Abstract
The effect of micafungin dose scheduling on the treatment of candidemia is unknown. Neutropenic mice with disseminated Candida glabrata infection were treated with single intraperitoneal micafungin doses of 0 to 100 mg/kg of body weight and sacrificed 7 days later. The maximal decline in kidney fungal burden was 5.8 log10 CFU/g. A 1-week pharmacokinetic-pharmacodynamic study revealed a micafungin serum half-life of 6.13 h. In mice treated with ≥50 mg/kg, there was maximal fungal decline without regrowth during the 1-week dosing interval. Next, doses associated with 34% (34% effective dose [ED34]) and 50% (ED50) of maximal kill were administered at one of three dose schedules: a single dose at t = 0, two equal doses at t = 0 and t = 3.5 days, and 7 equal doses daily. Some mice received a single dose of 100 mg/kg. Fungal burden was examined on days 1, 5, and 7. In mice treated with the ED34, microbial kill with the daily therapy initially lagged behind the intermittent doses but exceeded it by day 7. In mice treated with the ED50, daily and intermittent doses had equivalent day 7 effects. In mice treated with 100 mg/kg, there was no regrowth. The relative likelihoods that the area under the concentration-time curve/MIC ratio was linked to microbial kill versus peak concentration/MIC ratio or time above the MIC was 10.3 and 10,161.2, respectively. In all the experiments, no paradoxical increase in fungal burden was observed with high micafungin doses. However, only a single Candida isolate was tested. Regimens that simulated micafungin concentration-time profiles in patients treated with a single micafungin dose of 1,400 mg once a week demonstrated maximal fungal decline. Once-weekly micafungin therapy is as efficacious as daily therapy in a murine model of disseminated candidiasis.
Candidemia is associated with a high mortality (34), especially in the absence of the vital fungicidal role of polymorphonuclear leukocytes (7, 9, 19). In patients with persistent neutropenia, fluconazole- and amphotericin B-based therapies have failure rates as high as 76% (9), are sometimes associated with breakthrough candidemia (2), and may be associated with relapse many months to years after apparent cure (17). These limitations, as well as the toxicity associated with amphotericin B (36), necessitated the development of new classes of antifungal compounds, most notably echinocandins. Echinocandins are effective agents for the management of candidemia and invasive candidiasis (8, 25, 26, 31a). However, in common with other antifungal compounds used to treat candidemia, echinocandins are administered on a daily basis for at least 2 weeks (28). The daily therapy is administered via intravenous catheters. Unfortunately, intravenous catheters are associated with mechanical complications, venous thrombosis, bleeding complications, and bacterial and fungal bloodstream infections (16, 30, 31, 33). Thus, development of effective intermittent therapy that obviates the need for long-term intravenous catheters would be a major advantage.
One approach is to examine the pharmacodynamic indices that optimize echinocandin microbial kill. If a drug's microbial kill is best explained by the total drug exposure, defined by the ratio of the area under the concentration-time curve (AUC) to MIC (AUC/MIC ratio), or is best explained by the peak drug concentration (Cmax)-to-MIC (Cmax/MIC) ratio, then administration of the same cumulative dose intermittently would not compromise efficacy (3, 12, 14). On the other hand, if the drug's efficacy is best explained by the duration of the drug concentration above the MIC (TMIC), then intermittent dosing would reduce efficacy (15). In the current study, we examined the effect of once-weekly micafungin (Mycamine) dose scheduling on fungal kill in mice with persistent neutropenia. Intermittent doses which mimic human pharmacokinetics were then examined so that the efficacy of intermittent doses in patients could be predicted.
(Portions of these data were presented as a late breaker at the 46th Interscience Conference on Antimicrobial Agents and Chemotherapy [19a].)
MATERIALS AND METHODS
Organism.
We used a Candida glabrata strain isolated from the blood cultures of a patient with candidemia. The isolate was donated to us by Gerri Hall of the Cleveland Clinic Foundation. The isolate has been shown in our previous studies (18) to have a fluconazole MIC of 32 mg/liter (susceptible dose dependent) and an amphotericin B MIC of 1 mg/liter, when examined by Clinical and Laboratory Standards Institute (CLSI)-recommended methods (11). The isolate was stored at −70°C in 10% glycerol stocks. Prior to use, the C. glabrata isolate was thawed, plated on Sabouraud dextrose agar, and incubated overnight at 35°C. Several colonies were selected on the day of study and transferred to pyrogen-free saline. The optical density was determined, and the desired fungal density was achieved by further dilution in saline. This final suspension was used as the inoculum, with quantitative cultures performed to confirm the fungal density.
Drugs.
Micafungin was provided by Astellas Pharma USA, Inc. (Deerfield, Ill). Cyclophosphamide was purchased from Sigma-Aldrich (St. Louis, MO). The drugs were dissolved in pyrogen-free normal saline to the specific drug concentration required for each experiment.
MICs.
Micafungin MIC determination was performed using test conditions described by the CLSI (11).
Neutropenic model of disseminated Candida glabrata infection.
Animal experiments were approved by the Institutional Animal Care and Use Committee of the David Axelrod Institute (New York Department of Health), where all mouse experiments were performed. Six-week-old 22- to 24-g outbred Swiss Webster female mice (Taconic Farms, Taconic, NY) were rendered neutropenic (absolute neutrophil count < 100/ml) by administration of 150 mg/kg of intraperitoneal (i.p.) cyclophosphamide 4 days prior to infection, followed by a second dose of 100 mg/kg i.p. cyclophosphamide 1 day prior to infection, and then 100 mg/kg i.p. cyclophosphamide every other day thereafter. Twenty-four hours after the second dose of cyclophosphamide was delivered, mice were intravenously inoculated with 2 × 107 CFU of C. glabrata through a lateral tail vein. Mice were euthanized by CO2 asphyxiation, and the left kidney, spleen, and lungs were aseptically collected. The organs were weighed, homogenized, and washed twice in saline to prevent drug carryover, as described in our prior studies (18). The infected tissues were then serially diluted in saline and cultured on Sabouraud dextrose agar plates. Agar plates were incubated at 35°C for 48 h, after which C. glabrata colonies were counted. Assay limits of quantitation were as described in our prior studies (18).
Dose-effect study.
Mice were rendered neutropenic and infected with C. glabrata. Four hours after inoculation, mice were divided into 10 groups of 4 mice and treated with a single i.p. dose of micafungin of 0, 2.1, 4.2, 10, 15, 20, 30, 50, 75, and 100 mg/kg. Doses were chosen by multiplying daily doses from a prior study (19b) by a factor of 7 for the 7-day cumulative dose. Mice were sacrificed 168 h (7 days) after treatment, and the kidney, spleen, and lung fungal burdens were determined as described above. The relationship between dose and effect was examined using an inhibitory sigmoid Emax model.
Pharmacokinetic-pharmacodynamic study.
To more precisely delineate pharmacokinetic-pharmacodynamic indices, we performed another study in which at least 3 doses lay on the steep section of the inhibitory sigmoid Emax curve identified in the dose-effect studies above. Mice were treated with a single dose of 0, 5, 10, 15, 25, 50, and 100 mg/kg of i.p. micafungin. Since we were interested in the concentration-time profile for the entire 168-h dosing interval, 3 mice per group were sacrificed by CO2 asphyxiation at 1, 3, 6, 10, 18, 24, 48, 72, 96, 120, 144, and 168 h after drug administration. Blood was collected from each mouse by cardiac puncture and allowed to clot on ice, and the serum was immediately stored at −70°C to be used subsequently to measure micafungin concentration. At 0, 24, 96, and 168 h after drug treatment, the right kidney was aseptically collected from each mouse for quantitative C. glabrata cultures.
Dose-scheduling studies.
When progressively increasing doses of a drug are administered, there often is a colinearity of Cmax, AUC, and TMIC. We performed dose-scheduling studies to minimize this colinearity, so that the index (Cmax/MIC ratio, AUC/MIC ratio, and TMIC) associated with effect could be better delineated. Optimal Fisher information is expected to occur between the 20% effective dose (ED20) and ED80. Therefore, we performed the dose-scheduling studies using cumulative weekly micafungin doses deemed to be the ED34 and ED50, based on the inhibitory sigmoid Emax analysis performed for the dose-effect studies above. Each of these doses was administered at one of three dose schedules: a single dose at t = 0 h, two equal divided doses at 0 h and 84 h, or seven equally divided doses administered every 24 h. In addition, a group of mice received 100 mg/kg as a single dose, while one group consisted of saline-treated mice (controls). Mice were sacrificed at 0, 72, 120, and 168 h for kidney quantitative cultures.
Effect of a once-weekly dose with human concentration-time profile.
We were interested in examining if concentration-time profiles of once-weekly micafungin therapy encountered in humans receiving doses of 700 mg would be associated with maximum fungal decline, and no regrowth, during the 1-week dosing interval. This human dose was chosen because 700 mg corresponds with the cumulative weekly dose for the micafungin 100-mg-a-day dose used to treat candidemia (26). First, we performed a population pharmacokinetic analysis of the pharmacokinetic data reported by Hiemenz et al. (20). The population pharmacokinetic parameters obtained were then utilized in Monte Carlo simulations to predict the concentration-time profile expected in 9,999 patients treated with a single micafungin dose of 700 mg. We then simulated the human concentration-time profile by administering a micafungin dose to mice at t = 0 h, followed by a second smaller dose on day 4, so that the concentrations achieved would always be within the 5th and 95th percentile of that achieved in the 9,999 virtual patients for the entire time period of 1 week. The doses administered to mice were 20 mg/kg (i.p.) at t = 0 h, followed by a supplementary dose of 0.3 mg/kg on day 4, to simulate 700 mg once a week. In a second group of mice, 100 mg/kg of micafungin was administered, which is a dose that had been demonstrated to achieve maximal fungal decline in the mice. A third group of mice was treated with i.p. saline (controls). There were four C. glabrata-infected mice in each group. Mice were sacrificed on day 7. In a subsequent study, 40 mg/kg was administered at t = 0 h and supplemented with 0.6 mg/kg on day 4 to simulate 1,400 mg once a week, which is the cumulative weekly dose for the micafungin dose of 200 mg a day, which has also been used to treat candidemia in humans (26). A second group of mice was treated with a single dose of 100 mg/kg and a third group with saline, as described above. Mice were sacrificed on days 3 and 7.
Drug assay.
Micafungin concentrations in mouse serum were determined by protein precipitation using acetonitrile and analyzed by high-performance liquid chromatography/fluorescence. Chromatographic separation was performed using a Zorbax XDB-C8 column (150 by 4.6 mm) with a gradient mobile phase system comprised of 90% 0.02 M KH2PO4 and 10% acetonitrile at 1.0 ml/min. The analytes were detected fluorometrically at excitation and emission wavelengths of 260 and 464 nm, respectively. Micafungin eluted at approximately 8.9 min, and the internal standard eluted at 9.3 min. The assay was linear over a range of 0.01 to 10 mg/liter (r2 > 0.99). The lower limit of quantitation was 0.01 mg/liter. Comparison of samples assayed on three separate days yielded coefficients of variation ranging from 0.5 to 4.5%.
Mathematical modeling.
Serum drug concentrations were analyzed as a four-compartment open model with first-order input and elimination using the nonparametric adaptive grid program software (23), as described before (18, 24). The four pharmacokinetic compartments were the site of administration (peritoneum), the central compartment (serum or compartment 1), the kidney, which is the site of infection (compartment 2), and the rest of the peripheral tissues (compartment 3).
Akaike's information criteria for model discrimination.
Yamato et al. have demonstrated that the micafungin protein binding in both human and mouse sera is 99.8% (37). Therefore, the free drug fraction of 0.2% was utilized to calculate free drug pharmacodynamic indices. Data were examined by fitting the inhibitory sigmoid Emax model to day 7 fungal burden data versus free drug exposure. The likelihood that a particular pharmacodynamic index, free Cmax/MIC ratio, free AUC/MIC ratio, or free TMIC, was related to fungal burden was examined using Akaike's information criteria (AIC) (1). The relative likelihood (or evidence ratio) that a particular pharmacodynamic index, compared to another, was associated with microbial kill was calculated using the following formula: relative likelihood = 1/(e−0.5 × ΔAIC), where ΔAIC is the difference between AIC scores of the two indices being compared.
RESULTS
Micafungin MIC.
The micafungin MIC was 0.02 mg/liter at both the 24-h and 48-h time points. The MIC was the same whether a 50% or >95% reduction in turbidity was used.
Dose-effect studies.
There was no paradoxical increase (Eagle effect) in fungal burden with high doses. The inhibitory sigmoid Emax relationship for micafungin dose and kidney fungal density was as follows: log10 CFU/g = 6.38 to 5.80 × dose1.12/(14.691.12 + dose1.12) (P < 0.001), where 14.69 mg/kg is the ED50 (95% confidence intervals, 2.39 and 26.99 mg/kg). The inhibitory sigmoid Emax modeling for lung and spleen microbial response revealed ED50 of 17.29 mg/kg for lung fungal response and 7.38 mg/kg for splenic fungal response. Therefore, the ED50 for spleen and lung were within the 95% confidence bounds of the ED50 derived for the kidney fungal response. For infection in all three organs, doses of 75 mg/kg or higher were on the maximal effect portion of the dose-effect curve. This means that the use of the kidney fungal densities as an endpoint would not underestimate the micafungin doses needed to optimally kill Candida in other organs involved in disseminated candidiasis. Therefore, subsequent studies were carried out using studies of kidney fungal burden.
In vivo time-kill study and pharmacokinetic study.
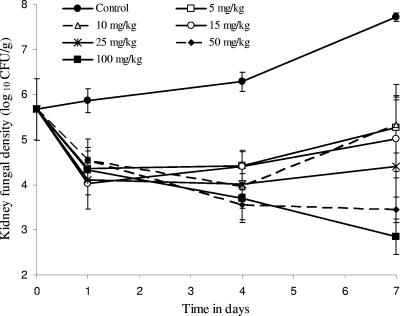
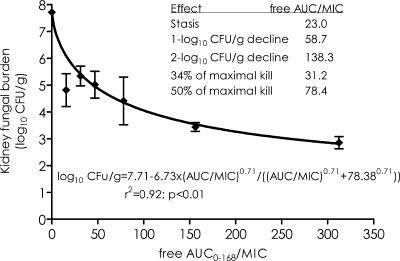
We examined the pattern of fungal decline that occurs after a single micafungin dose. Figure 1 demonstrates that micafungin doses of <50 mg/kg were associated with regrowth, while doses of ≥50 mg/kg led to a decline in fungal burden that was not accompanied by any regrowth during the entire 7-day dosing interval. The peak micafungin concentrations achieved by these doses in serum varied proportionately with dose (r = 0.994), indicating linear pharmacokinetics between 5 and 100 mg/kg. Micafungin population pharmacokinetic parameters derived from these concentrations are shown in Table 1. Using these pharmacokinetic parameters, we calculated that the micafungin serum half-life was 6.13 h. The parameters were also used to calculate the pharmacodynamic indices (AUC from 0 to 168 h [AUC0-168]/MIC ratio, Cmax/MIC ratio, and TMIC) associated with each dose. The pharmacodynamic indices were then related to microbial response. An example is the relationship between AUC0-168/MIC ratio and day 7 kidney fungal burden shown in Fig. 2. From this relationship, micafungin AUC0-168/MIC ratio exposures associated with stasis (fungal density equal to that in the kidney at time of treatment), ED34, and ED50 were calculated, with results shown in Fig. 2. These exposures were then used in the dose-scheduling study.
FIG. 1.
Changes in mouse kidney fungal burden after a single dose of micafungin.
TABLE 1.
Micafungin population pharmacokinetic parameter estimates in mice
| Parametera | Mean | SD |
|---|---|---|
| Vc (liter) | 0.0068 | 0.0020 |
| SCL (liter/h) | 0.0008 | 0.0002 |
| Ka (h−1) | 4.4418 | 3.0669 |
| K12 (h−1) | 2.2494 | 1.0800 |
| K21 (h−1) | 19.6142 | 5.5527 |
| K13 (h−1) | 2.9619 | 1.4910 |
| K31 (h−1) | 25.4929 | 4.2651 |
Vc, volume of the central compartment (compartment 1); SCL, serum clearance; Ka, absorption constant from peritoneum into compartment 1; K12 and K21, transfer constant from compartment 1 to 2 (kidney) and vice versa; K13 and K31, transfer constants from compartment 1 to 3 (peripheral tissues) and vice versa.
FIG. 2.
The inhibitory sigmoid Emax relationship between micafungin exposure and kidney fungal density.
Micafungin dose-scheduling study.
We examined the effect of different dose schedules, daily, twice a week, and once a week, for the ED34 (5 mg/kg) and ED50 (20 mg/kg) doses of micafungin. Results are shown in Fig. 3. In mice treated with the ED34, the day 3 microbial kill with the daily therapy lagged behind the intermittent doses. However, the intermittent doses ran out of persistent effect and the isolate regrew so that after day 5, the effect of the daily therapy exceeded that of intermittent therapy. On the other hand, in mice treated with the ED50, the regrowth in the intermittent doses started late so that, even by day 7, the microbial kill for the daily therapy regimen had only just caught up with the intermittent doses. In mice treated with a single dose of 100 mg/kg, there was no regrowth at all throughout the 7-day dosing interval, confirming the observations in the time-kill study. The relative likelihood that the AUC0-168/MIC ratio was linked to microbial kill compared to the Cmax/MIC ratio and TMIC was 10.3 and 10,161.2 times, respectively.
FIG. 3.
Effect of dose scheduling on micafungin efficacy. Changes in kidney fungal density with time in mice treated with 5 mg/kg (ED34) (A), 20 mg/kg (ED50) (B), and 100 mg/kg (C) of intraperitoneal micafungin are shown.
Effect of once-weekly dose with human concentration-time profile.
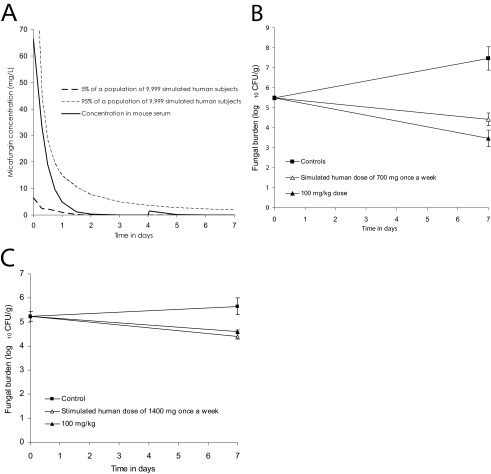
The mathematical simulation of the micafungin concentration-time profiles expected in patients treated with a single micafungin dose of 700 mg and how closely the dosing in mice mimics this is shown in Fig. 4a. Figure 4b shows the microbial response in mice treated with such a dosing scheme. The microbial kill on day 7 fell short of the maximal effect, as indicated by less microbial kill than the 100-mg/kg dose. Therefore, we simulated the human concentration-time profile of 1,400 mg, which demonstrated a microbial response equivalent to 100 mg/kg (Fig. 4c).
FIG. 4.
Microbial effect of mimicking micafungin concentration-time profiles encountered with once-weekly doses in humans. (A) Dosing regimen in mice that mimics the mathematically derived pharmacokinetic profile of humans treated with 700 mg once a week; (B and C) effect of mimicking human concentration-time profile of 700-mg (B) and 1,400-mg (C) once-weekly doses compared to the 100-mg/kg dose.
DISCUSSION
The currently recommended dose schedule for micafungin is daily therapy. We chose to examine intermittent therapy with micafungin for several reasons. First, micafungin continues to inhibit fungal regrowth for several days after concentrations at the site of infection have declined below the MIC for the Candida isolate (19b). Second, one study has demonstrated that patients may be able to tolerate micafungin doses as high as 896 mg administered each day for at least 7 days (32), which suggests that intermittent micafungin therapy may be possible in patients. However, the tolerability of such high intermittent doses needs to be confirmed in larger clinical studies. Third, micafungin serum concentrations closely parallel those in tissues, which makes it easier to translate exposures derived in animal models to human clinical dosing via comparison of serum exposures. On the other hand, others have noted a paradoxical increase in fungal burden when some Candida isolates were exposed to high concentrations of caspofungin in vitro and in mice (10). This suggests that some high echinocandin doses could be detrimental. In the series of experiments that were part of our current study, we noted no such effect when up to 100 mg/kg of micafungin was administered to mice. However, we only examined one Candida isolate, and the paradoxical fungal response is known to occur with some Candida isolates and not others (10). Thus, our results need to be confirmed with a larger number of Candida isolates.
In the dose-scheduling studies, the intermittent micafungin dose schedules achieved an early maximal reduction in Candida fungal burden and then exhibited some persistent growth inhibition. This is more striking given that the mice were severely neutropenic, with an absolute neutrophil count of <100/ml, so that the antifungal effect was predominantly due to the micafungin. Thus, as long as large enough doses are administered, micafungin could be administered intermittently without regrowth of the fungi. Micafungin's microbial kill was associated with the AUC/MIC ratio. This is similar to our findings for caspofungin (24) but different from studies by others, who found that the microbial kill by other echinocandins was linked to the Cmax/MIC ratio (6, 35). Differences may be due to differences in study design or simply because different pathogens were utilized. However, implications for intermittent dosing are the same. When a drug's effect is linked to the AUC/MIC ratio, it is the size of the cumulative dose during the dosing interval which is important. We speculate that if patients could tolerate even higher doses, it is conceivable that the whole cumulative dose for the 2 weeks of standard therapy could be administered as a single dose. On the other hand, if it were found that patients are unable to tolerate the high once-weekly doses, lower doses administered twice every week would still be effective. Indeed, with an AUC/MIC ratio-linked drug, doses could even be given every other day to the same efficacy as daily dosing, as long as the same cumulative dose is administered. For example, in a recent clinical trial, intravenous micafungin was administered as 150 mg per day versus 300 mg per day for the treatment of esophageal Candida (9a). Efficacy rates were similar in patients treated with either dosing strategy, consistent with AUC/MIC ratio-driven activity. Thus, conclusions on micafungin dosing schedules derived from our mouse studies were borne out in the clinical trial.
While the serum half-life of micafungin in mice was 6.13 h, it is known to be 11.3 to 20 h in humans (20) and is thus much longer. Simulating antimicrobials' concentration-time profiles to mimic that encountered in humans improves the clinical predictive power of in vitro and animal model studies (13). Our simulations with mice demonstrate that once-weekly micafungin doses of 700 mg will be effective but will not achieve optimal kill. On the other hand, simulating the higher dose of 1,400 mg once a week was associated with optimal effect. This is somewhat tempered by the fact that, in this last experiment, the total microbial kill by the 1,400-mg dose was less than the maximal kill achieved in all prior experiments, and the growth of Candida in control animals was less than in prior experiments. Thus, less kill might have resulted from poor fungal growth in this group of animals. However, the 100-mg/kg dose, established to achieve maximum fungal kill in prior experiments, produced similar microbial kill to the 1,400-mg dose. Moreover, we would like to reiterate that the simulation of these high doses was in mice and the tolerability of 1,400 mg of intravenous micafungin administered once a week to patients is currently unknown. Therefore, dose-ranging studies need to be performed to establish the safety of the once-weekly dosing regimens in patients.
Once-weekly micafungin dosing would be associated with several advantages. First, once-weekly dosing would obviate the need for long-dwelling intravenous catheters for drug administration and the resultant patient morbidity associated with long-dwelling intravenous catheters. Second, once-weekly therapy would make the lack of a micafungin oral formulation less relevant. Third, provided patients respond to therapy, there could be potential for a decreased length of hospitalization. Finally, there is potential for reduction in the nursing and pharmacy time associated with daily preparation and administration of the drug.
A limitation of our study is that we did not examine the effect of the micafungin dosing schedule on drug resistance. Micafungin resistance arising during therapy has already been demonstrated in the clinical arena (22). Recently, Andes et al. demonstrated that fluconazole, a drug with AUC/MIC ratio-linked microbial kill, suppresses resistance by optimizing the TMIC (5). This may not be the case for drugs that are fungicidal against Candida (5), such as micafungin. Nevertheless, the effect of dose scheduling on micafungin resistance will need to be examined in future. Finally, we studied only one isolate of C. glabrata. It may be that our findings are strain dependent. Therefore, our findings will need to be confirmed in different Candida species. For example, Candida parapsilosis has been associated with high echinocandin MICs (21, 27). On the other hand, echinocandin therapy has been as effective against C. parapsilosis as against the more susceptible species such as Candida albicans and C. glabrata in clinical trials (21, 26, 29). Moreover, the pharmacodynamic index associated with an antifungal drug's optimal fungal kill is often the same for the different Candida species (4), so we expect that our conclusions will be borne out when other Candida species are examined in future.
Acknowledgments
This study was funded by an educational grant from Astellas Pharma, Inc., Deerfield, IL.
Footnotes
Published ahead of print on 28 December 2006.
REFERENCES
- 1.Akaike, H. 1974. A new look at the statistical model identification. IEEE Trans. Automated Control 19:716-723. [Google Scholar]
- 2.Alexander, B. D., W. A. Schell, J. L. Miller, G. D. Long, and J. R. Perfect. 2005. Candida glabrata fungemia in transplant patients receiving voriconazole after fluconazole. Transplantation 80:868-871. [DOI] [PubMed] [Google Scholar]
- 3.Ambrose, P. G., S. J. Bhavnani, C. M. Rubino, A. Louie, T. Gumbo, A. Forrest, and G. L. Drusano. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin. Infect. Dis. 44:79-86. [DOI] [PubMed] [Google Scholar]
- 4.Andes, D. 2003. In vivo pharmacodynamics of antifungal drugs in treatment of candidiasis. Antimicrob. Agents Chemother. 47:1179-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andes, D., A. Forrest, A. Lepak, J. Nett, K. Marchillo, and L. Lincoln. 2006. Impact of antimicrobial dosing regimen on evolution of drug resistance in vivo: fluconazole and Candida albicans. Antimicrob. Agents Chemother. 50:2374-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andes, D., K. Marchillo, J. Lowther, A. Bryskier, T. Stamstad, and R. Conklin. 2003. In vivo pharmacodynamics of HMR 3270, a glucan synthase inhibitor, in a murine candidiasis model. Antimicrob. Agents Chemother. 47:1187-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertagnolio, S., D. K. de Gaetano, E. Tacconelli, G. Scoppettuolo, B. Posteraro, G. Fadda, R. Cauda, and M. Tumbarello. 2004. Hospital-acquired candidemia in HIV-infected patients. Incidence, risk factors and predictors of outcome. J. Chemother. 16:172-178. [DOI] [PubMed] [Google Scholar]
- 8.Betts, R., A. Glasmacher, J. Maertens, G. Maschmeyer, J. A. Vazquez, H. Teppler, A. Taylor, R. Lupinacci, C. Sable, and N. Kartsonis. 2006. Efficacy of caspofungin against invasive Candida or invasive Aspergillus infections in neutropenic patients. Cancer 106:466-473. [DOI] [PubMed] [Google Scholar]
- 9.Bodey, G. P., M. Mardani, H. A. Hanna, M. Boktour, J. Abbas, E. Girgawy, R. Y. Hachem, D. P. Kontoyiannis, and I. I. Raad. 2002. The epidemiology of Candida glabrata and Candida albicans fungemia in immunocompromised patients with cancer. Am. J. Med. 112:380-385. [DOI] [PubMed] [Google Scholar]
- 9a.Buell, D., L. Kovanda, T. Drake, and C. Frisco. 2005. Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-79.
- 10.Clemons, K. V., M. Espiritu, R. Parmar, and D. A. Stevens. 2006. Assessment of the paradoxical effect of caspofungin in therapy of candidiasis. Antimicrob. Agents Chemother. 50:1293-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. 1997. Clinical and Laboratory Standards Institute reference method for dilution antifungal susceptibility testing of yeast; approved standards. Clinical and Laboratory Standards Institute, Wayne, PA.
- 12.Craig, W. A. 2003. Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect. Dis. Clin. N. Am. 17:479-501. [DOI] [PubMed] [Google Scholar]
- 13.Deziel, M. R., H. Heine, A. Louie, M. Kao, W. R. Byrne, J. Basset, L. Miller, K. Bush, M. Kelly, and G. L. Drusano. 2005. Effective antimicrobial regimens for use in humans for therapy of Bacillus anthracis infections and postexposure prophylaxis. Antimicrob. Agents Chemother. 49:5099-5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drusano, G. L. 2004. Antimicrobial pharmacodynamics: critical interactions of ‘bug and drug’. Nat. Rev. Microbiol. 2:289-300. [DOI] [PubMed] [Google Scholar]
- 15.Eagle, H., R. Fleischman, and A. D. Musselman. 1950. Effect of schedule of administration on the therapeutic efficacy of penicillin; importance of the aggregate time penicillin remains at effectively bactericidal levels. Am. J. Med. 9:280-299. [DOI] [PubMed] [Google Scholar]
- 16.Fatkenheuer, G., D. Buchheidt, O. A. Cornely, H. G. Fuhr, M. Karthaus, J. Kisro, M. Leithauser, H. Salwender, T. Sudhoff, H. Szelenyi, and F. Weissinger. 2003. Central venous catheter (CVC)-related infections in neutropenic patients-guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO). Ann. Hematol. 82(Suppl. 2):S149-S157. [DOI] [PubMed] [Google Scholar]
- 17.Gumbo, T., R. F. Chemaly, C. M. Isada, G. S. Hall, and S. M. Gordon. 2002. Late complications of Candida (Torulopsis) glabrata fungemia: description of a phenomenon. Scand. J. Infect. Dis. 34:817-818. [DOI] [PubMed] [Google Scholar]
- 18.Gumbo, T., G. L. Drusano, W. Liu, L. Ma, M. R. Deziel, M. F. Drusano, and A. Louie. 2006. Anidulafungin pharmacokinetics and microbial response in neutropenic mice with disseminated candidiasis. Antimicrob. Agents Chemother. 50:3695-3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gumbo, T., C. M. Isada, G. Hall, M. T. Karafa, and S. M. Gordon. 1999. Candida glabrata fungemia. Clinical features of 139 patients. Medicine (Baltimore) 78:220-227. [DOI] [PubMed] [Google Scholar]
- 19a.Gumbo, T., W. Liu, C. Fregeau, V. Hsu, G. L. Drusano, and A. Louie. 2006. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-0310a.
- 19b.Gumbo, T., A. Louie, W. Liu, M. R. Deziel, M. Drusano, L. Turner, C. Fregeau, D. Brown, and G. L. Drusano. 2004. Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-1875.
- 20.Hiemenz, J., P. Cagnoni, D. Simpson, S. Devine, N. Chao, J. Keirns, W. Lau, D. Facklam, and D. Buell. 2005. Pharmacokinetic and maximum tolerated dose study of micafungin in combination with fluconazole versus fluconazole alone for prophylaxis of fungal infections in adult patients undergoing a bone marrow or peripheral stem cell transplant. Antimicrob. Agents Chemother. 49:1331-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kartsonis, N., J. Killar, L. Mixson, C. M. Hoe, C. Sable, K. Bartizal, and M. Motyl. 2005. Caspofungin susceptibility testing of isolates from patients with esophageal candidiasis or invasive candidiasis: relationship of MIC to treatment outcome. Antimicrob. Agents Chemother. 49:3616-3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laverdiere, M., R. G. Lalonde, J. G. Baril, D. C. Sheppard, S. Park, and D. S. Perlin. 2006. Progressive loss of echinocandin activity following prolonged use for treatment of Candida albicans oesophagitis. J. Antimicrob. Chemother. 57:705-708. [DOI] [PubMed] [Google Scholar]
- 23.Leary, R., R. Jellife, A. Schumitzky, and M. Van Guilder. 2001. An adaptive grid non-parametric approach to pharmacokinetic and dynamic (PK/PD) models. IEEE Computer Society, Bethesda, MD.
- 24.Louie, A., M. Deziel, W. Liu, M. F. Drusano, T. Gumbo, and G. L. Drusano. 2005. Pharmacodynamics of caspofungin in a murine model of systemic candidiasis: importance of persistence of caspofungin in tissues to understanding drug activity. Antimicrob. Agents Chemother. 49:5058-5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mora-Duarte, J., R. Betts, C. Rotstein, A. L. Colombo, L. Thompson-Moya, J. Smietana, R. Lupinacci, C. Sable, N. Kartsonis, and J. Perfect. 2002. Comparison of caspofungin and amphotericin B for invasive candidiasis. N. Engl. J. Med. 347:2020-2029. [DOI] [PubMed] [Google Scholar]
- 26.Ostrosky-Zeichner, L., D. Kontoyiannis, J. Raffalli, K. M. Mullane, J. Vazquez, E. J. Anaissie, J. Lipton, P. Jacobs, J. H. van Rensburg, J. H. Rex, W. Lau, D. Facklam, and D. N. Buell. 2005. International, open-label, noncomparative, clinical trial of micafungin alone and in combination for treatment of newly diagnosed and refractory candidemia. Eur. J. Clin. Microbiol. Infect. Dis. 24:654-661. [DOI] [PubMed] [Google Scholar]
- 27.Ostrosky-Zeichner, L., J. H. Rex, P. G. Pappas, R. J. Hamill, R. A. Larsen, H. W. Horowitz, W. G. Powderly, N. Hyslop, C. A. Kauffman, J. Cleary, J. E. Mangino, and J. Lee. 2003. Antifungal susceptibility survey of 2,000 bloodstream Candida isolates in the United States. Antimicrob. Agents Chemother. 47:3149-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pappas, P. G., J. H. Rex, J. D. Sobel, S. G. Filler, W. E. Dismukes, T. J. Walsh, and J. E. Edwards. 2004. Guidelines for treatment of candidiasis. Clin. Infect. Dis. 38:161-189. [DOI] [PubMed] [Google Scholar]
- 29.Pfaller, M. A., D. J. Diekema, L. Boyken, S. A. Messer, S. Tendolkar, R. J. Hollis, and B. P. Goldstein. 2005. Effectiveness of anidulafungin in eradicating Candida species in invasive candidiasis. Antimicrob. Agents Chemother. 49:4795-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polderman, K. H., and A. R. Girbes. 2002. Central venous catheter use. Part 1: mechanical complications. Intensive Care Med. 28:1-17. [DOI] [PubMed] [Google Scholar]
- 31.Polderman, K. H., and A. R. Girbes. 2002. Central venous catheter use. Part 2: infectious complications. Intensive Care Med. 28:18-28. [DOI] [PubMed] [Google Scholar]
- 31a.Reboli, A., C. Rotstein, P. G. Pappas, J. Schranz, D. Krause, and T. Walsh. 2005. Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother. abstr. M-718.
- 32.Sirohi, B., R. L. Powles, R. Chopra, N. Russell, J. L. Byrne, H. G. Prentice, M. Potter, and S. Koblinger. 2006. A study to determine the safety profile and maximum tolerated dose of micafungin (FK463) in patients undergoing haematopoietic stem cell transplantation. Bone Marrow Transplant. 38:47-51. [DOI] [PubMed] [Google Scholar]
- 33.Tacconelli, E., M. Tumbarello, D. K. de Gaetano, S. Bertagnolio, M. Pittiruti, F. Leone, G. Morace, and R. Cauda. 2000. Morbidity associated with central venous catheter-use in a cohort of 212 hospitalized subjects with HIV infection. J. Hosp. Infect. 44:186-192. [DOI] [PubMed] [Google Scholar]
- 34.Wenzel, R. P. 1995. Nosocomial candidemia: risk factors and attributable mortality. Clin. Infect. Dis. 20:1531-1534. [DOI] [PubMed] [Google Scholar]
- 35.Wiederhold, N. P., D. P. Kontoyiannis, J. Chi, R. A. Prince, V. H. Tam, and R. E. Lewis. 2004. Pharmacodynamics of caspofungin in a murine model of invasive pulmonary aspergillosis: evidence of concentration-dependent activity. J. Infect. Dis. 190:1464-1471. [DOI] [PubMed] [Google Scholar]
- 36.Wingard, J. R., P. Kubilis, L. Lee, G. Yee, M. White, L. Walshe, R. Bowden, E. Anaissie, J. Hiemenz, and J. Lister. 1999. Clinical significance of nephrotoxicity in patients treated with amphotericin B for suspected or proven aspergillosis. Clin. Infect. Dis. 29:1402-1407. [DOI] [PubMed] [Google Scholar]
- 37.Yamato, Y., H. Kanedo, T. Hashimoto, M. Katashima, K. Ishibashi, A. Kawamura, M. Terakawa, and A. Kagayama. 2002. Pharmacokinetics of the antifungal drug micafungin in mice, rats, and dogs and in its in vitro protein binding and distribution to blood cells. Jpn. J. Chemother. 50:74-79.