Abstract
Quinolone-induced chondrotoxicity in juvenile rats and multiple other species has been demonstrated previously. Identical damages can be induced in immature rats by feeding them a magnesium-deficient diet. The objective of the present study was to investigate whether, in reverse, oral supplementation with magnesium, vitamin E, or both can diminish the typical quinolone-induced arthropathy in juvenile Wistar rats. Four groups of 12 (6 male, 6 female) 24-day-old Wistar rats were each fed either normal feed (group A), a vitamin E-enriched diet (group B), a magnesium-enriched diet (group C), or a diet enriched with both vitamin E and magnesium (group D) for 10 days. All rats received two subcutaneous ciprofloxacin doses of 600 mg/kg of body weight on postnatal day 32. Two days later, the rats were sacrificed and cartilage samples from knee joints were examined under a light microscope for the presence of typical quinolone-induced joint cartilage lesions. In addition, magnesium, calcium, and vitamin E concentrations in cartilage and plasma were determined. In the samples from rats fed a normal diet (group A), 17 quinolone-induced joint cartilage lesions were observed. In groups fed an enriched diet, the incidence of specific lesions (n) was significantly lower: group B, n = 10 (41% reduction compared to the incidence for group A; P < 0.05); group C, n = 6 (65% reduction; P < 0.01); and group D, n = 3 (82% reduction; P < 0.01). In comparison to the standard diet, diets with magnesium and vitamin E supplementation resulted in significantly higher magnesium and vitamin E concentrations in plasma and articular cartilage. Supplementation with magnesium and vitamin E alone or in combination may relevantly diminish joint cartilage lesions induced by quinolones in immature rats, with an additive effect of combined supplementation. The data further support the proposed pathomechanism of quinolone-induced arthropathy and the crucial role of magnesium in immature joint cartilage.
Quinolones are widely used in clinical practice and are generally well tolerated. However, they are contraindicated for children and adolescents and during pregnancy and nursing due to the potential for joint cartilage lesions as found in immature animals (22, 23). Quinolone-induced chondrotoxicity in juvenile animals of multiple species has been demonstrated and shown to be irreversible under experimental conditions (5, 24), dose dependent (19), and developmental phase (age) specific (22, 23, 26). The relevance of those observations for humans is still unclear (3).
A review of quinolone use in children revealed no cases of arthropathy. The vast majority of these patients were treated with ciprofloxacin, and the authors of a previous report discussed the possibility that pefloxacin may be an exception to these findings (1). Recent experience with gatifloxacin administered to children with acute severe otitis media showed that this quinolone seems to be safe for children (13). On the other hand, some clinical case reports describe severe arthropathy in humans also and an extensive number of publications indicate that tendon disorders as a result of the toxic quinolone effects on connective tissue structures can be induced by quinolones under therapeutic conditions (12, 22).
A precise molecular mechanism for the induction of tendon or cartilage lesions by quinolones has not yet been identified, but there is some evidence that free-radical formation and oxidative stress may play a role (16). Pretreatment with the scavenger vitamin E can afford protection against such reactions in vitro (6). The formation of free radicals seems to be mediated via the lack of functionally available magnesium in joint cartilage as a result of the chelation of magnesium ions by quinolones. This idea is supported by several observations; for example, the joint cartilage lesions in juvenile rats induced either by quinolone treatment or by feeding with a magnesium-deficient diet were histologically identical (17). Treatment with low doses of quinolones, which are not chondrotoxic in control rats, acts synergistically with a mild, diet-induced magnesium deficiency (11). The developmental phases most sensitive for the induction of those lesions seem to be identical for both treatments (26).
Considering the role of free-radical formation and magnesium deficiency in quinolone-induced arthropathy, it is of special interest whether supplementation with magnesium and vitamin E may diminish the chondrotoxic effect of quinolones. Supplementation with magnesium can reduce the toxic effects of quinolones on chondrocytes in vitro (4). Preliminary data from our laboratory indicated that the administration of magnesium can reduce the incidence of quinolone-induced joint cartilage lesions in juvenile rats. Combined oral magnesium and vitamin E supplementation prevents quinolone-induced cartilage lesions in rats (21).
The objective of the present study was to investigate whether ciprofloxacin-induced chondrotoxicity in juvenile Wistar rats can be diminished by oral supplementation with either magnesium, vitamin E, or a combination. Magnesium and calcium concentrations in plasma and cartilage as well as vitamin E concentrations in plasma were determined in order to investigate specific concentration changes associated with different diet regimes.
MATERIALS AND METHODS
Animals and treatment.
Male and female 24-day-old Wistar rats were housed under controlled conditions in Macrolon cages at 21 ± 1°C with 50% ± 5% relative humidity and a constant light-dark schedule (light, 9 a.m. to 9 p.m.). Food and tap water was provided ad libitum. Four groups of 12 (6 male, 6 female) rats each were fed for 10 days (days 24 to 34) according to the following allocation: group A, normal feed (Altromin 1324 with 0.1% magnesium and 75 mg of vitamin E/kg; Altromin, Lage, Germany), and group B, vitamin E (alpha-tocopherol-acetate)-enriched diet (0.3% vitamin E); group C, magnesium-enriched diet (0.5% magnesium); and group D, vitamin E- and magnesium-enriched diet (0.5% magnesium and 0.3% vitamin E; all from Sniff, Soest, Germany). All 48 rats received ciprofloxacin hydrochloride on day 32 as two subcutaneous injections of 600 mg/kg of body weight administered 8 h apart. The injection solution was prepared using ciprofloxacin pure substance solved in Ciprobay 400 solution for infusion (Bayer Vital, Leverkusen, Germany) with a final ciprofloxacin strength of 20 mg/ml (corresponding to an injection volume of 30 ml/kg).
The animals were examined for clinical abnormalities including motility alterations and weighed during the treatment period. Rats were sacrificed by decapitation on day 34, and tissue samples were collected immediately as follows. Blood was collected and centrifuged to separate plasma. Cartilage samples from the right femoral hip joint were excoriated, rinsed in phosphate-buffered saline buffer, and dried, and sample weights were documented. Plasma and cartilage samples were frozen at −25°C until analysis. The knee joint of the right hind limb was removed, rinsed with phosphate-buffered saline buffer, and fixed in 10% formalin.
The research procedures have complied with all relevant guidelines. Animal tests were approved by the local authority.
Histopathology.
Joint samples were decalcified (EDTA solution, pH 7.4; Serva, Heidelberg, Germany), dehydrated in alcohol series, and embedded in paraffin (Paraplast Monoject Scientific, Kildare, Ireland). Sagittal sections of 7 μm were cut with a microtome (model no. 1140; Reichert-Jung, Heidelberg, Germany) and stained with 1% toluidine blue (Merck, Darmstadt, Germany). For each knee, 40 to 50 serial sections from the predilection sites were prepared and examined under a light microscope (Axiophot; Zeiss, Jena, Germany) and microscopic findings were documented on photographs.
The presence of typical quinolone-induced joint cartilage lesions (matrix swelling, cleft formation, the loss of chondrocytes and proteoglycans, and decreased matrix staining) as described before in detail (17, 20) as well as the lengths of those lesions on either femoral or tibial knee joint cartilage was documented and evaluated.
Analysis of magnesium, calcium, and vitamin E concentrations.
Magnesium and calcium concentrations in plasma and cartilage were determined by means of atomic absorption spectrophotometry (TYE Unicam SP9; Philips). Before analysis, cartilage from the femoral hip joint was lyophilized, cleansed, dryly incinerated to ash (at 110°C with a plasma processor 200-E; Technics, Munich, Germany), and diluted in 0.175% lanthanum solution. Plasma samples were deproteinized with 0.175% lanthanum-0.5% trichloroacetic acid solution. Vitamin E concentrations in plasma were determined using a fluorescence spectrophotometry method after hexane fluid-fluid extraction.
Data analysis.
Results were statistically presented as means ± standard deviations (SD). To calculate the statistical significance of results among treatment groups and the control, Pearson's chi-square test (comparison of numbers of cartilage lesions), Fisher's exact test (comparison of numbers of animals with lesions), and the two-sided Dunnett t test after a one-way analysis of variance (ANOVA; comparison of electrolyte and vitamin E concentrations) were employed using the statistical software SPSS 12.0 (SPSS Inc.) and StatXact 6.1 (Cytel Software Corp.). Statistical significance was assumed for P values of <0.05.
RESULTS
All 48 rats grew normally, and body weight increased continuously without relevant differences among groups maintained under the four diet regimes. Neither clinical abnormalities, gait alterations, nor other restrictions in the motility of the rats were observed.
Histopathology.
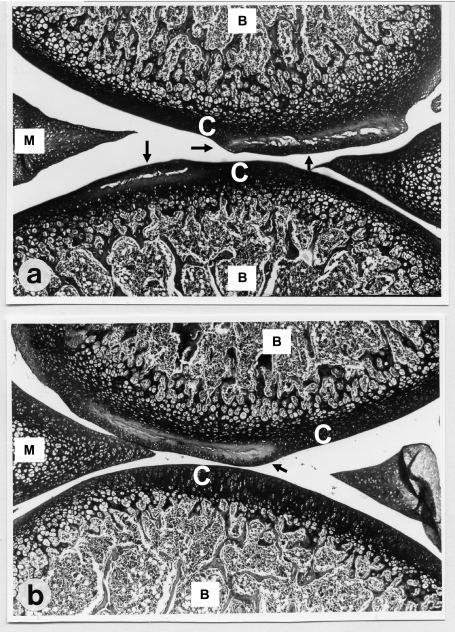
Typical quinolone-induced joint cartilage lesions as described before in detail (17, 20) were found in all four groups, with matrix swelling, cleft formation, the loss of chondrocytes, and reduced stainability (loss of proteoglycans). The histological appearance of lesions was not affected by diet supplementation with magnesium or vitamin E (Fig. 1).
FIG. 1.
Light microscopy image (magnification, ×100) of toluidine blue-stained articular cartilage (C, cartilage; B, bone) from knee joints of immature Wistar rats (34 days old) after subcutaneous administration of two doses of 600 mg of ciprofloxacin/kg of body weight to rats maintained under different diet regimes. Femoral and tibial parts as well as menisci (M) are recognizable. (a) Cartilage from rats fed a normal diet. Large lesions with cleft formations (arrows) in the tibial and femoral cartilage are present. (b) Cartilage from rats fed a diet with vitamin E and magnesium supplementation. Cleft formation is limited to the femoral cartilage (arrow); the tibial cartilage shows no pathological alterations, and its morphology is equivalent to that in untreated rats (17).
Altogether, 36 lesions were observed in samples from 30 of the 48 rats treated, 6 lesions on tibial joint cartilage and 30 lesions on femoral joint cartilage, after the administration of two subcutaneous ciprofloxacin injections. The incidence of knee joint cartilage lesions among all groups is summarized in Table 1. Among the 12 animals of group A, fed a normal diet, 17 cartilage lesions were found in 11 rats; six animals exhibited two lesions each, and five rats exhibited one lesion each (Fig. 2). In the groups fed a magnesium- or vitamin E-supplemented diet, both the number of lesions and the number of animals affected were lower than those in group A; 10 lesions occurred in 10 rats of group B (vitamin E-enriched diet), and six lesions occurred in six rats of group C (magnesium-enriched diet). These incidences correspond to a reduction of 41% (P < 0.05) for group B and 65% (P < 0.01) for group C compared to group A. Among animals of group D, which received a diet supplemented with both magnesium and vitamin E, the lowest incidence of cartilage lesions (three lesions in three rats) was observed. The number of lesions was reduced by 82% (P < 0.01) and the number of rats with lesions by 73% (P < 0.01) compared to those in group A (standard diet). The incidence of cartilage lesions and the number of animals affected were also significantly lower in group D (double supplementation) than in group B (vitamin E supplementation only; P < 0.05).
TABLE 1.
Incidence and lengths of tibial and femoral joint cartilage lesions and numbers of rats with lesionsa
| Group | No. of lesionsb
|
Length (mean ± SD) of lesion(s) (mm)
|
No. of rats with lesion(s)
|
|||||
|---|---|---|---|---|---|---|---|---|
| Tibial | Femoral | Total | Tibial | Femoral | Tibial | Femoral | Total | |
| A, standard diet | 4 | 13 | 17 | 0.72 ± 0.47 | 1.81 ± 0.57 | 4 | 11 | 11 |
| B, vitamin E-enriched diet | 0 | 10 | 10c | 1.53 ± 0.36 | 0 | 10 | 10 | |
| C, Mg2+-enriched diet | 1 | 5 | 6d | (3.02) | 0.87 ± 0.34d | 1 | 5 | 6 |
| D, vitamin E- and Mg2+-enriched diet | 1 | 2 | 3d,e | (1.07) | 1.04 ± 0.34c | 1 | 2 | 3f,g |
Data were determined after the subcutaneous administration of two doses of 600 mg of ciprofloxacin/kg of body weight to immature Wistar rats maintained under four different diet regimes (n, 12 per group). Values in parentheses indicate results from a single rat.
Two femoral lesions were observed in two rats (group A); all other cases showed one lesion per tibial or femoral cartilage sample.
P, <0.05 for comparison with value for standard-diet group (A); Pearson's chi-square test.
P, <0.01 for comparison with value for standard-diet group (A); Pearson's chi-square test.
P, <0.05 for comparison with value for tocopherol-enriched-diet group (B); Pearson's chi-square test.
P, <0.01 for comparison with value for standard-diet group (A); Fisher's exact test.
P, <0.05 for comparison with value for tocopherol-enriched-diet group (B); Fisher's exact test.
FIG. 2.
Number of rats with a tibial or femoral joint cartilage lesion(s) after subcutaneous administration of two doses of 600 mg of ciprofloxacin/kg of body weight to rats maintained under four different diet regimes (n, 12 immature Wistar rats per group).
The mean lengths of cartilage lesions were significantly lower for rats receiving a diet supplemented with magnesium (group C) and magnesium and vitamin E (group D) than for rats receiving a standard diet (group A) (Table 1).
Concentrations of magnesium, calcium, and vitamin E.
The results of the mineral and vitamin E analyses are summarized in Table 2. The magnesium concentration in plasma (mean ± SD) was 0.83 ± 0.10 mmol/liter for rats fed a standard diet (group A) and 0.88 ± 0.08 mmol/liter for rats fed a vitamin E-enriched diet (group B). Plasma magnesium levels were significantly higher in the rats fed a magnesium-enriched diet (group C, 1.28 ± 0.23 mmol/liter; P < 0.01) as well as in the rats fed a magnesium- and vitamin E-enriched diet (group D, 1.15 ± 0.20 mmol/liter; P < 0.01) than in the rats fed the standard diet. The magnesium concentrations in articular cartilage, 33.4 ± 6.4 mmol/kg (dry weight) for group C and 35.5 ± 10.4 mmol/kg for group D, were significantly (P, <0.05 for both groups) higher for rats receiving the magnesium- and magnesium- and vitamin E-enriched diets than for rats receiving the standard diet (group A, 27.7 ± 6.7 mmol/kg).
TABLE 2.
Magnesium, calcium, and tocopherol concentrations in plasma and cartilage after subcutaneous administration of two doses of 600 mg of ciprofloxacin/kg of body weight to rats maintained under four different diet regimesa
| Group | Magnesium concn in:
|
Calcium concn in:
|
Tocopherol concn in plasma (nmol/ml) | ||
|---|---|---|---|---|---|
| Plasma (mmol/liter) | Cartilage (mmol/kg, dry wt) | Plasma (mmol/liter) | Cartilage (mmol/kg, dry wt) | ||
| A, standard diet | 0.83 ± 0.10 | 27.7 ± 6.7 | 2.74 ± 0.18 | 683 ± 305 | 93.6 ± 21.1 |
| B, vitamin E-enriched diet | 0.88 ± 0.08 | 24.7 ± 4.2 | 2.77 ± 0.19 | 613 ± 333 | 146 ± 27.4c |
| C, Mg2+-enriched diet | 1.28 ± 0.23c | 33.3 ± 6.4 | 2.79 ± 0.11 | 595 ± 317 | 88.3 ± 21.8 |
| D, vitamin E- and Mg2+-enriched diet | 1.15 ± 0.20c | 35.5 ± 10.4b | 2.81 ± 0.11 | 763 ± 449 | 158 ± 25.8c |
Values are means ± SDs for 12 34-day-old Wistar rats per group.
P, <0.05 for comparison with value for standard-diet group (A); Dunnett t test (ANOVA).
P, <0.01 for comparison with value for standard-diet group (A); Dunnett t test (ANOVA).
No significant differences in calcium concentrations either in plasma or in cartilage tissue among samples from the four diet groups were observed.
Plasma tocopherol concentrations, 146 ± 27.4 nmol/ml for group B and 158 ± 25.8 nmol/ml for group D, were significantly (P, <0.01 for both groups) higher for rats receiving vitamin E or magnesium and vitamin E supplementation than for rats receiving the standard diet (group A, 93.6 ± 21.1 nmol/ml). No significant difference between group A and group C (magnesium supplementation; 88.3 ± 21.8 nmol/ml) was observed.
DISCUSSION
The toxic effect of quinolones on joint cartilage in the growing mammalian organism has been demonstrated with multiple species (5, 8, 10, 17, 18), but experiments designed to elucidate the mechanism of this unusual form of toxicity are rare. Quinolones accumulate in bone or cartilage tissue, revealing significantly higher concentrations in these samples than in plasma, and it has been hypothesized that due to their chelating activity they induce a lack of functionally available magnesium (7, 15, 19). It is well-known that magnesium is an essential mineral that is needed for numerous physiological functions. Changes in magnesium homeostasis concern mainly the extracellular space, as the intracellular magnesium concentration is well regulated and conserved (25).
The present study investigated the effect of two subcutaneous ciprofloxacin injections of 600 mg/kg in 34-day-old Wistar rats, fed either a standard diet or diets enriched with magnesium or vitamin E or both. In the group receiving standard feed, altogether 17 joint cartilage lesions in 11 of 12 (92%) rats were induced by the two doses of 600 mg of ciprofloxacin/kg. This result corresponded well with findings revealed from a previous experiment by our group, in which an incidence of 16 cartilage lesions in 11 of 12 rats treated with ciprofloxacin under similar conditions was found (21). In corresponding animal studies with ofloxacin, the following incidences were observed: two doses of ofloxacin at 600 mg/kg, 96% (5); single ofloxacin doses of 300, 600, and 1,200 mg/kg, 0%, 71%, and 100%, respectively (17).
In a study with daily intragastric administration of 400, 800, and 1,200 mg of ciprofloxacin/kg to 4-week-old rats for 7 consecutive days, doses of 800 and 1,200 but not 400 mg of ciprofloxacin/kg induced severe cartilage lesions, such as matrix swelling and the loss of chondrocytes. The thickness of cartilage of the femoral condyle in groups receiving ciprofloxacin doses of 800 and 1,200 mg/kg was significantly decreased compared to that in controls (10).
In previous studies, it was shown that in rats a magnesium-deficient diet can induce joint cartilage lesions that are identical to quinolone-induced cartilage lesions (17). The reduction of the magnesium concentration in joint cartilage via the chelating properties of quinolones was discussed as the underlying mechanism. Thus, it is of special interest whether, in reverse, supplementation with magnesium may diminish the chondrotoxic effect of quinolones in vivo. The promoting effect of vitamin E on cell proliferation and its ability to prevent the propagation of free-radical damage in biological membranes are well known. In an in vitro experiment, vitamin E provided a significant protective effect against the cytotoxicity as well as the lipid peroxidation induced by ciprofloxacin in fibroblast cells (6). Considering the role of free-radical formation in the quinolone-induced arthropathy as indicated by the data published by Simonin and colleagues (16), it was expected that supplementation with the scavenger vitamin E should also be beneficial in reducing the toxic effects of quinolones in immature animals.
In the present study, the number of ciprofloxacin-induced joint cartilage lesions was significantly reduced in the animals receiving a diet supplemented with vitamin E or magnesium or both for 10 days. The effects on lesion incidence of the diets with single supplementation (vitamin E, 41% reduction; magnesium, 65% reduction) were shown to be additive by the results for the group receiving combined supplementation (vitamin E plus magnesium, 82% reduction). The reduction of ciprofloxacin-induced joint lesions after combined magnesium-vitamin E supplementation for 10 days was higher than that in a previous experiment by our group with a 7-day supplementation, in which the incidence of those lesions was reduced by 56% only (21). In this previous study as well as in the present experiment, we used different routes of application for the quinolones and the magnesium (subcutaneous and oral) to avoid pharmacokinetic interactions.
The supplementation of the rats' diets with vitamin E and magnesium for 10 days resulted in magnesium concentrations in plasma and cartilage as well as tocopherol concentrations in plasma that were significantly higher than those in samples from animals receiving the standard diet. No difference in calcium concentrations among the groups was observed. In samples from animal groups fed a magnesium- or magnesium- and vitamin E-enriched diet for 10 days, mean magnesium concentrations were up to 54% (plasma) and 28% (cartilage) higher than magnesium concentrations in samples from animals fed a standard diet. For animals fed a vitamin E- or vitamin E- and magnesium-enriched diet, mean tocopherol concentrations in plasma were up to 67% higher than those for animals fed a standard diet.
The characteristic increases in magnesium and vitamin E concentrations in plasma and cartilage and the decreased incidence of ciprofloxacin-induced joint cartilage lesions after supplementation with magnesium and vitamin E support the suggested role of a (latent) magnesium deficiency in the pathogenesis of quinolone-induced cartilage lesions in rats. Magnesium supplementation seems to counterbalance effectively the magnesium deficiency in the extracellular matrix of cartilage initially induced by the formation of quinolone-magnesium chelates. Vitamin E inhibits lipid peroxidation and protects chondrocytes from oxidative stress (27). Thus, the consequences of a magnesium deficiency—increased lipid peroxidation, the formation of free radicals in several tissues, and a decrease in the content of the antioxidant vitamin E—may be diminished or even compensated for by either magnesium, vitamin E, or both.
The relevance of data regarding quinolone-induced arthropathy from animal studies for humans is still unclear. Rare but well-documented cases of joint damage in children treated with quinolones have been reported. The few pediatric trials for which data are available included too few patients to identify rare adverse effects on joints or to show a causal relationship between treatment and joint damage (2, 13, 14). The chondrotoxic potentials of different quinolones obviously differ, probably due to differences in pharmacokinetic behaviors as well as different affinities for magnesium (9). The highest incidence of arthropathy in humans reported to date is 14% in patients with cystic fibrosis treated with pefloxacin, whereas in a similar group of juvenile patients treated with ofloxacin, no such findings were observed (12).
In summary, the present study provides data indicating that supplementation with magnesium and vitamin E alone or in combination may relevantly diminish joint cartilage lesions induced by quinolones in immature rats. An additive effect of combined supplementation with magnesium and vitamin E was observed. The data further support the proposed pathomechanism of quinolone-induced arthropathy and its sequence with the lack of functionally available magnesium caused by chelation, which leads to increased oxidative stress and the characteristic destruction of cartilage.
Acknowledgments
We are indebted to S. Kühner, E. Lozo, C. Förster, and I. Baumann-Wilschke for their support.
Footnotes
Published ahead of print on 8 January 2007.
REFERENCES
- 1.Burkhardt, J. E., J. N. Walterspiel, and U. B. Schaad. 1997. Quinolone arthropathy in animals versus children. Clin. Infect. Dis. 25:1196-1204. [DOI] [PubMed] [Google Scholar]
- 2.Chysky, V., K. Kapila, R. Hullmann, G. Arcieri, P. Schacht, and R. Echols. 1992. Safety of ciprofloxacin in children: worldwide experience based on compassionate use. Emphasis on joint evaluation. Infection 19:289-296. [DOI] [PubMed] [Google Scholar]
- 3.Committee on Infectious Diseases. 2006. The use of systemic fluoroquinolones. Pediatrics 118:1287-1292. [DOI] [PubMed] [Google Scholar]
- 4.Egerbacher, M., B. Wolfesberger, and C. Gabler. 2001. In vitro evidence for effects of magnesium supplementation on quinolone-treated horse and dog chondrocytes. Vet. Pathol. 38:143-148. [DOI] [PubMed] [Google Scholar]
- 5.Foerster, C., K. Kociok, M. Shakibaei, H. J. Merker, and R. Stahlmann. 1996. Quinolone-induced cartilage lesions are not reversible in rats. Arch. Toxicol. 70:474-481. [DOI] [PubMed] [Google Scholar]
- 6.Guerbay, A., C. Garrel, M. Osman, M. J. Richard, A. Favier, and F. Hincal. 2002. Cytotoxicity in ciprofloxacin-treated human fibroblast cells and protection by vitamin E. Hum. Exp. Toxicol. 21:635-641. [DOI] [PubMed] [Google Scholar]
- 7.Kastner, M., U. Rahm, I. Baumann-Wilschke, A. Bello, and R. Stahlmann. 2004. Concentrations of the des-F(6)-quinolone garenoxacin in plasma and joint cartilage of immature rats. Arch. Toxicol. 78:61-67. [DOI] [PubMed] [Google Scholar]
- 8.Kato, M., and T. Onodera. 1988. Morphological investigation of cavity formation in articular cartilage induced by ofloxacin in rats. Fundam. Appl. Toxicol. 11:110-119. [DOI] [PubMed] [Google Scholar]
- 9.Lecomte, S., M. H. Baron, M. T. Chenon, C. Coupry, and M. J. Moreau. 1994. Effect of magnesium complexation by the fluoroquinolones on their antibacterial properties. Antimicrob. Agents Chemother. 38:2810-2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li, P., N. N. Cheng, B. Y. Chen, and Y. M. Wang. 2004. In vivo and in vitro chondrotoxicity of ciprofloxacin in juvenile rats. Acta Pharmacol. Sin. 25:1262-1266. [PubMed] [Google Scholar]
- 11.Lozo, E., K. Riecke, R. Schwabe, J. Vormann, and R. Stahlmann. 2002. Synergistic effect of ofloxacin and magnesium deficiency on joint cartilage in immature rats. Antimicrob. Agents Chemother. 46:1755-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pertuiset, E., G. Lenoir, M. Jehanne, F. Douchan, M. Guillot, and C. J. Menkes. 1989. Tolerance articulaire de la pefloxacin et de l'ofloxacin chez les enfants et adolescents attains de mucoviscidose. Rev. Rhum. 56:735-740. [PubMed] [Google Scholar]
- 13.Pichichero, M. E., A. Arguedas, R. Dagan, L. Sher, X. Saez-Llorens, K. Hamed, and R. Echols. 2005. Safety and efficacy of gatifloxacin therapy for children with recurrent acute otitis media (AOM) and/or AOM treatment failure. Clin. Infect. Dis. 41:470-478. [DOI] [PubMed] [Google Scholar]
- 14.Schaad, U. B., and J. Wedgwood. 1992. Lack of quinolone-induced arthropathy in children. J. Antimicrob. Chemother. 30:414-416. [DOI] [PubMed] [Google Scholar]
- 15.Schwabe, R., E. Lozo, I. Bauman-Wilschke, and R. Stahlmann. 1999. Chondrotoxicity and target tissue kinetics of ofloxacin in immature rats after multiple doses. Drugs 58:385-387. [Google Scholar]
- 16.Simonin, M. A., P. Gegout-Pottie, A. Minn, P. Gillet, P. Netter, and B. Terlain. 1999. Proteoglycan and collagen biochemical variations during fluoroquinolone-induced chondrotoxicity in mice. Antimicrob. Agents Chemother. 43:2915-2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stahlmann, R., C. Förster, M. Shakibaei, J. Vormann, T. Günther, and H. J. Merker. 1995. Magnesium deficiency induces joint cartilage lesions in juvenile rats which are identical to quinolone-induced arthropathy. Antimicrob. Agents Chemother. 39:2013-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stahlmann, R., J. Vormann, T. Günther, C. Förster, U. Zippel, E. Lozo, R. Schwabe, K. Kociok, M. Shakibaei, and H. J. Merker. 1997. Effects of quinolones, magnesium deficiency or zinc deficiency on joint cartilage in rats. Magnes. Bull. 19:7-22. [Google Scholar]
- 19.Stahlmann, R., and H. Lode. 1998. Safety overview—toxicity, adverse effects, and drug interactions, p. 369-415. In V. T. Andriole (ed.), The quinolones, 2nd ed. Academic Press, San Diego, CA.
- 20.Stahlmann, R., U. Zippel, C. Foerster, J. Vormann, T. Günther, and H. J. Merker. 1998. Chondrotoxicity and toxicokinetics of sparfloxacin in juvenile rats. Antimicrob. Agents Chemother. 42:1470-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stahlmann, R., R. Schwabe, K. Pfister, E. Lozo, M. Shakibaei, and J. Vormann. 1999. Supplementation with magnesium and tocopherol diminishes quinolone-induced chondrotoxicity in immature rats. Drugs 58(Suppl. 2):393-394. [Google Scholar]
- 22.Stahlmann, R. 2003. Effects on connective tissue structures, p. 441-449. In D. C. Hooper and E. Rubinstein (ed.), Quinolone antimicrobial agents, 3rd ed. ASM Press, Washington, DC.
- 23.Stahlmann. R. 2003. Children as a special population at risk—quinolones as an example for xenobiotics exhibiting skeletal toxicity. Arch. Toxicol. 77:7-11. [DOI] [PubMed] [Google Scholar]
- 24.von Keutz, E., C. Ruhl-Fehlert, W. Drommer, and M. Rosenbruch. 2004. Effects of ciprofloxacin on joint cartilage in immature dogs immediately after dosing and after a 5-month treatment-free period. Arch. Toxicol. 78:418-424. [DOI] [PubMed] [Google Scholar]
- 25.Vormann, J. 2003. Magnesium: nutrition and metabolism. Mol. Aspects Med. 24:27-37. [DOI] [PubMed] [Google Scholar]
- 26.Vormann, J., C. Foerster, U. Zippel, E. Lozo, T. Günther, H. J. Merker, and R. Stahlmann. 1997. Effects of magnesium deficiency and calcium content in bone and cartilage in developing rats in correlation to chondrotoxicity. Calcif. Tissue Int. 61:230-238. [DOI] [PubMed] [Google Scholar]
- 27.Watkins, B. A., H. Xu, and J. J. Turek. 1996. Linoleate impairs collagen synthesis in primary cultures of avian chondrocytes. Proc. Soc. Exp. Biol. Med. 212:153-159. [DOI] [PubMed] [Google Scholar]