine resistance of LePentR50.
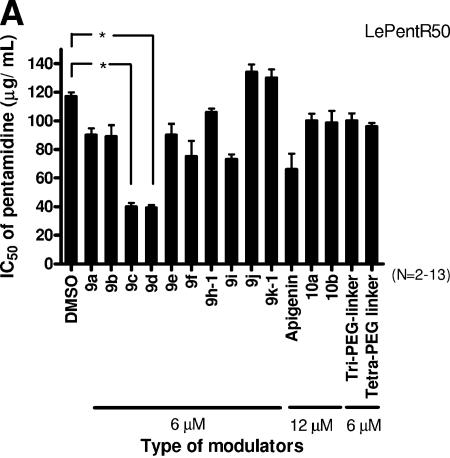
Dimethyl sulfoxide (DMSO)-treated LePentR50 has an IC50 of pentamidine of about 117.0 ± 3.0 μg/ml (Fig. 3A). A 6 μM concentration of compound 9c (n = 3; IC50 = 40.0 ± 2.7 μg/ml; P < 0.01) and of 9d (n = 4; IC50 = 39.2 ± 2.1 μg/ml; P < 0.01) significantly reduced the IC50 of LePentR50 by ∼3-fold (Fig. 3A). Other flavonoid dimers with either shorter linker lengths (9a [IC50 = 90 ± 4.88 μg/ml] and 9b [IC50 = 89.2 ± 8.92 μg/ml]) or longer linker lengths (9e [IC50 = 90 ± 7.88 μg/ml], 9f [IC50 = 75 × 10.99 μg/ml], 9 h-1 [IC50 × 106 ± 2.7 μg/ml], 9i [IC50 = 73 ± 3.54 μg/ml], 9j [IC50 = 134 ± 5.4 μg/ml], and 9k-1 [IC50 = 130 ± 6.1 μg/ml]) gave less than half or no modulating activity (Fig. 3A). The “U”-shaped relationship between the linker length and modulating activity of the flavonoid dimers suggests that the targets of the apigenin moiety are separated by a relatively defined distance. The control compounds of apigenin monomer with three or four ethylene glycol units (10a and 10b) did not give any modulating activity even when used at double the concentration (12 μM) (Fig. 3A; IC50 = 100.0 ± 5.0 μg/ml and 98.5 ± 8.5 μg/ml, respectively). This suggests that the modulating activity of compounds 9c and 9d is indeed due to their dimeric nature. A simple molar increase in the number of apigenin moiety did not result in any significant modulating activity. As a control, the linkers with n = 3 and 4 (Tri-PEG-linker and Tetra-PEG linker) did not have any reversing effect (Fig. 3A).
FIG. 3.
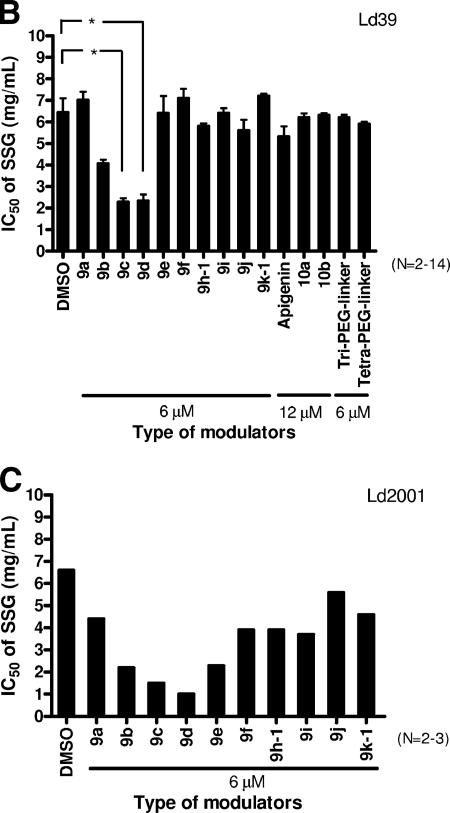
Modulation of drug resistance by synthetic flavonoid dimers with different ethylene glycol lengths. The modulating activity of synthetic flavonoid dimers with different lengths of ethylene glycol units (from one to thirteen units) on the resistance of pentamidine-resistant L. enriettii LePentR50 (A), SSG resistance of SSG-resistant L. donovani Ld39 and Ld2001 (B and C), and wild-type L. donovani LdAG83 (D) was studied. The IC50 (that is, the concentration of pentamidine or SSG that can reduce the survival to 50% of the untreated control) was determined by MTS essay (mean ± standard error mean). Promastigotes were seeded at 105 cells per well and incubated at 27°C for 72 h with various concentrations of pentamidine or SSG in the presence of DMSO solvent control or various modulators. The modulators used included flavonoid dimers (9a to 9k-1), monomers (apigenin and apigenin with three or four ethylene glycol units added), and 10a and 10b (refer to Fig. 1 for structures). Tri-PEG-linker and Tetra-PEG-linker are linkers with three or four ethylene glycol units. The asterisk in panels A and B indicates that the differences between the IC50s of the DMSO control and 9c and 9d are statistically significant (Student t test; P < 0.01).
Effect of synthetic flavonoid dimers on modulating the SSG resistance of Ld39 and Ld2001.
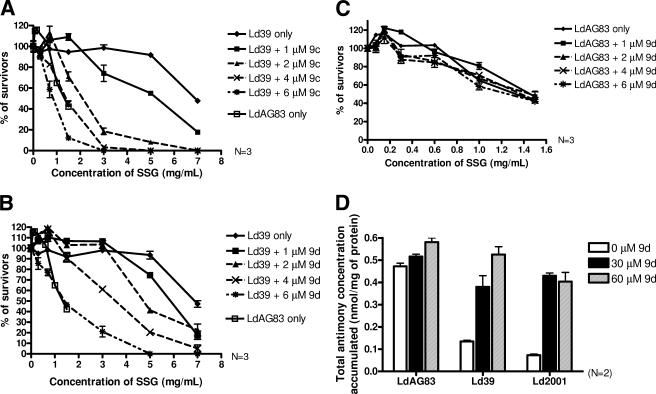
We have also measured the effect of synthetic flavonoid dimers on modulating SSG resistance of Ld39 and Ld2001 promastigotes. Among the synthetic flavonoid dimers (used at 6 μM), 9c and 9d were the most effective in modulating the SSG resistance of L. donovani Ld39 promastigotes. The IC50 of SSG of Ld39 was reduced from 6.4 ± 0.7 mg/ml (DMSO treated) to 2.3 ± 0.2 mg/ml (9c treated) and 2.3 ± 0.3 mg/ml (9d treated) (Fig. 3B). Similar to the pentamidine resistance in LePentR50, compounds with shorter linkers (9a and 9b) or longer linkers 9e to 9k-1) did not show any significant SSG resistance modulating activity (Fig. 3B). Apigenin, 10a, and 10b, even when used at double the concentration (12 μM), also did not show any significant modulating activity (Fig. 3B). The control linkers with n = 3 (Tri-PEG-linker) or n = 4 (Tetra-PEG-linker) did not demonstrate any effect as well (Fig. 3B).
Essentially, a similar pattern was observed when the other SSG-resistant L. donovani strain Ld2001 was studied (Fig. 3C). Compounds 9c and 9d were the most effective and can decrease the IC50 of SSG of Ld2001 from 6.6 mg/ml (DMSO control) to 1.5 mg/ml (9c) and 1.0 mg/ml (9d), respectively (Fig. 3C).
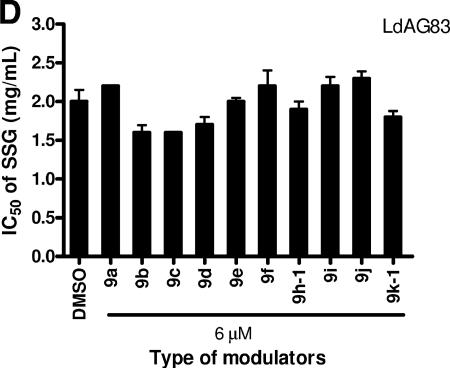
Interestingly, all synthetic flavonoid modulators, including 9c and 9d, had no modulatory effect on SSG-sensitive wild-type L. donovani LdAG83. The IC50 values remained almost the same with or without any modulators (Fig. 3D). This suggests that 9c and 9d specifically target a protein that is uniquely or sufficiently present in SSG-resistant parasite but absent or rarely expressed in SSG-sensitive parasite.
Synthetic flavonoid dimers 9c and 9d show a dose-dependent modulating activity on pentamidine resistance and accumulation in LePentR50.
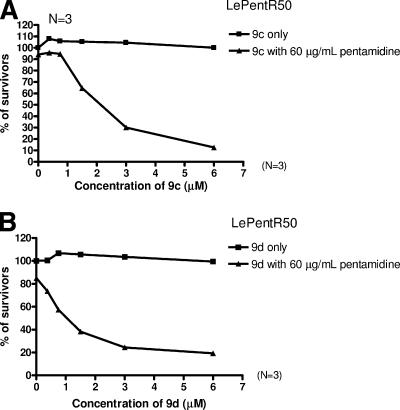
We have studied the dosage effect of the two most effective modulators, namely, 9c (containing three ethylene glycol units) and 9d (containing four ethylene glycol units) on modulating the pentamidine resistance of LePentR50. When treated with only 60 μg of pentamidine/ml, the survival of LePentR50 was only slightly decreased (94.0% ± 2.3% of untreated). Cotreatment of 60 μg of pentamidine/ml with increasing concentrations of 9c, however, resulted in a gradual decrease in the survival of LePentR50, suggesting that 9c can modulate the pentamidine resistance of LePentR50 in a dose-dependent manner (Fig. 4A). The EC50 for 9c (the effective concentration of 9c that results in 50% survival of LePentR50 at 60 μg of pentamidine/ml) is about 1.85 μM. A similar observation was made for compound 9d (Fig. 4B). No toxicity was observed for 9d up to a concentration of 6 μM. The EC50 for 9d is about 0.94 μM. Compound 9d is therefore about twice as effective as 9c in modulating the pentamidine resistance of LePentR50.
FIG. 4.
Dose-dependent modulating activity of flavonoid dimers 9c and 9d on the pentamidine resistance of LePentR50. LePentR50 promastigotes were seeded at 105 cells/well in 100 μl and then incubated at 27°C for 72 h with either flavonoid dimers only (9c only [A] or 9d only [B]) or together with pentamidine (9c with 60 μg of pentamidine/ml [A] or 9d with 60 μg of pentamidine/ml [B]). The percentage of survivors was measured by using an MTS assay. Each concentration of 9c or 9d was tested in triplicate, and analyses were repeated three times.
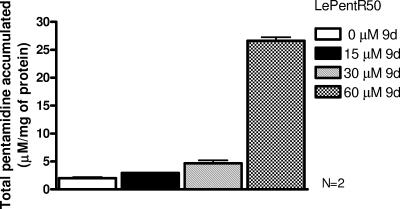
We investigated the effect of 9d on the pentamidine accumulation of LePentR50. Here we have used higher concentrations of 9d (15, 30, and 60 μM), together with a shorter incubation time (3 h), to measure the pentamidine accumulation. Compound 9d can increase the pentamidine accumulation of LePentR50 in a dose-dependent manner (Fig. 5). The intracellular pentamidine concentration of LePentR50 was gradually increased from 2.0 ± 0.2 to 2.95 ± 0.01, 4.69 ± 0.51, and 26.6 ± 0.6 μM pentamidine/mg of protein when the concentration of 9d was increased from 0 to 15, 30, and 60 μM, respectively (Fig. 5). This result suggests that 9d is modulating the pentamidine resistance of LePentR40 by increasing the pentamidine accumulation. Incubation of LePentR50 with 60 μM 9d for 3 h did not result in any cytotoxicity (data not shown). Therefore, the dose-dependent increase in pentamidine accumulation is really due to the modulatory effect of 9d and not to its cytotoxic effect on LePentR50.
FIG. 5.
Effect of 9d on pentamidine accumulation of LePentR50. A 1-ml portion of 4-day-old LePentR50 promastigotes (2 × 108 cells/ml) was incubated at 27°C for 3 h with 0.84 mM pentamidine in the presence of various concentrations of 9d (0, 15, 30, and 60 μM). After incubation, cells were washed with PBS, and the pentamidine concentration was determined by HPLC. Each concentration of 9d was tested in triplicate, and analyses were repeated twice.
Synthetic flavonoid dimers 9c and 9d show a dose-dependent modulating activity on SSG resistance and accumulation in Ld39 cells.
Similar to LePentR50, both 9c and 9d showed a dose-dependent modulating effect on the SSG resistance of Ld39 promastigotes (Fig. 6A and B). A 4 μM concentration of 9c or 9d can reduce the SSG resistance level of Ld39 back to the level of the sensitive strain of LdAG83 (Fig. 6A and B). The modulating effect of 9d was specific to a target protein present only on Ld39 because 9d did not have any modulating effect on the SSG sensitivity of LdAG83 even when used up to 6 μM (Fig. 6C).
FIG. 6.
Dose-dependent modulating activity of 9c and 9d on the SSG resistance of Ld39. SSG-resistant Ld39 promastigotes were seeded at 105 cells/well in 100 μl and incubated at 27°C for 72 h in the presence of serial dilutions of SSG and 9c (A) or 9d (B). The ⧫, ▪, ▴, ×, and ✠ symbols represent 0, 1, 2, 4, and 6 μM 9c or 9d, respectively. Wild-type LdAG83, without 9c, was included as control (□). SSG-sensitive LdAG83 promastigotes were studied by using the same protocol as described above. (C) The percentage of survivors was quantified by using MTS assay after 72 h of incubation at 27°C. Each datum point was tested in triplicate, and analyses were repeated three times in separate experiments. Note that the concentration of SSG used for Ld39 is different from that of LdAG83. The effect of 9d on the SSG accumulation of LdAG83, Ld39, and Ld2001 was studied in panel D. A 1-ml portion of 4-day-old axenic amastigotes at a cell density of 2 × 108 cells/ml was incubated at 37°C for 3 h with 0.05 mM SSG and various concentrations of 9d (0, 30, or 60 μM). After incubation, the cells were washed with PBS, and the total antimony concentration was determined by ICP-MS. Each concentration of 9d was tested in triplicate, and analyses were repeated twice in separate experiments. The total antimony concentration is presented as nmol per mg of protein (mean ± standard error mean, n = 2). The white bar, black bar, and striped bar represent 0, 30, and 60 μM 9d, respectively.
We investigated the effect of 9d on the SSG accumulation of L. donovani amastigotes. Axenic amastigotes were produced by adapting the parasites to 37°C for 24 h. Light microscopy showed that the cells have rounded up (data not shown). We assumed that the parasites changed into the amastigote form. Other researchers have demonstrated that this adaptation method resulted in biochemical changes that were associated with the amastigote formation (39).
In the SSG accumulation experiment, we used higher concentrations of 9d (30 and 60 μM), together with a shorter incubation time (3 h) to measure the SSG accumulation. In the absence of 9d, the accumulations of SSG of Ld39 and Ld2001 were 28 and 15% of that of LdAG83, respectively (Fig. 6D). When treated with 30 μM 9d, the SSG accumulations of Ld39 and Ld2001 were increased to 74 and 83% of that of LdAG83, respectively (Fig. 6D). When the concentration of 9d was further increased to 60 μM, the SSG accumulations of Ld39 and Ld2001 were 90 and 69% of that of LdAG83, respectively (Fig. 6D). In contrast, the accumulation of SSG in SSG-sensitive LdAG83 treated with 9d (30 or 60 μM) did not significantly differ from its accumulation in cells without any treatment, indicating that the dimer 9d specifically inhibited the function of the ABC transporters present only in an SSG-resistant strain (Fig. 6D). Compound 9d did not have any cytotoxicity to L. donovani at 60 μM when treated for 3 h (data not shown), confirming that the increase in SSG accumulation was due to the modulating effect of 9d and not to its cytotoxic effect.
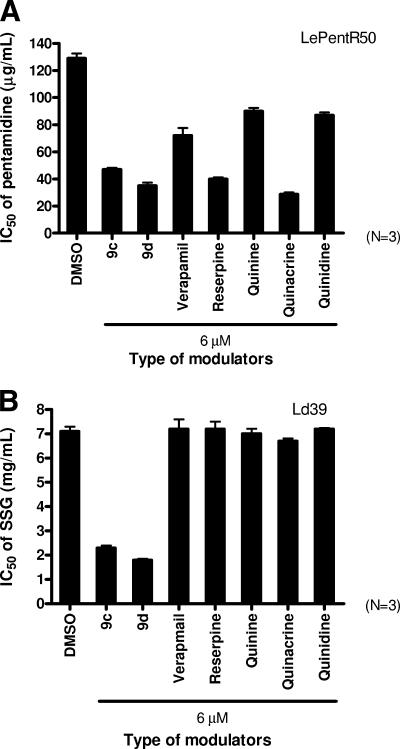
Comparison of the modulating activities of 9c and 9d with other traditional MDR modulators.
We compared the modulating activities of 9c and 9d with verapamil, reserpine, quinine, quinacrine, and quinidine. For LePentR50, the modulating activities of modulators of 9c (IC50 = 47 ± 1.2 μg/ml) and 9d (IC50 = 35 ± 2.3 μg/ml) were similar to those of reserpine (IC50 = 40 ± 1.3 μg/ml) and quinacrine (IC50 = 28.7 ± 1.3 μg/ml), with about 2.7-, 3.7-, 3.2-, and 4.5-fold pentamidine sensitizations, respectively (Fig. 7A). In contrast, only less than a half-fold sensitization was demonstrated when verapamil, quinine, and quinidine were used (Fig. 7A). Regarding the modulating activity of SSG resistance in Ld39, only 9c and 9d were effective (IC50 = 2.3 ± 0.1 mg/ml and 1.8 ± 0.05 mg/ml, respectively), representing 3.1- and 3.9-fold SSG sensitization (Fig. 7B). None of the other traditional MDR chemosensitizers exhibited any modulating effect (IC50 = 7.2 ± 0.54, 7.2 ± 0.3, 7.0 ± 0.21, 6.7 ± 0.11, and 7.2 ± 0.04 mg/ml for verapamil, reserpine, quinine, quinacrine, and quinidine, respectively) (Fig. 7B).
FIG. 7.
Comparison of the modulating activity of 9c and 9d with other MDR modulators on the pentamidine resistance of LePentR50 (A) and SSG resistance of Ld39 (B). Promastigotes were seeded at 105 cells/well in 100 μl and incubated at 27°C for 72 h in the presence of either 9c, 9d, or other traditional MDR modulators. All modulators were used at the concentration of 6 μM. The IC50 values were determined by using MTS assay. Each sample was tested in triplicate, and analyses were repeated three times in separate experiments.
The target of the synthetic flavonoid dimers is not LeMDR1.
We are interested in identifying the target of the synthetic flavonoid dimers. Other researchers have suggested that flavonoid monomers can bind to the NBD of ABC transporter of L. tropica (36). It is possible that our synthetic flavonoid dimers will also bind to the ABC transporters via the two NBDs. We have investigated whether the ABC transporter, LeMDR1, in L. enriettii is the target of the synthetic flavonoid dimers. We have previously demonstrated that LeMDR1 is an ABC transporter that can mediate resistance to vinblastine and puromycin and sensitivity to rhodamine 123 (11, 16). Here we studied the modulating effect of the synthetic flavonoid dimers on three L. enriettii cell lines, namely, wild-type Le, LeMDR1 knockout (LeMDR1−/−), and LeMDR1 overexpressed (LeV160). We found that pentamidine resistance was inversely related to the copy number of LeMDR1. The pentamidine IC50s for LeMDR1−/−, Le wild type, and LeV160 are 18.9 ± 0.8, 12.0 ± 0.8, and 9.0 ± 0.1 μg/ml, respectively (Table 2). When the panel of synthetic flavonoid dimers was tested for their modulating activity on the pentamidine resistance of LeMDR1−/−, we found that 9c and 9d were effective in reducing the IC50s of pentamidine to 5 ± 0.3 μg/ml and 4.6 ± 0.4 μg/ml, respectively, representing 3.8- and 4.1-fold sensitizations (Table 2). Compounds 9b (IC50 = 9.4 ± 0.4 μg/ml) and 9 h-1 (IC50 = 8.2 ± 0.5 μg/ml) showed 2.0- and 2.3-fold sensitizations, respectively. However, 9a (IC50 = 18 ± 1.0 μg/ml), 9e (IC50 = 12.5 ± 0.1 μg/ml), 9f (IC50 = 12.5 ± 0.8 μg/ml), 9i (IC50 = 13.8 ± 0.7 μg/ml), 9j (IC50 = 20.9 ± 1.3 μg/ml), and 9k-1 (IC50 = 20.9 ± 3 μg/ml) gave less than half or no sensitization effect (Table 2). When all of the flavonoid dimers were analyzed, a “U”-shaped relationship was found between the ethylene glycol linker length and the pentamidine resistance modulating activity. This is similar to what we found in LePentR50 (Fig. 3A).
TABLE 2.
Effect of synthetic flavonoid dimers on pentamidine resistance of LeMDR1 mutantsa
| Compound | IC50 of pentamidine (μg/ml) ± SD
|
Mean IC50 (μg/ml) ± SD
|
|||
|---|---|---|---|---|---|
| LeMDR1−/− | Le wild type | LeV160 | Vinblastine (LeV160) | Puromycin (LeV160) | |
| No modulator | 18.9 ± 0.8 | 12.0 ± 0.8 | 9.0 ± 0.1 | 167.0 ± 3.6 | 16.0 ± 1.0 |
| 9a | 18.0 ± 1.0 | ND | ND | 170.0 ± 7.0 | ND |
| 9b | 9.4 ± 0.4 | ND | ND | 160.0 ± 6.0 | ND |
| 9c | 5.0 ± 0.3 | ND | 5.0 ± 0.4 | 134.0 ± 6.0 | 13.0 ± 0.5 |
| 9d | 4.6 ± 0.4 | 4.0 ± 0.3 | 4.7 ± 0.1 | 140.0 ± 2.3 | 15.0 ± 0.6 |
| 9e | 12.5 ± 0.1 | ND | 7.5 ± 0.3 | 170.0 ± 2.3 | 19.0 ± 0.4 |
| 9f | 12.5 ± 0.8 | ND | 7.2 ± 0.3 | 165.0 ± 2.3 | 17.0 ± 1.0 |
| 9h-1 | 8.2 ± 0.5 | ND | ND | 160.0 ± 8.0 | ND |
| 9i | 13.8 ± 0.7 | ND | 6.8 ± 0.2 | 165.0 ± 2.3 | 19.0 ± 0.8 |
| 9j | 20.9 ± 1.3 | ND | ND | 170.0 ± 4.0 | ND |
| 9k-1 | 20.9 ± 3.0 | ND | ND | 150.0 ± 6.0 | ND |
The IC50 values for each drug were determined by a MTS-based proliferation assay. Each IC50 value was derived from at least three independent experiments with triplicates in each experiment. ND, not determined.
In Le wild-type cells, 9d (IC50 = 4 ± 0.3 μg/ml) significantly reduced the IC50 of pentamidine from 12.0 ± 0.8 μg/ml to 4.0 ± 0.8 μg/ml (∼3-fold decrease) (Table 2). In LeMDR1-overexpressed LeV160, 9c (IC50 = 5.0 ± 0.4 μg/ml) and 9d (IC50 = 4.7 ± 0.1 μg/ml) slightly decreased the IC50s of pentamidine from 9.0 ± 0.1 μg/ml to 5.0 ± 0.4 and 4.7 ± 0.1 μg/ml, respectively (approximately 1.8-fold and 1.9-fold decreases) (Table 2). Compounds 9e (IC50 = 7.5 ± 0.3 μg/ml), 9f (IC50 = 7.2 ± 0.3 μg/ml), and 9i (IC50 = 6.8 ± 0.2 μg/ml), however, had no sensitization effect.
The observation that the synthetic flavonoid dimers can modulate the pentamidine resistance irrespective of the copy number of LeMDR1 suggests that LeMDR1 is not the target for the synthetic flavonoid dimers. LeMDR1 is known to be responsible for vinblastine and puromycin resistance in L. enriettii (11, 16). When we tried to study the modulating activity of the flavonoid dimers on the vinblastine and puromycin resistance of LeV160, we found that none of the flavonoid dimers have any significant modulating activity (Table 2). This result further confirms that our synthetic flavonoid dimers cannot target LeMDR1.
DISCUSSION
Various ABC transporters in Leishmania have been implicated in mediating drug resistance (38). These include Ldmdr1 in L. donovani (23), Lamdr1 and Lamdr2 in L. amazonensis (21, 29), LtpgpA in L. tarentolae (20, 22, 33), Ltmdr1 in L. tropica (19), Lemdr1 in L. enriettii (11), LmepgpA in L. mexicana (13), LmpgpA in L. major (7), and PENr in L. major (12). Structurally, they can be grouped into the ABCB (Ldmdr1, Lamdr1, Lamdr2, Ltrmdr1, Lemdr1, and PENr) and ABCC (LtpgpA, LmepgpA, and LmpgpA) types. Both ABCB and ABCC transporters have two NBDs and therefore are potential targets of flavonoids. Indeed, flavonoids have been demonstrated to modulate the daunomycin resistance in L. tropica by binding to the NBDs of LtrMDR1 (36).
Success in overcoming MDR has been limited by a lack of specificity and a low affinity of MDR modulators for the drug binding sites of ABC transporter. An application of polyvalency in drug design has recently been studied which exploits the cooperativity effect during molecular recognition and binding, resulting in a polyvalent ligand binding more tightly than equivalent monovalent system when the target protein has multiple binding sites (10, 40, 42, 46). The existence of several drug-binding sites in the homo- or -heterodimeric ABC transporter suggests that polyvalency may be an invaluable approach to enhance the efficacy of MDR modulators. In the present study, we used dimers of flavonoids that differ only in the length of ethylene glycol (from one ethylene glycol unit to thirteen ethylene glycol units) to investigate whether polyvalency is a practical strategy to develop inhibitors for the ABC transporter-mediated pentamidine and SSG resistance in the parasite Leishmania.
Pentamidine resistance in Leishmania may be caused by the exclusion of pentamidine from mitochondria in L. mexicana (3) and in L. donovani (32). A genetic approach has identified an ABC transporter PRP1 that may be involved in pentamidine resistance (12). It is possible that multiple factors are involved in pentamidine resistance. Here we have used a stepwise selected pentamidine-resistant L. enriettii cell line (LePentR50) to investigate the molecular mechanism of pentamidine resistance. First, the pentamidine resistance factor may be an ABC transporter because our synthetic flavonoid dimer, particularly 9c and 9d, can modulate the pentamidine resistance of LePentR50 in a dose-dependent manner. Flavonoids have been demonstrated to be an efficient MDR modulator in both mammalian P-gp (15) and Leishmania ABC transporters (36) by binding to the NBDs. We assume that our synthetic apigenin dimers may also bind to the NBDs, although we do not have any direct proof yet. We have previously demonstrated that our synthetic flavonoid dimers can inhibit P-gp-mediated anticancer drug resistance in mammalian cells by increasing drug accumulation (8). Such observations support our hypothesis that our synthetic flavonoid dimers might indeed bind to a putative ABC transporter in Leishmania. Second, this putative ABC transporter is not LeMDR1 because our flavonoid dimers can modulate the pentamidine resistance irrespective of the LeMDR1 copy number (LeMDR1−/−, Le, or LeV160). This is further confirmed by the observation that the LeMDR1-mediated vinblastine and puromycin resistance are not affected by the flavonoid dimers. The identity of this ABC transporter remains elusive. In addition to this ABC transporter, there may be other factors that might contribute to pentamidine resistance in L. enriettii. LeMDR1 could be one of the factors. Here we found that pentamidine resistance is inversely associated with LeMDR1 copy number. The IC50 of pentamidine of LeMDR1−/−, Le, and LeV160 are 18.9 ± 0.8, 12.0 ± 0.8, and 9.0 ± 0.1 μg/ml, respectively (Table 2). We have previously reported that LeMDR1 is inversely associated with rhodamine resistance, and LeMDR1 may be sequestering to a multivesicular tubules that could connect to mitochondria (16). Therefore, LeMDR1 overexpression results in concentrating rhodamine 123 with its mitochondrial target and causes hypersensitivity. Based on our observation here, we hypothesize that LeMDR1 may be working similarly on causing pentamidine hypersensitivity by concentrating pentamidine with its mitochondrial target(s). In such a case, LeMDR1 may be the factor involved in the accumulation of pentamidine indirectly into mitochondria. In summary, we hypothesize that the pentamidine resistance in L. enriettii may be caused by an ABC transporter involved in lowering pentamidine accumulation. In addition, LeMDR1 is involved in importing pentamidine indirectly into mitochondria, possibly via multivesicular tubules.
Regardless of the identity of the putative ABC transporter that causes pentamidine resistance in LePentR50, our synthetic flavonoid dimers can inhibit it and reverse the pentamidine resistance. Compounds 9c or 9d with two apigenins connected by three or four ethylene glycol units exhibited the highest modulating activity of both pentamidine and SSG resistance, with an ∼3-fold decrease in IC50. Other flavonoid dimers with longer or shorter linker lengths showed a lower activity or no modulating activity. The apigenin monomers with the same number of ethylene glycols in the linker (10a and 10b) did not have any modulating activity, even when twice the concentration was used (12 μM). This clearly demonstrates that the modulatory activity of 9c and 9d is not due to the doubled concentration of the flavonoid binding to the ABC transporters but rather due to the chain length effect of the ethylene glycol units between the two apigenins. The optimal chain length is three to four ethylene glycol units. This result suggests that the two apigenin targets of the transporter have a relatively optimal distance between them. Only when the flavonoid dimers have the suitable length (three to four ethylene glycol units) will they be able to bind to them tightly. Previously, we have reported that the optimal linker length to modulate paclitaxel resistance in human breast cancer cells was also three to four ethylene glycols, suggesting the transporter in L. enriettii and L. donovani involved in pentamidine and SSG resistance is likely to be an ABC transporter and may have a similar structure as the human P-gp (8). The distance between the two apigenin targets will have a similar distance between them.
9c and 9d work to reverse the pentamidine and SSG resistance by increasing drug accumulation in the resistant cells. Treatment with 9c and 9d resulted in a dose-dependent increase in the accumulation of pentamidine and SSG. This result also indirectly suggests that an efflux transporter is mediating pentamidine and SSG resistance by lowering the drug accumulation. We are assuming that such an efflux transporter is an ABC transporter and that it is the target of 9c and 9d. At this point, we do not know where the flavonoid dimers are binding to the putative ABC transporter. Flavonoids have been demonstrated to bind to a region that is overlapped by the ATP-binding and the steroid-binding region. However, we have no experimental evidence to show that the flavonoid dimer is binding to the same site at which the monomer binds. The target could either be the NBD or the drug binding site. In the former case, the flavonoid dimer will inhibit the ATPase activity, whereas in the latter case the flavonoid dimer will act as a competitive inhibitor.
In comparison with other traditional MDR modulators, 9c and 9d exhibited a pentamidine resistance reversal activity comparable to that of reserpine and quinacrine. In the case of SSG resistance, only 9c and 9d have significant modulating activity, whereas none of the traditional MDR modulators work. This demonstrates that polyvalency is indeed a powerful approach in designing novel MDR modulators. An application of polyvalency in drug design has recently been studied that exploits the cooperativity effect in molecular recognition and binding (10, 40, 42, 46). Our study now demonstrates that the bivalent nature of flavonoid synthesized in the present study can dramatically increase the reversal activity of modulators, so it is of great significance for future clinical application.
In summary, our study demonstrates that dimerization of flavonoids using spacers of a defined ethylene glycol units can enhance the reversal activity of modulators on antileishmanial drug resistance. The flavonoid dimers with three or four ethylene glycol units (9c or 9d) displayed the greatest modulatory activity, with ∼3-fold sensitizations of pentamidine and SSG resistance, respectively, and in a dose-dependent manner. Moreover, their reversal activity on antileishmanial drug resistance was explained by the increase in intracellular accumulation of pentamidine and total antimony. Although the present study demonstrates that flavonoid dimers are effective modulators in vitro, animal experiments are required to determine whether the flavonoid dimers have the potential to be developed as an effective chemosensitizer for inhibiting pentamidine and SSG resistance in Leishmania.
Acknowledgments
This study was supported by the Areas of Strategic Development fund under The Hong Kong Polytechnic University and the Areas of Excellence Scheme established under the University Grants Committee of the Hong Kong Administrative Region, China (project no. AOE/P-10/01); RGC CERG (grants B-Q540, B-Q423, and B-Q762); and the Hong Kong Polytechnic Internal Grant (G-T835).
Footnotes
Published ahead of print on 28 December 2006.
REFERENCES
- 1.Ashford, R. W., P. Desjeux, and P. Deraadt. 1992. Estimation of population at risk of infection and number of cases of leishmaniasis. Parasitol. Today 8:104-105. [DOI] [PubMed] [Google Scholar]
- 2.Ashutosh, G., S. Ramesh, S. Sundar, and N. Goyal. 2005. Use of Leishmania donovani field isolates expressing the luciferase reporter gene in in vitro drug screening. Antimicrob. Agents Chemother. 49:3776-3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basselin, M., H. Denise, G. H. Coombs, M. P. Barrett, M. A. Badet-Denisot, and M. Robert-Gero. 2002. Resistance to pentamidine in Leishmania mexicana involves exclusion of the drug from the mitochondrion. Antimicrob. Agents Chemother. 46:3731-3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basselin, M., F. Lawrence, and M. Robert-Gero. 1996. Pentamidine uptake in Leishmania donovani and Leishmania amazonensis promastigotes and axenic amastigotes. Biochem. J. 315(Pt. 2):631-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berman, J. D. 1997. Human leishmaniasis: clinical, diagnostic, and chemotherapeutic developments in the last 10 years. Clin. Infect. Dis. 24:684-703. [DOI] [PubMed] [Google Scholar]
- 6.Brochu, C., J. Wang, G. Roy, N. Messier, X. Y. Wang, N. G. Saravia, and M. Ouellette. 2003. Antimony uptake systems in the protozoan parasite Leishmania and accumulation differences in antimony-resistant parasites. Antimicrob. Agents Chemother. 47:3073-3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Callahan, H. L., and S. M. Beverley. 1991. Heavy metal resistance: a new role for P-glycoproteins in Leishmania. J. Biol. Chem. 266:18427-18430. [PubMed] [Google Scholar]
- 8.Chan, K. F., Y. Zhao, B. A. Burkett, I. L. K. Wong, L. M. C. Chow, and T. H. Chan. 2006. Flavonoid dimers as bivalent modulators for P-glycoproteins based multidrug resistance (MDR): synthetic apigenin homodimers linked with defined-length polyethylene glycol spacers increase drug retention and enhance chemosensitivity in resistant cancer cells. J. Med. Chem. 49:6742-6759. [DOI] [PubMed] [Google Scholar]
- 9.Chiquero, M. J., J. M. Perez-Victoria, F. O'Valle, J. M. Gonzalez-Ros, R. G. del Moral, J. A. Ferragut, S. Castanys, and F. Gamarro. 1998. Altered drug membrane permeability in a multidrug-resistant Leishmania tropica line. Biochem. Pharmacol. 55:131-139. [DOI] [PubMed] [Google Scholar]
- 10.Cho, Y. R., A. J. Maguire, A. C. Try, M. S. Westwell, P. Groves, and D. H. Williams. 1996. Cooperativity and anti-cooperativity between ligand binding and the dimerization of ristocetin A: asymmetry of a homodimer complex and implications for signal transduction. Chem. Biol. 3:207-215. [DOI] [PubMed] [Google Scholar]
- 11.Chow, L. M., A. K. Wong, B. Ullman, and D. F. Wirth. 1993. Cloning and functional analysis of an extrachromosomally amplified multidrug resistance-like gene in Leishmania enriettii. Mol. Biochem. Parasitol. 60:195-208. [DOI] [PubMed] [Google Scholar]
- 12.Coelho, A. C., S. M. Beverley, and P. C. Cotrim. 2003. Functional genetic identification of PRP1, an ABC transporter superfamily member conferring pentamidine resistance in Leishmania major. Mol. Biochem. Parasitol. 130:83-90. [DOI] [PubMed] [Google Scholar]
- 13.Detke, S., K. Katakura, and K. P. Chang. 1989. DNA amplification in arsenite-resistant Leishmania. Exp. Cell Res. 180:161-170. [DOI] [PubMed] [Google Scholar]
- 14.de Wet, H., D. B. McIntosh, G. Conseil, H. Baubichon-Cortay, T. Krell, J. M. Jault, J. B. Daskiewicz, D. Barron, and A. Di Pietro. 2001. Sequence requirements of the ATP-binding site within the C-terminal nucleotide-binding domain of mouse P-glycoprotein: structure-activity relationships for flavonoid binding. Biochemistry 40:10382-10391. [DOI] [PubMed] [Google Scholar]
- 15.Di Pietro, A., G. Conseil, J. M. Perez-Victoria, G. Dayan, H. Baubichon-Cortay, D. Trompier, E. Steinfels, J. M. Jault, H. de Wet, M. Maitrejean, G. Comte, A. Boumendjel, A. M. Mariotte, C. Dumontet, D. B. McIntosh, A. Goffeau, S. Castanys, F. Gamarro, and D. Barron. 2002. Modulation by flavonoids of cell multidrug resistance mediated by P-glycoprotein and related ABC transporters. Cell Mol. Life Sci. 59:307-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dodge, M. A., R. F. Waller, L. M. Chow, M. M. Zaman, L. M. Cotton, M. J. McConville, and D. F. Wirth. 2004. Localization and activity of multidrug resistance protein 1 in the secretory pathway of Leishmania parasites. Mol. Microbiol. 51:1563-1575. [DOI] [PubMed] [Google Scholar]
- 17.Ferriola, P. C., V. Cody, and E. Middleton, Jr. 1989. Protein kinase C inhibition by plant flavonoids: kinetic mechanisms and structure-activity relationships. Biochem. Pharmacol. 38:1617-1624. [DOI] [PubMed] [Google Scholar]
- 18.Formica, J. V., and W. Regelson. 1995. Review of the biology of quercetin and related bioflavonoids. Food Chem. Toxicol. 33:1061-1080. [DOI] [PubMed] [Google Scholar]
- 19.Gamarro, F., M. J. Chiquero, M. V. Amador, D. Legare, M. Ouellette, and S. Castanys. 1994. P-glycoprotein overexpression in methotrexate-resistant Leishmania tropica. Biochem. Pharmacol. 47:1939-1947. [DOI] [PubMed] [Google Scholar]
- 20.Grondin, K., A. Haimeur, R. Mukhopadhyay, B. P. Rosen, and M. Ouellette. 1997. Co-amplification of the gamma-glutamylcysteine synthetase gene gsh1 and of the ABC transporter gene pgpA in arsenite-resistant Leishmania tarentolae. EMBO J. 16:3057-3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gueiros-Filho, F. J., J. P. Viola, F. C. Gomes, M. Farina, U. Lins, A. L. Bertho, D. F. Wirth, and U. G. Lopes. 1995. Leishmania amazonensis: multidrug resistance in vinblastine-resistant promastigotes is associated with rhodamine 123 efflux, DNA amplification, and RNA overexpression of a Leishmania mdr1 gene. Exp. Parasitol. 81:480-490. [DOI] [PubMed] [Google Scholar]
- 22.Haimeur, A., C. Brochu, P. Genest, B. Papadopoulou, and M. Ouellette. 2000. Amplification of the ABC transporter gene PGPA and increased trypanothione levels in potassium antimonyl tartrate (SbIII)-resistant Leishmania tarentolae. Mol. Biochem. Parasitol. 108:131-135. [DOI] [PubMed] [Google Scholar]
- 23.Henderson, D. M., C. D. Sifri, M. Rodgers, D. F. Wirth, N. Hendrickson, and B. Ullman. 1992. Multidrug resistance in Leishmania donovani is conferred by amplification of a gene homologous to the mammalian mdr1 gene. Mol. Cell. Biol. 12:2855-2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirano, T., K. Oka, and M. Akiba. 1989. Effects of synthetic and naturally occurring flavonoids on Na+,K+-ATPase: aspects of the structure-activity relationship and action mechanism. Life Sci. 45:1111-1117. [DOI] [PubMed] [Google Scholar]
- 25.Jinsart, W., B. Ternai, and G. M. Polya. 1992. Inhibition of rat liver cyclic AMP-dependent protein kinase by flavonoids. Biol. Chem. Hoppe-Seyler's 373:205-211. [DOI] [PubMed] [Google Scholar]
- 26.Juliano, R. L., and V. Ling. 1976. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim. Biophys. Acta 455:152-162. [DOI] [PubMed] [Google Scholar]
- 27.Kandaswami, C., E. Perkins, G. Drzewiecki, D. S. Soloniuk, and E. Middleton, Jr. 1992. Differential inhibition of proliferation of human squamous cell carcinoma, gliosarcoma, and embryonic fibroblast-like lung cells in culture by plant flavonoids. Anticancer Drugs 3:525-530. [DOI] [PubMed] [Google Scholar]
- 28.Kandaswami, C., E. Perkins, D. S. Soloniuk, G. Drzewiecki, and E. Middleton, Jr. 1993. Ascorbic acid-enhanced antiproliferative effect of flavonoids on squamous cell carcinoma in vitro. Anticancer Drugs 4:91-96. [DOI] [PubMed] [Google Scholar]
- 29.Katakura, K., M. Iwanami, H. Ohtomo, H. Fujise, and Y. Hashiguchi. 1999. Structural and functional analysis of the LaMDR1 multidrug resistance gene in Leishmania amazonensis. Biochem. Biophys. Res. Commun. 255:289-294. [DOI] [PubMed] [Google Scholar]
- 30.Legare, D., D. Richard, R. Mukhopadhyay, Y. D. Stierhof, B. P. Rosen, A. Haimeur, B. Papadopoulou, and M. Ouellette. 2001. The Leishmania ATP-binding cassette protein PGPA is an intracellular metal-thiol transporter ATPase. J. Biol. Chem. 276:26301-26307. [DOI] [PubMed] [Google Scholar]
- 31.Mahadevan, D., and A. F. List. 2004. Targeting the multidrug resistance-1 transporter in AML: molecular regulation and therapeutic strategies. Blood 104:1940-1951. [DOI] [PubMed] [Google Scholar]
- 32.Mukherjee, A., P. K. Padmanabhan, M. H. Sahani, M. P. Barrett, and R. Madhubala. 2006. Roles for mitochondria in pentamidine susceptibility and resistance in Leishmania donovani. Mol. Biochem. Parasitol. 145:1-10. [DOI] [PubMed] [Google Scholar]
- 33.Ouellette, M., F. Fase-Fowler, and P. Borst. 1990. The amplified H circle of methotrexate-resistant Leishmania tarentolae contains a novel P-glycoprotein gene. EMBO J. 9:1027-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ouellette, M., A. Haimeur, K. Grondin, D. Legare, and B. Papadopoulou. 1998. Amplification of ABC transporter gene pgpA and of other heavy metal resistance genes in Leishmania tarentolae and their study by gene transfection and gene disruption. Methods Enzymol. 292:182-193. [DOI] [PubMed] [Google Scholar]
- 35.Papadopoulou, B., G. Roy, S. Dey, B. P. Rosen, M. Olivier, and M. Ouellette. 1996. Gene disruption of the P-glycoprotein related gene pgpA of Leishmania tarentolae. Biochem. Biophys. Res. Commun. 224:772-778. [DOI] [PubMed] [Google Scholar]
- 36.Perez-Victoria, J. M., M. J. Chiquero, G. Conseil, G. Dayan, A. Di Pietro, D. Barron, S. Castanys, and F. Gamarro. 1999. Correlation between the affinity of flavonoids binding to the cytosolic site of Leishmania tropica multidrug transporter and their efficiency to revert parasite resistance to daunomycin. Biochemistry 38:1736-1743. [DOI] [PubMed] [Google Scholar]
- 37.Perez-Victoria, J. M., F. J. Perez-Victoria, A. Parodi-Talice, I. A. Jimenez, A. G. Ravelo, S. Castanys, and F. Gamarro. 2001. Alkyl-lysophospholipid resistance in multidrug-resistant Leishmania tropica and chemosensitization by a novel P-glycoprotein-like transporter modulator. Antimicrob. Agents Chemother. 45:2468-2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pradines, B., J. M. Pages, and J. Barbe. 2005. Chemosensitizers in drug transport mechanisms involved in protozoan resistance. Curr. Drug Targets Infect. Disord. 5:411-431. [DOI] [PubMed] [Google Scholar]
- 39.Rainey, P. M., T. W. Spithill, D. McMahon-Pratt, and A. A. Pan. 1991. Biochemical and molecular characterization of Leishmania pifanoi amastigotes in continuous axenic culture. Mol. Biochem. Parasitol. 49:111-118. [DOI] [PubMed] [Google Scholar]
- 40.Rao, J., J. Lahiri, L. Isaacs, R. M. Weis, and G. M. Whitesides. 1998. A trivalent system from vancomycin d-Ala-d-Ala with higher affinity than avidin biotin. Science 280:708-711. [DOI] [PubMed] [Google Scholar]
- 41.Rosenberg, M. F., A. B. Kamis, R. Callaghan, C. F. Higgins, and R. C. Ford. 2003. Three-dimensional structures of the mammalian multidrug resistance P-glycoprotein demonstrate major conformational changes in the transmembrane domains upon nucleotide binding. J. Biol. Chem. 278:8294-8299. [DOI] [PubMed] [Google Scholar]
- 42.Sauna, Z. E., M. B. Andrus, T. M. Turner, and S. V. Ambudkar. 2004. Biochemical basis of polyvalency as a strategy for enhancing the efficacy of P-glycoprotein (ABCB1) modulators: stipiamide homodimers separated with defined-length spacers reverse drug efflux with greater efficacy. Biochemistry 43:2262-2271. [DOI] [PubMed] [Google Scholar]
- 43.Sundar, S., S. G. Reed, S. Sharma, A. Mehrotra, and H. W. Murray. 1997. Circulating T helper 1 (Th1) cell- and Th2 cell-associated cytokines in Indian patients with visceral leishmaniasis. Am. J. Trop. Med. Hyg. 56:522-525. [DOI] [PubMed] [Google Scholar]
- 44.Thiyagarajah, P., S. C. Kuttan, S. C. Lim, T. S. Teo, and N. P. Das. 1991. Effect of myricetin and other flavonoids on the liver plasma membrane Ca2+ pump: kinetics and structure-function relationships. Biochem. Pharmacol. 41:669-675. [DOI] [PubMed] [Google Scholar]
- 45.Walker, J. E., M. Saraste, M. J. Runswick, and N. J. Gay. 1982. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1:945-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams, D. H., A. J. Maguire, W. Tsuzuki, and M. S. Westwell. 1998. An analysis of the origins of a cooperative binding energy of dimerization. Science 280:711-714. [DOI] [PubMed] [Google Scholar]
- 47.Zaman, G. J., M. J. Flens, M. R. van Leusden, M. de Haas, H. S. Mulder, J. Lankelma, H. M. Pinedo, R. J. Scheper, F. Baas, H. J. Broxterman, et al. 1994. The human multidrug resistance-associated protein MRP is a plasma membrane drug-efflux pump. Proc. Natl. Acad. Sci. USA 91:8822-8826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang, S., X. Yang, and M. E. Morris. 2004. Combined effects of multiple flavonoids on breast cancer resistance protein (ABCG2)-mediated transport. Pharm. Res. 21:1263-1273. [DOI] [PubMed] [Google Scholar]