Abstract
Coccidioidal meningitis (CM) is a devastating disease that requires long-term therapy and for which there is little hope of a cure. A model was used to compare the efficacies of itraconazole and fluconazole. CD-1 mice were infected intrathecally with 30 to 36 viable arthroconidia of Coccidioides. Oral therapy with cyclodextrin (control) or itraconazole or fluconazole at 10, 25, or 50 mg/kg of body weight twice daily (BID) was given for 12 days, from day 3 of infection. Treatment with both antifungals at all doses prolonged survival compared with that of the control treatment (P < 0.01 to 0.0001). At 50 mg/kg, itraconazole and fluconazole were equivalent, whereas itraconazole at 10 or 25 mg/kg prolonged survival compared to that achieved with fluconazole at these dosages (P < 0.05 and 0.01, respectively). Early histologic analysis (10 days of treatment) with 50 mg/kg BID itraconazole or fluconazole showed suppression of CM in all five animals per group; in quantitative cultures, three of three animals from each group had no detectable infection in the brain, spinal cord, or a site of secondary infection, the lungs. In contrast, four of seven controls showed mild to severe meningitis, with arteritis detected in three animals. In a short-term organ clearance study, 5 days of treatment with 10 or 50 mg/kg BID itraconazole or fluconazole reduced the tissue burdens in the brain and spinal cord compared to the tissue burdens in the controls (P < 0.02 to 0.0003). Fluconazole at 10 mg/kg did not reduce the fungal burden in secondary sites, the lungs and kidneys, whereas this itraconazole dose was more effective in clearing the fungi from both organs (P < 0.05 and P < 0.001, respectively). At 50 mg/kg, itraconazole and fluconazole were equivalent in clearing the fungi from the brain and kidney, but itraconazole was superior to fluconazole in clearing the fungi from the spinal cord and lungs (P < 0.05). Thus, both itraconazole and fluconazole were effective at controlling CM, but neither eliminated Coccidioides from tissues. Overall, itraconazole was more efficacious on an mg/kg basis; at high doses they were similarly effective.
Meningitis is the most severe form of coccidioidomycosis in humans and is associated with frequent relapses and high mortality (8, 9, 11, 14, 23, 33, 35, 41, 42). The traditional treatment for coccidioidal meningitis (CM) had been administration of amphotericin B directly into the cerebrospinal fluid (CSF) by the lumbar or cisternal route or into the ventricles or other sites through a reservoir (9, 24, 35). However, this therapy is poorly tolerated and is plagued by toxic side effects, and lifelong treatment is often required.
Azoles have increasingly been used as alternative therapies for CM. Data on the efficacy of fluconazole (FCZ) in the treatment of CM have accumulated, and FCZ currently plays an integral role in the treatment (2, 4, 8, 12, 13, 28, 38). Itraconazole (ICZ) has also shown impressive activities in a small series of patients with refractory disease (37). However, to date no studies have compared FCZ and ICZ for the treatment of CM in humans, and this treatment would be difficult to study in humans because of the relative infrequency of the disease (11, 23, 33).
We have established a murine model of CM by introducing arthroconidia intrathecally (22). This model differs from those reported previously (7, 15, 27) in the method of central nervous system (CNS) infection and the form of the organism used for infection. The model mimics the disease in humans and appears to be appropriate for the testing of therapy. Although arthroconidia are not believed to access the CNS directly in human disease, promptly after challenge the arthroconidia convert to the parasitic form, as occurs in the natural pulmonary infection. In the present study we compared the efficacies of FCZ and ICZ in this new murine model.
MATERIALS AND METHODS
Animals.
Outbred male CD-1 mice (age, 9 to 10 weeks; average weight, 35 g at the start of the study) were purchased from Charles River Laboratory, Hollister, CA. They were housed in microisolator cages with five mice/cage and were provided sterilized food and acidified water ad libitum.
Antifungal agents.
ICZ powder was obtained from Janssen (Beerse, Belgium) and was suspended in 2-hydroxypropyl-β-cyclodextrin (CD) to produce a stock solution of 25 mg/ml, as described previously (19, 20). The stock solution was diluted (in sterile water) to the desired concentration with CD. FCZ was obtained from Pfizer (Groton, CT). The drug was suspended in sterile water for injection, according to the manufacturer's recommendation. Each drug was given orally twice daily (BID) in a volume 0.10 to 0.15 ml (the drug dose was adjusted to the body weight of the mice every 2 days) by gavage.
Organism.
Coccidioides immitis strain Silveira (ATCC 28868) was used in all studies. All work with the organism was performed in biocontainment facilities appropriate for the biohazard represented by this organism. A suspension of arthroconidia was prepared as reported previously (22, 43). Prior to use, the CFU in the stock was quantitated by cultures of serial dilutions on Mycosel (Difco, Detroit, MI) agar plates at 37°C for a minimum of 3 days. The MIC and minimum fungicidal concentration (MFC) for this organism are 0.78 and 1.56 μg/ml, respectively, for ICZ, and 6.25 and > 100 μg/ml, respectively, for FCZ (5).
Infection and treatment studies.
These animal studies were approved by the Institutional Animal Care and Use Committee of the California Institute for Medical Research. On the day of infection, the stock of arthroconidia was adjusted to the desired number with sterile saline and the number of CFU was confirmed by plating of the arthroconidia after all animals were infected, as described above. Mice were injected with 50 μl of the inoculum intrathecally, as described previously (22). In untreated mice, this produces a progressive lethal infection (an outcome identical to untreated human coccidioidal meningitis [9, 41]). There is late dissemination from the CNS to visceral organs. The pathology at these disseminated sites is minor in comparison to that in the CNS, even when the numbers of CFU/organ are comparable; this is understandable, in view of the consequences of infection on a small and critical organ, i.e., the brain (22).
High-dose studies.
Initially, the efficacies of ICZ and FCZ given at 50 mg/kg of body weight BID were compared. Thirty mice were randomized to three groups of 10 mice each and were challenged with 30 viable arthroconidia of C. immitis. Treatment with antifungal agents or CD (controls) was initiated on day 3 postinfection and was continued for 12 days. The mice were observed BID, and deaths were recorded until day 36 postinfection. Two days after the last treatment dose, five mice from each treatment group were randomly selected and euthanized by CO2 asphyxiation. A necropsy was performed immediately; and the brain, spinal cord, and lungs of each animal were removed aseptically and weighed. Portions of each organ were homogenized in 5 ml of saline with a Tissumizer (Tekmar Co., Cincinnati, OH); and dilutions of the homogenates were plated for determination of the number of viable CFU of C. immitis, after incubation at 37°C for a minimum of 3 days, as described previously (22) (sensitivity of the assay for all organs, 5 CFU/g).
Histologic studies.
Three groups of eight mice each were challenged in the same fashion described above and treated with CD, ICZ, or FCZ with the same dosage regimens, starting on day 3, as described above, for 10 days. One day after the last treatment dose, five mice from each group were randomly selected and euthanized as described above. Their brains and spinal cords were removed and placed in 10% buffered formalin for histologic studies, as described previously (22). The remaining mice from the treatment groups were examined for residual fungal burdens in the brain, spinal cord, and lungs by quantitative plating of organ homogenates. The brains and spinal cords of seven control mice that died prior to the study end point were saved in 10% buffered formalin for histologic analysis.
Escalating-dose studies.
To define the comparative efficacies of lower doses of the two drugs, the mice were again challenged with 36 viable arthroconidia of C. immitis. On day 3 postinfection, groups of 11 to 15 mice each were randomized to one of the following treatments: ICZ at 10 or 25 mg/kg, FCZ at 10 or 25 mg/kg, or the control treatment. All treatments were given BID for 12 days. Deaths were tallied through 28 days of infection. At the end of this period, all surviving mice were euthanized by CO2 asphyxiation and the residual tissue burdens were determined by quantitative plating of serially diluted organ homogenates, as described above.
Short-term organ clearance study.
A short-term organ clearance assay was also conducted to compare the efficacies of ICZ and FCZ at 10- or 50-mg/kg doses. The drugs were given for 5 days in the same fashion described above. The organs (brain, spinal cord, lungs, and kidneys) were removed 24 h after the last dose for determination of the number of viable CFU of C. immitis in the entire organ (sensitivity of the assay, 1 CFU/organ).
Statistical analyses.
For the survival studies, a log rank or Wilcoxon test was used. The tissue burdens were analyzed by the Mann-Whitney U test. For the analysis of the fungal burdens in the surviving animals, a value of 8 log10 CFU per organ was given to missing datum points (due to the death of the animal). This value represents the approximate number of CFU in each organ just before death, as determined from prior studies (22). This assignment also ensures that in a nonparametric rank test, death is designated as an outcome worse than survival with any amount of residual infection (25, 31).
RESULTS
High-dose study.
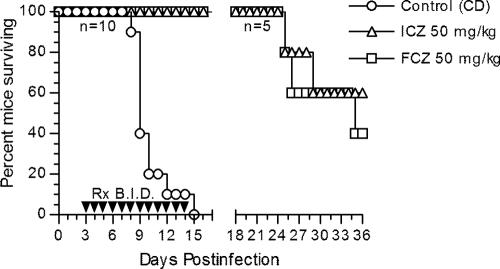
Most infected animals appeared normal through day 6, after which the control animals developed an overt acute disease characterized by generalized signs of illness (ruffled fur, weight loss, lethargy) and neurological abnormalities (ataxia, paresis, paralysis). Both ICZ and FCZ were effective in preventing many of the systemic and neurological signs. The therapeutic efficacies of the high dose (50 mg/kg BID) of ICZ and FCZ for the prolongation of survival against lethal coccidioidal meningitis are presented in Fig. 1. The control group had 100% mortality, with all deaths occurring between days 8 and 15 postinfection. In contrast, no deaths were observed in either of the treatment groups by day 16 (P < 0.001). On day 16, five mice from each treatment group were randomly selected and euthanized for the quantitation of tissue burdens. Within the ICZ group, two mice had positive brain tissue cultures (mean, 0.75 log10 CFU/g; 95% confidence interval, 0 to 2.21 log10 CFU/g) and three mice had positive spinal cord tissue cultures (mean, 1.73 log10 CFU/g; 95% confidence interval, 0 to 3.80 log10 CFU/g); lung tissue cultures were negative for all five mice. None of the five mice in the FCZ group had detectable infection in the three organs; this result was not significantly different from that for the ICZ group. Follow-up of the remaining mice in the treatment groups revealed two deaths in the ICZ group and three deaths in the FCZ group, which occurred at between 25 and 36 days postinfection, indicating that neither drug offered total protection at the dose tested (Fig. 1). Of the five survivors at day 36, none had residual brain infection; one of the three survivors in the ICZ group and one of the two survivors in the FCZ group had C. immitis-positive spinal cords.
FIG. 1.
Cumulative mortality of CD-1 mice infected intrathecally with 30 viable arthroconidia of C. immitis and given cyclodextrin (control), itraconazole, or fluconazole at the indicated doses. Therapies (Rx) were given BID by gavages on days 3 to 14 of infection. Five mice in each treatment group were randomly selected and euthanized on day 16 of infection for the quantitation of tissue burdens. n, numbers of mice per group; P < 0.001 (either treatment) versus the results for the control (log rank test).
Histopathologic studies and cultures.
Examination performed 10 days after treatment (day 13) revealed normal brains and spinal cords in all 10 treated animals examined (5 animals/group). The remaining mice from each treatment group (n = 3) had no detectable infection in the brain, spinal cord, or lungs, as demonstrated by quantitative tissue cultures. In contrast, four of seven control mice showed mild to severe meningitis, with meningeal vasculitis observed in three mice.
Escalating-dose study.
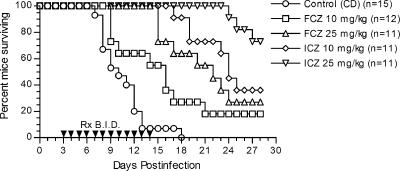
The survival results in a study comparing the lower doses of ICZ with those of FCZ are shown in Fig. 2. The control group had 100% mortality, with most deaths occurring between days 7 and 12 after infection. All treatment groups had significantly prolonged survival compared to that for the control group (P < 0.01 to P < 0.0001, depending on the comparison), and both antifungal agents showed a dose-dependent effect on the prolongation of survival. However, at the same doses, ICZ was superior to FCZ (P < 0.05 for ICZ versus FCZ at 10 mg/kg; P < 0.01 for ICZ versus FCZ at 25 mg/kg). Furthermore, ICZ at 10 mg/kg was equivalent to FCZ at 25 mg/kg (P = 0.3).
FIG. 2.
Cumulative mortality of CD-1 mice infected intrathecally with 36 viable arthroconidia of C. immitis and given cyclodextrin (control), itraconazole, or fluconazole at the indicated dosages. Therapies were given BID by gavages on days 3 to 14 of infection.
Enumeration of the fungal burden in the tissues of the surviving animals showed that only ICZ at 25 mg/kg significantly reduced the numbers of CFU in the brain, spinal cord, and lungs compared with the numbers of CFU in the control group (P < 0.001) (Fig. 3). This dose of ICZ was superior to FCZ given at the same dose in all organs examined (P < 0.05 to P < 0.005). Three of 11 mice in the 25-mg/kg ICZ group had no detectable infection in any of the three organs studied, whereas 0 of 11 mice in the 25-mg/kg FCZ group had no detectable infection in any of the three organs studied. ICZ at 10 mg/kg and FCZ at 10 or 25 mg/kg had minimal efficacy in clearing fungi from the organs of surviving animals.
FIG. 3.
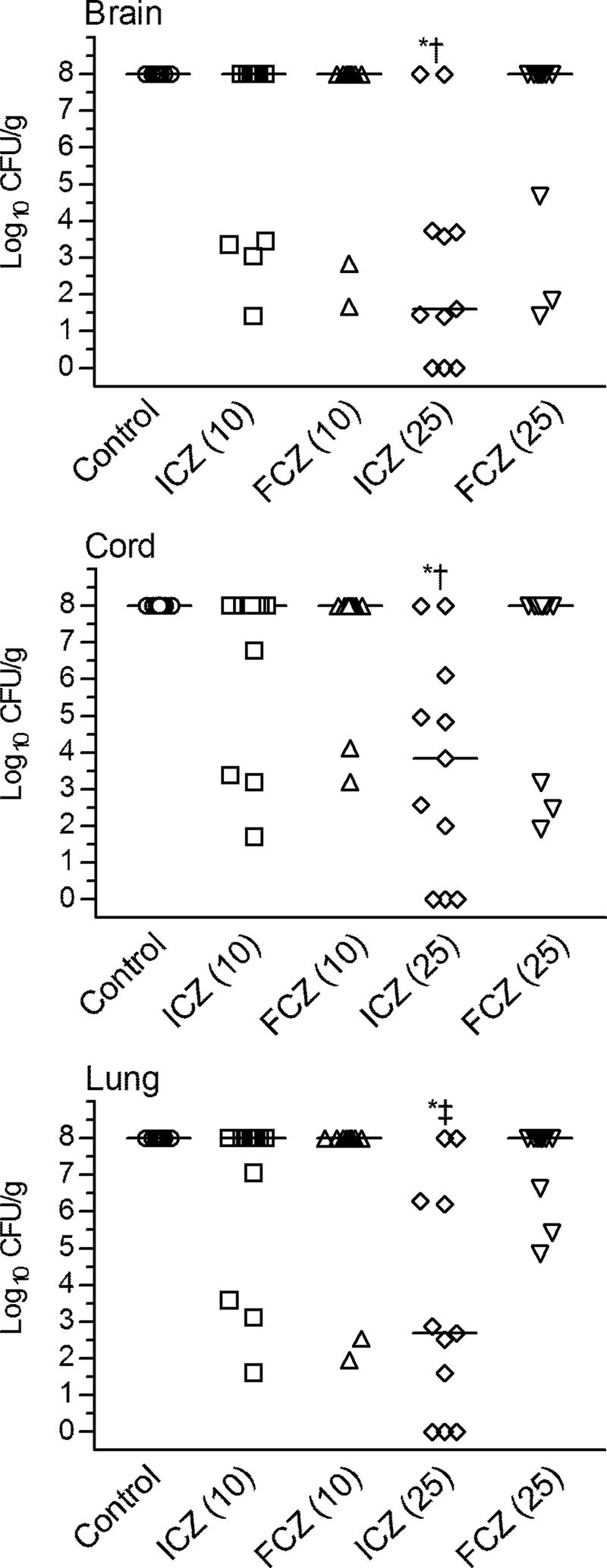
Scattergram plot of individual CFU burdens on day 28 postinfection in the brains, spinal cords, and lungs of mice with CM. Itraconazole, fluconazole, and cyclodextrin were given BID through days 3 to 14 of infection. For the analysis of the fungal burdens in the surviving animals, a value of 8 log10 CFU/g was given to missing datum points (due to the death of the animal) (21, 27). Horizontal lines, median log10 of numbers of CFU/g tissue; *, P < 0.001 versus the results for the control; †, P < 0.05 for the results for ICZ versus the results for FCZ at 25 mg/kg; ‡, P < 0.005 for the results for ICZ versus the results for FCZ at 25 mg/kg.
The findings with ICZ at 10 mg/kg and 25 mg/kg FCZ were substantiated in a separate study (J. Capilla, K. V. Clemons, and D. A. Stevens, unpublished data; data not shown).
Short-term organ clearance study.
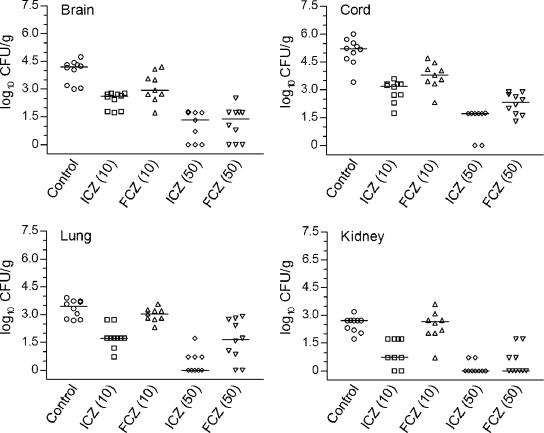
Further evaluation of efficacies was done by analysis of the clearance of C immitis from the tissue after 5 days of treatment with ICZ or FCZ given at 10 mg/kg or 50 mg/kg. Overall, ICZ and FCZ significantly reduced the fungal burdens in the brain, spinal cord, lung, and kidney tissues compared with those in the control group (P < 0.02 to 0.0003, depending on the comparison) in a dose-dependent fashion (Fig. 4), with the exception of FCZ at 10 mg/kg, which lacked efficacy in the lung and kidney. ICZ at 10 mg/kg was superior to FCZ given at the same dose (P < 0.05 to P < 0.001, depending on the organ compared). ICZ and FCZ given at 50 mg/kg were superior to the same agents at the lower dose (P < 0.01 and P < 0.003, respectively). In the brain and kidney, the efficacies of ICZ at 50 mg/kg and FCZ at 50 mg/kg were not significantly different (P > 0.05), but ICZ at 50 mg/kg was more effective in the spinal cord (P < 0.05) as well as the lungs (P < 0.002).
FIG. 4.
Scattergram plot of individual CFU burdens on day 8 postinfection in the brains, spinal cords, lungs, and kidneys of mice that were treated with cyclodextrin (control), itraconazole, or fluconazole at the indicated dosages. Therapies were given BID on days 3 to 7 of infection. Horizontal lines, median numbers of CFU/organ for 9 or 10 mice.
DISCUSSION
The present studies illustrate the efficacies of both ICZ and FCZ for therapy for coccidioidal meningitis in a mouse model, a conclusion consistent with clinical recommendations (12). The elimination of infection by either drug was rare, a conclusion also consistent with clinical evidence (8). These findings confirm the robustness of the model for extrapolation of the conclusions to the human disease. Although the two drugs were equivalent when both drugs were studied at the maximal dose here, our dose-finding comparisons indicated that ICZ is the more potent drug on an mg/kg basis of comparison. As ICZ produces lower serum concentrations than an equivalent dose of FCZ (3, 6, 10, 16-19, 21, 30, 32, 36, 39), the therapeutic superiority would be magnified if the drugs were compared on a μg/ml serum basis. The dose ranges used produce serum concentrations in mice similar to those obtained after the usual dosage (100 to 400 mg/day) in humans and were twofold or less lower than those at which toxicity has been noted in infected mice (3, 6, 7, 15-21, 26, 30, 36, 40). For comparison of the pharmacokinetics in rodents (at doses relevant to our studies) and humans, a 25-mg/kg dose of itraconazole in the mouse produced a maximum concentration in serum (Cmax) of 7 μg/ml and an area under the concentration-time curve (AUC) from time zero to 24 h (AUC0-24) of 38 μg · h/ml (20); in humans with coccidioidomycosis, we showed a maximum concentration in serum at steady state of 5.9 μg/ml (36), and a 200-mg BID dose in humans produces an AUC0-24 of 39.3 μg · h/ml (16). The levels of plasma protein binding are 99.8% (18) in humans and 99.7% in rats (17). We found that CSF concentrations were undetectable in coccidioidal meningitis patients receiving 400 mg (37) (concordant with what is seen in rabbits [29]), whereas, of interest, in the brain itself, 10 mg/kg in the rat produced a concentration that was 30% of the corresponding serum Cmax (17). For fluconazole, we showed a Cmax in coccidioidal meningitis patients receiving 400 mg/day of 21 μg/ml (with a corresponding CSF level of 19 μg/ml; levels in CSF are generally ∼83% of those in serum) (38), and 400 mg daily for 14 days produces an AUC0-24 of 349.9 mg · h/liter (3). In the mouse, 40 mg/kg produces a Cmax of 25 μg/ml (21) and an AUC from time zero to infinity of 264 μg · h/ml (30). The levels of serum protein binding are 11% in mice and 11% in humans (18, 21). The brain AUC/plasma AUC in the rat is 0.6 and is independent of the dose (44).
The isolate used in this study was more susceptible to ICZ than to FCZ. This is true of C. immitis in general; of 38 isolates concurrently tested by previously described methods (34), the MIC50s, MIC90s, MFC50s, and MFC90s were 25, >100, 25, and >100 μg/ml, respectively, for FCZ and 0.78, 1.56, 1.56, and 6.25 μg/ml, respectively, for ICZ.
Although extended survival comparisons indicated that cure was rarely achieved, even in the most intensively treated groups, early studies with treated mice did not document the presence of infection in some by culture or histology. This could possibly be explained by the insensitivity of the culture method at early, low levels of infection and/or sampling considerations related to the method of induction of infection in the model (lumbar injection), which requires ascending infection from the lower spinal cord to meningoencephalitis involving the whole neuraxis.
In a prior study, by using intracisternal infection of arthroconidia in the rabbit, the efficacies of FCZ and ICZ were deemed to be equivalent, but only one high dose of each drug was compared (32). A prior study also indicated that ICZ is superior to FCZ on an mg/kg basis (7), but that study used a method (direct intracranial implantation) to produce coccidioidal CNS infection different from that used in this study and mainly addressed alternative agents, thus precluding extensive dose finding for FCZ versus that for ICZ. Another study found that these two drugs are equivalent at the highest doses of each drug tested (27).
A recent consensus guideline (12) indicated that FCZ is “preferred by most clinicians,” although ICZ has been “reported to be comparably effective” (37). A reason for such a preference could be the superior penetration of FCZ into CSF (1, 6, 10, 17, 21, 29, 32, 39, 44); however, CSF in human CM is culture positive in a minority of cases (11, 23, 33), and thus, the disease appears to be related to infection in the meninges and not in the CSF. In human coccidioidal infection, ICZ appears to be superior to FCZ for the treatment of bone and joint infections and equivalent to FCZ for the treatment of other nonmeningeal manifestations (14).
A subsequently developed azole, posaconazole, is the most active agent against murine nonmeningeal coccidioidomycosis (26). It would be of interest to know whether this new drug is comparably active against meningitis.
Acknowledgments
This study was supported by grants from The Valley Foundation, Los Gatos, CA, and the Bank of Stockton, Stockton, CA.
Footnotes
Published ahead of print on 18 December 2006.
REFERENCES
- 1.Arndt, C. A. S., T. J. Walsh, C. L. McCully, F. M. Balis, P. A. Pizzo, and D. G. Poplack. 1988. Cerebrospinal fluid penetration of fluconazole: implications for antifungal therapy in patients with acquired immunodeficiency syndrome. J. Infect. Dis. 157:178-180. [DOI] [PubMed] [Google Scholar]
- 2.Ayala-Gaytan, J. J., B. A. Condarco-Cortes, J. A. Perez-Zuno, and P. Moreno-Guevara. 1997. Fluconazole in meningeal coccidioidomycosis. Rev. Investig. Clin. 49:205-208. [PubMed] [Google Scholar]
- 3.Bennett, J. (ed.). 1990. Fluconazole. An overview. ADIS Press International Inc., Langhorne, PA.
- 4.Classen, D. C., J. P. Burke, and C. B. Smith. 1988. Treatment of coccidioidal meningitis with fluconazole. J. Infect. Dis. 158:903-904. [DOI] [PubMed] [Google Scholar]
- 5.Clemons, K. V., M. E. Homola, and D. A. Stevens. 1995. Activities of the triazole SCH 51048 against Coccidioides immitis in vitro and in vivo. Antimicrob. Agents Chemother. 39:1169-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeBeule, K., and J. VanGestel. 2001. Pharmacology of itraconazole. Drugs 61(Suppl. 1):27-37. [DOI] [PubMed] [Google Scholar]
- 7.Defaveri, J., S. H. Sun, and J. N. Graybill. 1990. Treatment of murine coccidioidal meningitis with SCH39304. Antimicrob. Agents Chemother. 34:663-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dewsnup, D. H., J. N. Galgiani, J. R. Graybill, M. Diaz, A. Rendon, G. A. Cloud, and D. A. Stevens. 1996. Is it ever safe to stop azole therapy for Coccidioides immitis meningitis? Ann. Intern. Med. 124:305-310. [DOI] [PubMed] [Google Scholar]
- 9.Einstein, H. E., C. W. Holeman, L. L. Sandidge, and D. H. Holden. 1961. Coccidioidal meningitis. The use of amphotericin B in treatment. Calif. Med. 94:339-343. [PMC free article] [PubMed] [Google Scholar]
- 10.Foulds, G., D. R. Brennan, C. Wajszczuk, A. Catanzaro, D. C. Garg, W. Knopf, M. G. Rinaldi, and D. J. Weidler. 1988. Fluconazole penetration into cerebrospinal fluid in humans. J. Clin. Pharmacol. 28:363-366. [DOI] [PubMed] [Google Scholar]
- 11.Galgiani, J. N. 1997. Coccidioides immitis meningitis, p. 227-238. In P. K. Peterson and J. S. Remington (ed.), In defense of the brain. Blackwell Science, Boston, MA.
- 12.Galgiani, J. N., N. M. Ampel, J. E. Blair, A. Catanzaro, R. H. Johnson, D. A. Stevens, and P. L. Williams. 2005. Coccidioidomycosis. Clin. Infect. Dis. 41:1217-1223. [DOI] [PubMed] [Google Scholar]
- 13.Galgiani, J. N., A. Catanzaro, G. A. Cloud, J. Higgs, B. A. Friedman, R. A. Larsen, J. R. Graybill, and the NIAID-Mycoses Study Group. 1993. Fluconazole therapy for coccidioidal meningitis. Ann. Intern. Med. 119:28-35. [DOI] [PubMed] [Google Scholar]
- 14.Galgiani, J. N., A. Catanzaro, G. A. Cloud, R. H. Johnson, P. L. Williams, L. F. Mirels, F. Nassar, J. E. Lutz, D. A. Stevens, P. K. Sharkey, V. R. Singh, R. A. Larsen, K. L. Delgado, C. Flanigan, and M. G. Rinaldi. 2000. Comparison of oral fluconazole and itraconazole for progressive, nonmeningeal coccidioidomycosis. Ann. Intern. Med. 133:676-686. [DOI] [PubMed] [Google Scholar]
- 15.Graybill, R. J., S. Sun, and J. Ahrens. 1986. Treatment of murine coccidioidal meningitis with fluconazole (UK 49,858). J. Med. Vet. Mycol. 24:113-119. [DOI] [PubMed] [Google Scholar]
- 16.Hardin, T., J. Graybill, R. Fetchick, R. Woestenborghs, M. Rinaldi, and J. Kuhn. 1988. Pharmacokinetics of itraconazole following oral administration to normal volunteers. Antimicrob. Agents Chemother. 32:1310-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heykants, J., M. Michiels, W. Meuldermans, J. Monbaliu, K. Lavrijsen, A. Van Peer, J. C. Levron, R. Woestenborghs, and G. Cauwenbergh. 1987. The pharmacokinetics of itraconazole in animals and man: an overview, p. 223-249. In R. A. Fromtling (ed.), Recent trends in the discovery, development and evaluation of antifungal agents. J. R. Prous Science Publishers, Barcelona, Spain.
- 18.Heykants, J., A. Van Peer, K. Lavrijsen, W. Meuldermans, R. Woestenborghs, and G. Cauwenbergh. 1990. Pharmacokinetics of oral antifungals and their clinical implications. Br. J. Clin. Pract. 44(Suppl. 71):50-56. [PubMed] [Google Scholar]
- 19.Hostetler, J. S., L. H. Hanson, and D. A. Stevens. 1992. Effect of cyclodextrin on the pharmacology of antifungal oral azoles. Antimicrob. Agents Chemother. 36:477-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hostetler, J. S., L. H. Hanson, and D. A. Stevens. 1993. Effect of hydroxypropyl-beta-cyclodextrin on efficacy of oral itraconazole in disseminated murine cryptococcosis. J. Antimicrob. Chemother. 32:459-463. [DOI] [PubMed] [Google Scholar]
- 21.Humphrey, M. J., S. Jevons, and M. H. Tarbit. 1985. Pharmacokinetic evaluation of UK-49,858, a metabolically stable triazole antifungal drug, in animals and humans. Antimicrob. Agents Chemother. 28:648-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamberi, P., R. A. Sobel, K. V. Clemons, D. A. Stevens, D. Pappagianis, and P. L. Williams. 2003. A murine model of coccidioidal meningitis. J. Infect. Dis. 187:453-460. [DOI] [PubMed] [Google Scholar]
- 23.Kelly, P. C. 1980. Coccidioidal meningitis, p. 163-193. In D. A. Stevens (ed.), Coccidioidomycosis. Plenum Medical Book Company, New York, NY.
- 24.Labadie, E. L., and R. H. Hamilton. 1986. Survival improvement in coccidioidal meningitis by high-dose intrathecal amphotericin B. Arch. Intern. Med. 146:2013-2018. [PubMed] [Google Scholar]
- 25.Lachin, J. M. 1999. Worst-rank score analysis with informatively missing observations in clinical trials. Control. Clin. Trials 20:408-422. [DOI] [PubMed] [Google Scholar]
- 26.Lutz, J. E., K. V. Clemons, B. H. Aristizabal, and D. A. Stevens. 1997. Activity of the triazole SCH 56592 against disseminated murine coccidioidomycosis. Antimicrob. Agents Chemother. 41:1558-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pappagianis, D., B. L. Zimmer, G. Theodoropoulos, M. Plempel, and R. F. Hector. 1990. Therapeutic effect of the triazole Bay R 3783 in mouse models of coccidioidomycosis, blastomycosis, and histoplasmosis. Antimicrob. Agents Chemother. 34:1132-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perez, A. J., R. H. Johnson, J. W. Caldwell, E. L. Arsura, and P. Nemecheck. 1995. Fluconazole therapy in coccidioidal meningitis maintained with intrathecal amphotericin B. Arch. Intern. Med. 155:1665-1668. [DOI] [PubMed] [Google Scholar]
- 29.Perfect, J. R., and D. T. Durack. 1985. Penetration of imidazoles and triazoles into cerebrospinal fluid of rabbits. J. Antimicrob. Chemother. 16:81-86. [DOI] [PubMed] [Google Scholar]
- 30.Pfizer Central Research. 1989. Fluconazole investigator's brochure. Pfizer & Co., Groton, CT.
- 31.Shih, W. 2002. Problems in dealing with missing data and informative censoring in clinical trials. Curr. Control. Trials Cardiovasc. Med. 3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sorensen, K. N., R. A. Sobel, K. V. Clemons, D. Pappagianis, D. A. Stevens, and P. L. Williams. 2000. Comparison of fluconazole and itraconazole in a rabbit model of coccidioidal meningitis. Antimicrob. Agents Chemother. 44:1512-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stevens, D. A. 1995. Current concepts: coccidioidomycosis. N. Engl. J. Med. 332:1077-1082. [DOI] [PubMed] [Google Scholar]
- 34.Stevens, D. A., and B. H. Aristizabal. 1997. In vitro antifungal activity of novel azole derivatives with a morpholine ring, UR-9746 and UR-9751, and comparison with fluconazole. Diagn. Microbiol. Infect. Dis. 29:103-106. [DOI] [PubMed] [Google Scholar]
- 35.Stevens, D. A., and S. A. Shatsky. 2001. Intrathecal amphotericin in the management of coccidioidal meningitis. Semin. Respir. Med. 16:263-269. [DOI] [PubMed] [Google Scholar]
- 36.Tucker, R. M., D. W. Denning, E. G Arathoon, M. G. Rinaldi, and D. A. Stevens. 1990. Itraconazole therapy of non-meningeal cocccidioidomycosis: clinical and laboratory observations. J. Am. Acad. Dermatol. 23:593-601. [DOI] [PubMed] [Google Scholar]
- 37.Tucker, R. M., D. W. Denning, B. Dupont, and D. A. Stevens. 1990. Itraconazole therapy for chronic coccidioidal meningitis. Ann. Intern. Med. 112:108-112. [DOI] [PubMed] [Google Scholar]
- 38.Tucker, R. M., J. N. Galgiani, D. W. Denning, L. H. Hanson, J. R. Graybill, K. Sharkey, M. R. Eckman, C. Salemi, R. Libke, R. A. Klein, and D. A. Stevens. 1990. Treatment of coccidioidal meningitis with fluconazole. Rev. Infect. Dis. 12(Suppl. 3):S380-S389. [DOI] [PubMed] [Google Scholar]
- 39.Tucker, R. M., P. L. Williams, E. Arathoon, B. E. Levine, A. I. Hartstein, L. H. Hanson, and D. A. Stevens. 1988. Pharmacokinetics of fluconazole in cerebrospinal fluid and serum in human coccidioidal meningitis. Antimicrob. Agents Chemother. 32:369-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.VanCauteren, H., A. Lampo, J. Vandenberghe, P. Vanparys, W. Coussement, R. DeCoster, and R. Marsboom. 1989. Toxicological profile and safety evaluation of antifungal azole derivatives. Mycoses 32(Suppl. 1):60-66. [DOI] [PubMed] [Google Scholar]
- 41.Vincent, T., J. N. Galgiani, M. Huppert, and D. Salkin. 1993. The natural history of coccidioidal meningitis: VA-armed forces cooperative studies, 1955-1958. Clin. Infect. Dis. 16:247-254. [DOI] [PubMed] [Google Scholar]
- 42.Williams, P. L., R. H. Johnson, D. Pappagianis, H. Einstein, U. Slager, F. T. Koster, J. J. Eron, J. Morrison, J. Aguet, and M. E. River. 1992. Vasculitis and encephalitic complications associated with Coccidioides immitis infection of the central nervous system in humans: report of ten cases and review. Clin. Infect. Dis. 14:673-682. [DOI] [PubMed] [Google Scholar]
- 43.Williams, P. L., R. A. Sobel, K. N. Sorensen, K. V. Clemons, L. M. Shuer, S. S. Royaltey, Y. Yao, D. Pappagianis, J. E. Lutz, C. Reed, M. E. River, B. C. Lee, S. U. Bhatti, and D. A. Stevens. 1998. A model of coccidioidal meningoencephalitis and cerebrospinal vasculitis in the rabbit. J. Infect. Dis. 178:1217-1221. [DOI] [PubMed] [Google Scholar]
- 44.Yang, H., Q. Wang, and W. F. Elmquist. 1996. Fluconazole distribution to the brain: a crossover study in freely moving rats using in vivo microdialysis. Pharm. Res. 13:1570-1575. [DOI] [PubMed] [Google Scholar]