Abstract
Isolates of Salmonella enterica serovar Typhi that are multidrug resistant (MDR, resistant to chloramphenicol, ampicillin, and trimethoprim-sulfamethoxazole) and have reduced susceptibility to fluoroquinolones (nalidixic acid resistant, Nar) are common in Asia. The optimum treatment for infections caused by such isolates is not established. This study compared different antimicrobial regimens for the treatment of MDR/Nar typhoid fever. Vietnamese children and adults with uncomplicated typhoid fever were entered into an open randomized controlled trial. Ofloxacin (20 mg/kg of body weight/day for 7 days), azithromycin (10 mg/kg/day for 7 days), and ofloxacin (15 mg/kg/day for 7 days) combined with azithromycin (10 mg/kg/day for the first 3 days) were compared. Of the 241 enrolled patients, 187 were eligible for analysis (186 S. enterica serovar Typhi, 1 Salmonella enterica serovar Paratyphi A). Eighty-seven percent (163/187) of the patients were children; of the S. enterica serovar Typhi isolates, 88% (165/187) were MDR and 93% (173/187) were Nar. The clinical cure rate was 64% (40/63) with ofloxacin, 76% (47/62) with ofloxacin-azithromycin, and 82% (51/62) with azithromycin (P = 0.053). The mean (95% confidence interval [CI]) fever clearance time for patients treated with azithromycin (5.8 days [5.1 to 6.5 days]) was shorter than that for patients treated with ofloxacin-azithromycin (7.1 days [6.2 to 8.1 days]) and ofloxacin (8.2 days [7.2 to 9.2 days]) (P < 0.001). Positive fecal carriage immediately posttreatment was detected in 19.4% (12/62) of patients treated with ofloxacin, 6.5% (4/62) of those treated with the combination, and 1.6% (1/62) of those treated with azithromycin (P = 0.006). Both antibiotics were well tolerated. Uncomplicated typhoid fever due to isolates of MDR S. enterica serovar Typhi with reduced susceptibility to fluoroquinolones (Nar) can be successfully treated with a 7-day course of azithromycin.
Enteric fever, due to infection with Salmonella enterica subsp. enterica serotype Typhi or Salmonella enterica subsp. enterica serotype Paratyphi A, is estimated to cause more than 27 million infections each year worldwide with 216,000 deaths (13). Multidrug-resistant (MDR) strains of S. enterica serovar Typhi and serovar Paratyphi A (resistant to chloramphenicol, trimethoprim-sulfamethoxazole, and ampicillin) are endemic to many Asian countries (28). Expanded-spectrum cephalosporins and fluoroquinolones are used for treating such infections. Where fluoroquinolones, such as ciprofloxacin and ofloxacin, have become widely used, isolates of S. enterica serovar Typhi and serovar Paratyphi A with reduced susceptibility to fluoroquinolones have become common (27, 30). Infections with these isolates have been associated with treatment failures, particularly when very short durations of treatment have been used (31, 35). These isolates are ciprofloxacin susceptible by current disk testing criteria but are usually resistant to nalidixic acid (Nar) (12, 35). In ciprofloxacin-susceptible S. enterica isolates, nalidixic acid resistance has been proposed as an indicator that infection with such a strain may not respond to fluoroquinolone treatment (24). In some areas of endemicity, >75% of typhoid-infected patients admitted to the hospital are Nar (27, 30), and these infections are also seen in returning travelers (2).
The choice of oral antimicrobial regimens for uncomplicated typhoid fever caused by isolates of S. enterica serovar Typhi that are both MDR and Nar is unclear. A fluoroquinolone given in a high dose for 7 days is the most affordable first-line option for MDR/Nar infections in areas of endemicity, but at the time this study was planned, the efficacy of such a regimen had not been examined in a randomized controlled trial. The expanded-spectrum cephalosporins would be effective for the treatment of such infections, and resistance to these agents is uncommon (15, 32). However, expense and the need for parenteral therapy limit their usefulness as first-line treatments. The azalide antimicrobial azithromycin is a further option. Treatment courses of 500 mg per day (10 mg/kg of body weight/day) for 7 days and 1 g per day (20 mg/kg/day) for 5 days have proved successful for adults and children (8, 18, 19, 21), including adults with MDR/Nar infections (10). A combination of a fluoroquinolone with another antimicrobial directed against a different target is another option that may improve the efficacy compared with the fluoroquinolone alone and potentially reduce the chance of fluoroquinolone-resistant mutants emerging. However, there is no controlled trial evidence to support this approach.
We have conducted a three-way comparison of 7 days of ofloxacin (20 mg/kg/day), 7 days of azithromycin (10 mg/kg/day), and 7 days of ofloxacin (15 mg/kg/day) combined with azithromycin (10 mg/kg/day) for the first 3 days for the treatment of uncomplicated enteric fever. The combination of ofloxacin and azithromycin was empirically designed to match the different pharmacokinetics of the two antimicrobials. The lower dose of ofloxacin and a shorter duration of azithromycin were chosen to see if it was possible to reduce cost but maintain the efficacy of the regimen.
MATERIALS AND METHODS
Study site and ethical compliance.
The study was conducted on the infection ward at Dong Thap Provincial Hospital, Cao Lanh Town, Dong Thap Province, Vietnam. The hospital is a 300-bed provincial hospital for Dong Thap Province in the Mekong Delta. The study had received approval from the Scientific and Ethical Committees of Dong Thap Provincial Hospital and the Hospital for Tropical Diseases, Ho Chi Minh City. Patients or a parent or guardian, for children, gave informed verbal consent before entry into the study. The study was conducted in compliance with ICH and Declaration of Helsinki guidelines.
Study population.
Children and adults with the clinical features of enteric fever were enrolled in the study. Eligibility for enrollment required that the patient have a documented fever (temperature ≥ 38°C) and a history of fever for at least 4 days plus at least one of the following criteria: abdominal pain/tenderness, diarrhea or constipation, hepatomegaly, splenomegaly, and/or rose spots. Patients were excluded if they had evidence of severe or complicated disease (severe gastrointestinal bleeding, intestinal perforation, visible jaundice, myocarditis, pneumonia, renal failure, shock, or an altered conscious level), inability to swallow oral medication, a history of significant underlying disease or of hypersensitivity to either of the trial drugs, or were pregnant or lactating. Patients who gave a history of treatment with a fluoroquinolone or expanded-spectrum cephalosporin or macrolide within 1 week of hospital admission were also excluded.
Randomization and treatment.
Patients were allocated to one of three treatment groups in an open randomized comparison. The computer-generated randomization list was produced by a member of the staff not otherwise involved in the study. The treatment allocations were kept in serially numbered sealed envelopes that were only opened after the patient had been enrolled in the study. Patients were randomized to receive one of the following three regimens: (i) ofloxacin (Oflocet; Hoescht Marion Roussel, Paris, France) 20 mg/kg/day orally in two divided doses (maximum, 400 mg twice daily) for 7 days; (ii) azithromycin suspension (Zithromax; Pfizer International) 10 mg/kg/day orally once a day (maximum, 500 mg daily) for 7 days (tablets were used for adults); (iii) ofloxacin (Oflocet; Hoescht Marion Roussel, Paris, France) 15 mg/kg/day orally in two divided doses (maximum, 300 mg twice daily) for 7 days combined with azithromycin suspension (Zithromax; Pfizer International) 10 mg/kg/day orally once per day (maximum, 500 mg daily) for the first 3 days. Care was taken that calcium-containing foods or drugs (e.g., milk or antacids) were not given at the same time as the antimicrobials to avoid problems with ofloxacin absorption.
Laboratory investigations.
Hematocrit, white cell, differential, platelet, serum aspartate transaminase, alanine transaminase, and creatinine counts and urinalysis were performed before therapy. The aspartate transaminase and alanine transaminase counts were repeated 1 day after the end of therapy. The full blood count was repeated if there had been evidence of gastrointestinal bleeding or clinical evidence of anemia. A chest X-ray and other radiological investigations, including abdominal ultrasound, were performed as clinically indicated. Blood, bone marrow, and fecal cultures were obtained before therapy. A blood culture was taken in all patients a day after the end of treatment. In addition, three fecal specimens were cultured between 2 and 4 days after the end of treatment.
Isolates of Salmonella were identified by standard biochemical tests and agglutination with Salmonella-specific antisera (Murex diagnostics, Dartford, United Kingdom). Antimicrobial sensitivities were examined by the modified Bauer-Kirby disk diffusion method with zone size interpretation based on CLSI (formerly NCCLS) guidelines (24). Antibiotic disks tested were chloramphenicol (30 μg), ampicillin (10 μg), trimethoprim-sulfamethoxazole (1.25/23.75 μg), ceftriaxone (30 μg), ofloxacin (5 μg), azithromycin (15 μg), and nalidixic acid (30 μg). Isolates were stored in protect beads (Prolabs, Oxford, United Kingdom) at −20°C for later MIC testing by agar plate dilution (25). Antibiotic powders were purchased from Sigma, United Kingdom. The azithromycin MIC was determined by E-test (AB Biodisk, Solna, Sweeden) according to the manufacturer's instructions. Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 25923 were used as control strains for these assays. An isolate was defined as MDR if it was resistant to chloramphenicol (≥32 μg/ml), ampicillin (≥32 μg/ml), and trimethoprim-sulfamethoxazole (≥8 and 152 μg/ml). An isolate was defined as nalidixic acid resistant (Nar) if it was resistant to nalidixic acid (≥32 μg/ml). The CLSI breakpoints for ofloxacin were ≤2 μg/ml (susceptible) and ≥8 μg/ml (resistant), but there are none for azithromycin (24).
Definitions.
Patients were examined daily until discharge from the hospital, with particular reference to clinical symptoms, side effects of the drug, and complications of the disease. Body temperature was measured every 6 h. The response to treatment was assessed by clinical parameters (resolution of clinical symptoms and signs), fever clearance time (time from the start of treatment until the body temperature reached ≤37.5°C and remained at ≤37.5°C for 48 h), development of complications, and evidence of relapse of infection. A clinical treatment failure was defined as the persistence of fever and at least one other typhoid-related symptom for more than 7 days after the start of treatment or the development of severe complications (severe gastrointestinal bleeding, intestinal perforation, visible jaundice, myocarditis, pneumonia, renal failure, shock, or an altered conscious level) during treatment requiring a change in therapy. Microbiological treatment failure was defined as isolation of S. enterica serovar Typhi or serovar Paratyphi A from blood or a sterile site after the completion of treatment. Those who failed treatment and, in the opinion of the treating physician, required retreatment received 60 mg/kg/day ceftriaxone for 7 to 10 days. Early fecal carriage was defined as a positive fecal culture, with an isolate having the same susceptibility pattern as the original isolate, after the end of the initial 7-day treatment and before hospital discharge.
Patients were requested to return for a follow-up assessment at 4 weeks or earlier if their symptoms recurred. Further follow-up was performed at 3 months and 6 months posttreatment. Those patients who did not return were visited at their home by one of the study team members. At the first follow-up, clinical evidence of relapse was sought, the patient was asked about joint symptoms, and one stool culture was performed. A blood culture was performed if the symptoms and signs suggested relapse. A relapse was defined as a recurrence of symptoms and signs suggestive of enteric fever within the 4-week period after the patient had been discharged well from the hospital accompanied by a blood culture positive for S. enterica serovar Typhi or serovar Paratyphi A.
Sample size and statistical analysis.
We assumed that the failure rate for the patients treated with azithromycin would be 5%. A sample size of 59 patients in each group would give a power of 80% at a 5% significance level to detect a failure rate of 25% in the patients treated with ofloxacin (i.e., a difference between the two failure rates of 20%). Detailed analysis of the outcome was confined to those patients in whom the pretreatment culture of blood or bone marrow was positive with S. enterica serovar Typhi or serovar Paratyphi A. Proportions were compared with the chi-square test or Fisher's exact test. Normally distributed data were compared using a one-way analysis of variance with Tukey's HSD post hoc multiple-comparison test, nonnormally distributed data using the Kruskal-Wallis test. The fever clearance time and duration of admission after the start of treatment were compared using survival analysis and the log rank test. The independent associations of clinical, laboratory, and treatment variables with an outcome of clinical failure were determined using multivariable logistic regression analysis, including all variables significantly associated with univariate analysis (P < 0.05). Statistical analysis was performed using the EpiInfo version 6 (CDC, Atlanta, GA) and SPSS for Windows version 11 (SPSS, Inc., Chicago, IL).
RESULTS
Two hundred forty-one patients with suspected enteric fever were entered into the study between 1998 and 2002. Eighty patients were randomized to treatment with ofloxacin, 81 to the ofloxacin and azithromycin combination, and 80 to azithromycin. One hundred ninety-three patients had culture-positive results from the blood or bone marrow, including 192 with S. enterica serovar Typhi and 1 with serovar Paratyphi A. One hundred thirty-seven patients had positive results from the blood and bone marrow, 32 from bone marrow alone and 24 from blood alone, and 40/161 (24.8%) patients with blood or bone marrow culture-positive results also had positive fecal cultures. Four of the culture-positive randomized patients had been treated with a fluoroquinolone prior to admission and two self discharged before the completion of treatment, leaving 187 eligible patients with a positive blood or bone marrow culture. Of the remaining 55 patients who completed treatment, all were cured, with an average duration of admission of 10 days. One patient randomized to the ofloxacin treatment group had a prolonged fever clearance lasting 12 days, and an additional patient randomized to the ofloxacin treatment group had a positive fecal culture for S. enterica serovar Typhi at the 6-month follow-up visit.
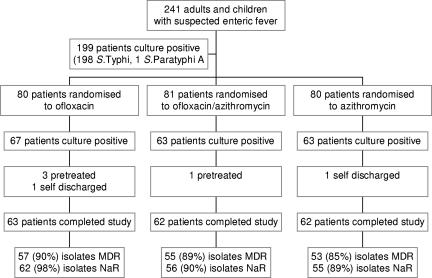
Sixty-three of the eligible patients were randomized to the ofloxacin group, 62 to ofloxacin and azithromycin group, and 62 to the azithromycin group (Fig. 1). Of 187, 163 (87%) were children (aged <15 years); 165/187 (88%) were infected with a MDR isolate and 173/187 (93%) with a Nar isolate. All isolates were susceptible to ofloxacin and ceftriaxone by disk test and MIC. For the isolates, the MIC90 (range) of azithromycin was 16 μg/ml (4 to 32 μg/ml) and that of ofloxacin was 1.00 μg/ml (0.03 to 1.00 μg/ml). The MIC90 (range) of ofloxacin for the Nas isolates was 0.06 μg/ml (0.03 to 0.125 μg/ml) and that for the Nar isolates was 1.0 μg/ml (0.25 to 1.0 μg/ml). The majority of isolates in the study had an ofloxacin MIC of 0.5 or 1.0 μg/ml. There were no important differences between the admission characteristics of patients from the three groups (Table 1).
FIG. 1.
Flow chart for recruitment of patients into the study.
TABLE 1.
Epidemiological, clinical, and laboratory features in the 187 patients with culture-confirmed enteric fever
| Parameter | Result for patients treated with:
|
||
|---|---|---|---|
| Ofloxacin | Ofloxacin + azithromycin | Azithromycin | |
| No. of patients | 63 | 62 | 62 |
| No. of males/females | 33/30 | 31/31 | 22/40 |
| Mean age (yr) (95% CI, range) | 8.8 (7.8-9.7, 3-22) | 10.2 (8.4-12.0, 4-36) | 10.5 (8.9-12.1, 4-42) |
| Mean wt (kg) (95% CI, range) | 20 (19-22, 10-50) | 23 (20-26, 10-56) | 24 (21-27, 12-58) |
| Mean duration of fever before admission (days) (95% CI, range) | 9.6 (8.5-10.7, 4-30) | 9.9 (8.6-11.1, 4-25) | 9.2 (8.1-10.2, 4-30) |
| Patients with (n [%]): | |||
| Abdominal pain | 49 (78) | 43 (69) | 39 (63) |
| Vomiting | 32 (51) | 24 (39) | 28 (45) |
| Diarrhea | 52 (83) | 54 (87) | 49 (79) |
| Hepatomegaly | 43 (68) | 41 (66) | 41 (66) |
| Splenomegaly | 3 (5) | 5 (8) | 4 (6) |
| Mean % hematocrit (95% CI, range) | 34 (33-35, 26-47) | 34 (33-34, 25-45) | 34 (33-35, 23-43) |
| Mean white cell count (109/liter) (95% CI, range) | 7.2 (6.6-7.8, 2.9-14.1) | 6.3 (5.9-6.7, 3.2-10.9) | 7.2 (6.7-7.8, 1.7-13.4) |
| Mean platelet count (109/liter) (95% CI, range) | 191 (171-212, 84-442) | 194 (172-217, 50-480) | 211 (184-239, 60-477) |
| Mean AST (IU/liter) (95% CI, range)a | 165 (132-198, 34-788) | 166 (135-197, 37-592) | 150 (125-176, 35-443) |
| Mean ALT (IU/liter) (95% CI, range)b | 109 (86-132, 16-437) | 97 (78-115, 15-477) | 103 (84-123, 10-471) |
| Characteristics of organism isolated | |||
| Serovar Typhi/serovar Paratyphi A (n) | 63/0 | 61/1 | 62/0 |
| Multiresistant (n [%]) | 57 (90) | 55 (89) | 53 (85) |
| Nalidixic acid resistant (n [%]) | 62 (98) | 55 (89) | 55 (89) |
| No. of patients with positive pretreatment fecal culture/no. of patients with a pretreatment fecal culture (%) | 13/50 (26) | 11/51 (22) | 16/60 (27) |
AST, aspartate transaminase (normal range, 20 to 40 IU/liter).
ALT, alanine transaminase (normal range, 20 to 45 IU/liter).
There were 49 treatment failures; 23 in the ofloxacin-treated patients, 15 in the ofloxacin-azithromycin-treated patients, and 11 in the azithromycin-treated patients (Table 2). The 23 ofloxacin-treated patients failed because of persistent fever and symptoms after the end of treatment, and repeat blood culture was positive for two of them. Fourteen patients required retreatment, while symptoms in the remaining nine resolved during the 3-day period posttreatment. The 15 patients who failed treatment with ofloxacin-azithromycin did so because of persistent fever and symptoms in 14, with a positive repeat blood culture in one, and one developed a gastrointestinal bleed on day 7. Five patients required retreatment, and symptoms resolved in the others in the 3 days posttreatment. All of the patients who failed treatment with ofloxacin or with ofloxacin plus azithromycin were infected with isolates that had an ofloxacin MIC of 0.5 or 1.0 μg/ml and were nalidixic acid resistant. Nine of the 11 patients who failed with azithromycin treatment did so because of persistent fever and symptoms, with a repeat blood culture positive in one, and two patients developed gastrointestinal bleeding on day 4 and day 6 of treatment. Five patients were retreated, while symptoms in the remaining six resolved over the succeeding 3 days. The posttreatment blood culture was positive in one further patient treated with azithromycin. In this patient, the symptoms had already completely resolved and no further treatment was given. Although blood culture was not repeated while the patient was still in the hospital, at the 1-, 3-, and 6-month follow-up visits, the patient was completely well without negative fecal cultures. All of the patients requiring retreatment responded promptly.
TABLE 2.
Clinical and microbiological outcomes in 187 patients with culture-confirmed uncomplicated typhoid fever
| Parameter | Result for patients treated with:
|
||
|---|---|---|---|
| Ofloxacin | Ofloxacin + azithromycin | Azithromycin | |
| No. of patients | 63 | 62 | 62 |
| Patients with clinical failures (n [%]) | 23 (36.5) | 15 (24.2) | 11 (17.8) |
| Persistent fever and symptoms | 23 (36.5) | 14 (22.6) | 9 (14.6) |
| Gastrointestinal hemorrhage | 0 (0) | 1 (1.6) | 2 (3.2) |
| Positive blood culture posttreatment (n [%]) | 2 (3.2) | 1 (1.6) | 2 (3.2) |
| Fecal carriage immediately posttreatment (n [%]) | 12/62 (19.4) | 4/62 (6.5) | 1/62 (1.6) |
| Mean fever clearance time (days) (95% CI, range) | 8.2 (7.2-9.2, 2-24) | 7.1 (6.2-8.1, 1-27) | 5.8 (5.1-6.5, 1-13) |
| Mean duration of hospitalization after starting treatment (days) (95% CI, range) | 13.7 (12.7-14.6, 9-32) | 12.8 (12.0-13.7, 8-33) | 12.6 (12.1-13.2, 10-19) |
| Mean posttreatment AST (IU/liter) (95% CI, range)a | 87 (67-108, 12-489) | 111 (76-146, 27-632) | 102 (79-125, 27-338) |
| Mean posttreatment ALT (IU/liter) (95% CI, range)b | 75 (62-88, 26-203) | 83 (60-106, 12-426) | 92 (73-110, 14-301) |
| Relapse (n [%]) | 0 (0) | 0 (0) | 0 (0) |
| No. with fecal carriage/no. tested during convalescence (%) at: | |||
| 1 mo | 2/54 (3.7) | 2/54 (3.7) | 3/52 (5.8) |
| 3 mo | 2/54 (3.7) | 3/54 (5.8) | 1/48 (2.6) |
| 6 mo | 0/48 (0) | 1/55 (1.9) | 0/48 (0) |
| Any time during 6-mo follow-up period | 4/58 (6.9) | 6/58 (10.3) | 4/56 (7.1) |
AST, aspartate transaminase (normal range, 20 to 40 IU/liter).
ALT, alanine transaminase (normal range, 20 to 45 IU/liter).
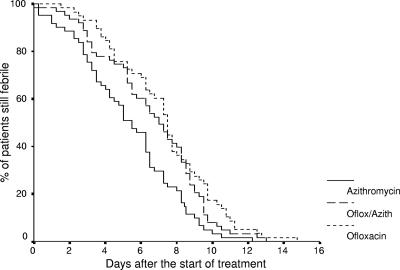
Table 3 shows the differences between the responses to treatment with each regimen. Clinical failure was more common with ofloxacin-treated patients than with those treated with azithromycin and those treated with ofloxacin-azithromycin. Overall, these differences were on the borderline of significance (P = 0.053). However, the confidence intervals for the ofloxacin comparison with azithromycin suggests an important difference in this instance. There were no significant differences in the proportion of patients in each group that were blood culture positive at the end of treatment (P = 0.818). Patients treated with ofloxacin were more likely than the patients treated with ofloxacin-azithromycin and those treated with azithromycin to be fecal culture positive immediately posttreatment (P = 0.006). The detection of positive fecal carriage at any time during the 6-month follow-up period did not differ between the three groups (P = 0.908). Figure 2 shows the Kaplan-Meier survival curve for the fever clearance time in the three groups of patients. The average fever clearance time among the patients treated with azithromycin was 1.3 days shorter than that for the patients treated with the ofloxacin and azithromycin combination and 2.4 days shorter than that of the patients treated with ofloxacin (P < 0.001). On average, the patients treated with azithromycin were hospitalized for a day less than those treated with ofloxacin, although the differences were not significant (P = 0.166). Upon multivariate analysis, the presence of diarrhea (odds ratio [OR], 5.8; 95% confidence interval [CI], 1.2 to 28.3; P = 0.029), hepatomegaly (OR, 3.7; 95% CI, 1.4 to 9.6; P = 0.007), and randomization to the ofloxacin treatment arm (OR, 3.0; 95% CI, 1.4 to 6.5; P = 0.004) were variables independently associated with clinical failure.
TABLE 3.
Differences in clinical and microbiological outcomes between each antibiotic treatment groupa
| Parameter | Result for:
|
P valueb | ||
|---|---|---|---|---|
| Ofloxacin compared with:
|
Ofloxacin-azithromycin compared with azithromycin | |||
| Ofloxacin-azithromycin | Azithromycin | |||
| Clinical failures (%) | 12.3 (−47.4, 28.3) | 18.8 (3.6, 34.0) | 6.4 (−7.9, 20.7) | 0.053 |
| Blood culture positive posttreatment (%) | 1.6 (−36.6, 39.8) | −0.6 (−41.3, 41.5) | 1.6 (−38.6, 38.2) | 0.818 |
| Fecal carriage immediately posttreatment (%) | 12.9 (1.4, 28.0) | 17.8 (7.6, 28.0) | 4.9 (−2.0, 11.8) | 0.006 |
| Fever clearance time (days) | 1.06 (−0.29, 2.41) | 2.44 (1.25, 3.63) | 1.38 (0.21, 2.55) | <0.001 |
| Duration of hospitalization after starting treatment (days) | 0.85 (−0.41, 2.11) | 1.04 (−0.003, 2.11) | 0.19 (−0.81, 1.18) | 0.166 |
| Fecal carriage during convalescence at any time during 6 mo follow-up period | −3.4 (−13.2, 6.6) | −0.2 (−9.1, 8.7) | 3.2 (−6.7, 13.1) | 0.908 |
Data are differences, and 95% CI are shown in parentheses.
Overall P value using chi-squared or log rank test.
FIG. 2.
Kaplan-Meier survival curve showing the percentage of patients still febrile following the start of treatment. The patients who failed treatment and required retreatment with a further course of antimicrobial are excluded. Oflox, ofloxacin; Azith, azithromycin.
A total of 172/187 (92%) of the patients were seen on at least one occasion at follow-up. There were no relapses. Fourteen (8.1%) of 172 patients were fecal culture positive on at least one occasion (Table 2). There were no significant differences in positive fecal carriage between the three groups at each visit (Table 3). No patient was fecal culture positive at more than one visit. For each patient with a positive isolate at follow-up, there was no increase in the ofloxacin MIC of the follow-up isolate compared with the pretreatment isolate. The two patients who were fecal culture positive at the 6-month visit were lost to further follow-up.
Self-limiting gastrointestinal side effects were reported for a small number of patients in each treatment arm but did not require any interruption of therapy. The mean levels of aspartate transaminase and alanine transaminase fell in all treatment groups during treatment (Table 2). Three patients (aged 5, 11, and 12 years), one in each treatment arm, described joint discomfort during follow-up that had resolved by the next visit. There were no other significant side effects attributable to either antibiotic.
DISCUSSION
Patients with uncomplicated typhoid fever due to infection with S.enterica serovar Typhi fully susceptible to fluoroquinolones (MIC ≤ 0.06 μg/ml) treated with ciprofloxacin for 7 days have a clinical and microbiological success rate approaching 100% (20, 21, 36). Such infections even respond well to shorter courses of ofloxacin. In two studies conducted in Vietnam, a 5-day regimen of ofloxacin at 8 to 10 mg/kg/day cured 100% of 22 adults, with a mean fever clearance time of 3.4 days (33), and 97% of 38 children, with a mean fever clearance time of 4.4 days (9), with no microbiological failures in either study. In contrast, patients with uncomplicated typhoid fever due to infection with S. enterica serovar Typhi with reduced susceptibility to fluoroquinolones (MIC of 0.25 to 1.0 μg/ml) have an impaired response when treated with short courses (<7 days) of ofloxacin (27, 35). Suggestions that such infections will respond better to longer courses of ofloxacin treatment have not previously been studied in a controlled trial. This study shows that the clinical response to ofloxacin given at 20 mg/kg/day for 7 days is significantly impaired in patients infected with isolates of S. enterica serovar Typhi with reduced susceptibility to ofloxacin. Only 64% of patients treated with ofloxacin were cured, with a mean fever clearance time of 8.2 days. There were two (3.2%) microbiological failures indicated by a positive blood culture posttreatment. Subsequent to the period when this study was being conducted, a case series from south India found 8 of 38 patients with S. enterica serovar Typhi infection had a positive blood culture after 6 days of ciprofloxacin treatment at a dose of 500 mg orally or 400 mg intravenously twice daily (31). All of the failure isolates had a ciprofloxacin MIC of 0.25 to 1.0 μg/ml. These results suggest that fluoroquinolones should only be used to treat typhoid fever caused by isolates of S. enterica serovar Typhi with reduced fluoroquinolone susceptibility with considerable caution.
Although there are several potential reasons for treatment failure, pharmacokinetic and pharmacodynamic (PK-PD) parameters are likely to be an important factor. A PK-PD parameter now widely used for fluoroquinolones is the free drug area under the concentration-time curve from 0 to 24 h/MIC ratio (AUC/MIC ratio) (16). The PK-PD parameters determining the optimum response in fluoroquinolone-treated typhoid fever has not been studied in humans. However, one study has attempted to use an in vitro model with S. enterica serovar Typhi and Monte Carlo simulations to explore PK-PD parameters that were predictive of efficacy (6). This study found that the AUC/MIC ratio was the parameter most predictive of efficacy and that a ratio of 105 corresponded to 90% of maximal activity. In a pharmacokinetic study of ofloxacin (7.5 mg/kg body weight twice daily) in the treatment of children, a total area under the concentration-time curve from 0 to 12 h of 26.5 mg/h/liter was observed (3). Using this value, assuming that ofloxacin is approximately 35% protein bound, the calculated AUC/MIC ratio for an isolate with an MIC of ≤0.06 μg/ml would be ≥574. For an isolate with an MIC of ≥0.5 μg/ml, however, the calculated AUC/MIC ratio would be ≤69. Although the ofloxacin dosages used in the two studies were slightly different, these in vitro data are generally consistent with the good clinical response to ofloxacin with infections caused by isolates with an ofloxacin MIC of ≤0.06 μg/ml (9, 33) and the poor clinical response to ofloxacin observed in the current study with infections caused by isolates with an ofloxacin MIC of 0.5 to 1.0 μg/ml. It should be noted that these calculations use a mean AUC value, whereas the degree of exposure to fluoroquinolones in individual patients is variable, and this may explain why not all patients that have an organism with an MIC of ≥0.5 μg/ml will fail therapy.
There has been ongoing discussion concerning appropriate fluoroquinolone breakpoints for invasive Salmonella infections (1, 12). The presence of nalidixic acid resistance has been suggested as a laboratory marker of isolates with reduced susceptibility to fluoroquinolones and an indicator that invasive infections may fail to respond to fluoroquinolone therapy (24). In this study, isolates with an ofloxacin MIC of 0.25 to 1.0 μg/ml were detected by the presence of nalidixic acid resistance with 100% sensitivity and specificity. The ofloxacin MIC of the nalidixic acid-susceptible isolates was between 0.03 to 0.125 μg/ml. However, some recent data highlighted a significant number of S. enterica serovar Typhi isolates with reduced susceptibility to fluoroquinolones that were not nalidixic acid resistant (11). This suggests that nalidixic acid resistance may no longer be a reliable marker of reduced fluoroquinolone susceptibility.
Nearly 20% of patients treated with ofloxacin had positive fecal cultures immediately posttreatment, although convalescent-phase fecal carriage after discharge from the hospital was not significantly different from that of patients in the other treatment arms. This transient fecal carriage posttreatment has the potential to allow further transmission of S. enterica serovar Typhi among the family and close contacts. A slightly curious feature of this study was the absence of relapses in any of the treatment groups. This cannot be attributed to poor follow-up, as 86% of patients were seen at the first follow-up at 1 month and 92% of patients were seen on at least one occasion during the 6-month follow-up period.
Azithromycin at 10 mg/kg/day for 7 days cured more than 80% of patients with an average fever clearance time of 5.8 days in this study. The microbiological failure rate was 3.2%. In a similar study of Egyptian adults, azithromycin (1 g on day 1 followed by 500 mg on the succeeding 6 days) was compared with ciprofloxacin for 7 days (21). Symptoms and signs had resolved in all patients treated with azithromycin by day 7, with a mean (standard deviation) fever clearance time of 3.8 (1.1) days. In Egyptian children, azithromycin (10 mg/kg/day; maximum dose, 500 mg/day) for 7 days was compared with ceftriaxone for 7 days (19). A total of 91% (31/34) of the azithromycin-treated children were clinically cured by day 7, and the mean duration of fever after starting therapy was 4.1 (1.1) days. Two studies have examined a regimen of azithromycin at a dose of 20 mg/kg/day (maximum, 1 g/day) in children or 1 g/day in adults given for 5 days. Of the Egyptian children, 94% (30/32) were cured, with a mean duration of fever of 4.5 days (18). Of Vietnamese adults, 96% (42/44) were cured, with a mean duration of fever of 5.4 days (10). The slightly lower cure rate in this study compared with those in these other studies may be due to the higher doses of azithromycin used in the other studies. The response to azithromycin in this study compares favorably with the results reported for ceftriaxone and cefixime when given for 7 days for uncomplicated enteric fever (5, 9, 18, 19, 36).
The in vitro activity of azithromycin against S. enterica serovar Typhi in this study (MIC90, 16 μg/ml; range, 4 to 32 μg/ml) was similar to that of other reports (8). The MIC is above the reported peak serum level of 0.4 μg/ml following a 500-mg oral dose of azithromycin (17). However, azithromycin achieves intracellular concentrations up to 50 to 100 times that in serum, and at an alkaline pH and with a low inoculum, conditions that may reflect the in vivo situation, the MIC is lower (7, 26, 29). The discordance between in vitro susceptibility and in vivo effectiveness is probably explained by the predominantly intracellular location of S. enterica serovar Typhi. However, an estimated one-third of S. enterica serovar Typhi isolates in the blood of patients with typhoid are extracellular (34) and consequently may be exposed to inadequate concentrations of azithromycin that could result in slow clearance of bacteremia. Of note, therefore, is that one of the patients treated with azithromycin in this study was blood culture positive posttreatment despite apparent resolution of symptoms. Exposure of the organism to subtherapeutic levels of azithromycin may encourage the emergence of resistance, and this is an issue that merits further study.
The addition of 3 days of azithromycin treatment to 7 days of ofloxacin treatment improved the overall cure rate compared with ofloxacin treatment alone, despite the use of a lower dose of ofloxacin, although the difference was not statistically significant. There was no evidence that the combination discouraged the emergence of fluoroquinolone-resistant strains, although the study was too small to properly address this question. The average fever clearance time with azithromycin was about one and a half days shorter than that of the ofloxacin-azithromycin combination and two and a half days shorter than that of ofloxacin alone. Azithromycin alone and the ofloxacin-azithromycin combination were more effective than ofloxacin alone in eradicating the early posttreatment fecal carriage, although there was no difference in fecal carriage rates during convalescence. Both antimicrobials were well tolerated, and no significant joint problems were reported in the children treated with ofloxacin in the 6-month follow-up period. This acceptable safety profile is in keeping with the observations of other studies in which fluoroquinolones have been used for children (4, 14, 37).
For a 20-kg child in Vietnam, a 7-day course of ofloxacin (20 mg/kg/day) costs $2 to $10, depending on the manufacturer, the ofloxacin-azithromycin combination used in this study costs $8 to $14, 7 days of azithromycin costs $15, and a 10-day course of intravenous ceftriaxone costs $23 to $93. The use of ofloxacin or ciprofloxacin for 7 days as a first-line therapy for typhoid infections caused by isolates that are MDR and with reduced susceptibility to fluoroquinolones will result in one-third of patients remaining symptomatic at the end of treatment. They will require further treatment, and patients will be at increased risk of developing severe or complicated disease. Furthermore, prolonged fecal carriage could lead to increased transmission and may also encourage the appearance and dissemination of fully resistant isolates (22, 23). In this study, a 7-day course of azithromycin was more effective as an initial oral treatment for uncomplicated typhoid fever. Whether widespread adoption of azithromycin as a first-line treatment in areas where MDR strains with reduced susceptibility to fluoroquinolones are common will in turn lead to the emergence of azithromycin resistance remains to be seen.
Acknowledgments
We thank the Directors of the Dong Thap Provincial Hospital and the staff of the infection ward and the microbiology laboratory and the Directors of the Hospital for Tropical Diseases, Ho Chi Minh City, for their support of this study and Brian Faragher, Liverpool School of Tropical Medicine, for statistical advice.
The Wellcome Trust United Kingdom funded this study.
Footnotes
Published ahead of print on 4 December 2006.
REFERENCES
- 1.Aarestrup, F. M., C. Wiuff, K. Mølbak, and E. J. Threlfall. 2003. Is it time to change fluoroquinolone breakpoints for Salmonella spp.? Antimicrob. Agents Chemother. 47:827-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ackers, M.-L., N. D. Puhr, R. V. Tauxe, and E. D. Mintz. 2000. Laboratory-based surveillance of Salmonella serotype Typhi infections in the United States. JAMA 283:2668-2673. [DOI] [PubMed] [Google Scholar]
- 3.Bethell, D. B., N. P. J. Day, M. D. Nguyen, C. McMullin, T. L. Ha, T. H. T. Dong, T. N. M. Le, T. M. L. Nguyen, Q. D. Nguyen, V. Ha, A. P. MacGowan, L. O. White, and N. J. White. 1996. Pharmacokinetics of oral and intravenous ofloxacin in children with multidrug-resistant typhoid fever. Antimicrob. Agents Chemother. 40:2167-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bethell, D. B., T. T. Hien, L. T. Phi, N. P. Day, H. Vinh, N. M. Duong, N. V. Len, L. V. Chuong, and N. J. White. 1996. Effects on growth of single short courses of fluoroquinolones. Arch. Dis. Child. 74:44-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhutta, Z. A., I. A. Khan, and M. Shadmani. 2000. Failure of short-course ceftriaxone chemotherapy for multidrug-resistant typhoid fever in children: a randomized controlled field trial in Pakistan. Antimicrob. Agents Chemother. 44:450-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Booker, B. M., P. F. Smith, A. Forrest, J. Bullock, P. Kelchlin, S. M. Bhavnani, R. N. Jones, and P. G. Ambrose. 2005. Application of an in vitro infection model and simulation for reevaluation of fluoroquinolone breakpoints for Salmonella enterica serotype Typhi. Antimicrob. Agents Chemother. 49:1775-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler, T., R. W. Frenck, R. B. Johnson, and R. Khakhria. 2001. In vitro effects of azithromycin on Salmonella typhi: early inhibition by concentrations less than the MIC and reduction of MIC by alkaline pH and small inocula. J. Antimicrob. Chemother. 47:455-458. [DOI] [PubMed] [Google Scholar]
- 8.Butler, T., C. Sridhar, M. Daga, K. Pathak, R. B. Pandit, R. Khakhria, C. N. Potkar, M. T. Zelasky, and R. B. Johnson. 1999. Treatment of typhoid fever with azithromycin versus chloramphenicol in a randomized multicentre trial in India. J. Antimicrob. Chemother. 44:243-250. [DOI] [PubMed] [Google Scholar]
- 9.Cao, X. T. P., R. Kneen, N. T. Anh, T. D. Luat, N. J. White, and C. M. Parry. 1999. A comparative study of ofloxacin and cefixime for treatment of typhoid fever in children. Pediatr. Infect. Dis. J. 18:245-248. [DOI] [PubMed] [Google Scholar]
- 10.Chinh, N. T., C. M. Parry, N. T. Ly, H. D. Ha, M. X. Thong. T. S. Diep, J. Wain, N. J. White, and J. J. Farrar. 2000. A randomised controlled comparison of azithromycin and ofloxacin for treatment of multidrug-resistant or nalidixic acid-resistant enteric fever. Antimicrob. Agents Chemother. 44:1855-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooke, F. J., J. Wain, and E. J. Threlfall. 2006. Fluoroquinolone resistance in Salmonella typhi. Br. Med. J. 333:353-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crump, J. A., T. J. Barrett, J. T. Nelson, and F. J. Angulo. 2003. Reevaluating fluoroquinolone breakpoints for Salmonella enterica serotype Typhi and for non-Typhi salmonellae. Clin. Infect. Dis. 37:75-81. [DOI] [PubMed] [Google Scholar]
- 13.Crump, J. A., S. P. Luby, and E. D. Mintz. 2004. The global burden of typhoid fever. Bull. W. H. O. 82:346-353. [PMC free article] [PubMed] [Google Scholar]
- 14.Doherty, C. P., S. K. Saha, and W. M. Cutting. 2000. Typhoid fever, ciprofloxacin and growth in young children. Ann. Trop. Paediatr. 20:297-303. [DOI] [PubMed] [Google Scholar]
- 15.Dutta, P., U. Mitra, S. Dutta, A. De, M. K. Chatterjee, and S. K. Bhattacharya. 2001. Ceftriaxone therapy in ciprofloxacin treatment failure in children. Indian J. Med. Res. 113:210-213. [PubMed] [Google Scholar]
- 16.Forrest, A. D., E. D. Nix, C. H Ballow, T. F Goss, M. C. Birmingham, and J. J. Schentag. 1993. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob. Agents. Chemother. 37:1073-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foulds, G., R. M. Shepard, and R. B. Johnson. 1990. The pharmacokinetics of azithromycin in human serum and tissues. J. Antimicrob. Chemother. 25(Suppl. A):73-82. [DOI] [PubMed] [Google Scholar]
- 18.Frenck, R. W., Jr., A. Mansour, I. Nakhla, I. Y. Sultan, S. Putnam, T. Wierzba, M. Morsy, and C. Knirsch. 2004. Short-course azithromycin for the treatment of uncomplicated typhoid fever in children and adolescents. Clin. Infect. Dis. 38:951-957. [DOI] [PubMed] [Google Scholar]
- 19.Frenck, R. W., Jr., I. Nakhla, Y. Sultan, S. B. Bassily, Y. F. Girgis, J. David, T. C. Butler, N. I. Girgis, and M. Morsy. 2000. Azithromycin versus ceftriaxone for the treatment of uncomplicated typhoid fever in children. Clin. Infect. Dis. 31:1134-1138. [DOI] [PubMed] [Google Scholar]
- 20.Gasem, M. H., M. Keyter, W. M. V. Dolmans, J. V. van der Ven-Jongekrijg, R. Djokomoeljanto, and J. W. M. van der Meer. 2003. Persistence of salmonellae in blood and bone marrow: randomized controlled trial comparing ciprofloxacin and chloramphenicol treatments against enteric fever. Antimicrob. Agents Chemother. 47:1727-1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Girgis, N. I., T. Butler, R. W. Frenck, Y. Sultan, F. M. Brown, D. Tribble, and R. Khakhria. 1999. Azithromycin versus ciprofloxacin for treatment of uncomplicated typhoid fever in a randomized trial in Egypt that included patients with multidrug resistance. Antimicrob. Agents Chemother. 43:1441-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasan, R., F. J. Cooke, S. Nair, B. N. Harish, and J. Wain. 2005. Typhoid and paratyphoid fever. Lancet 366:1603-1604. [DOI] [PubMed] [Google Scholar]
- 23.Mehta, G., V. S. Randhawa, and N. P. Mohapatra. 2001. Intermediate susceptibility to ciprofloxacin in Salmonella typhi strains in India. Eur. J. Clin. Microbiol. Infect. Dis. 20:760-761. [DOI] [PubMed] [Google Scholar]
- 24.National Committee for Clinical Laboratory Standards. 2003. Performance standards for antimicrobial sensitivity testing; disc diffusion. Supplemental tables. M100-S13 (M2). NCCLS, Wayne, PA.
- 25.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically-sixth edition. Approved standard. NCCLS document M7-A6. NCCLS, Wayne, PA.
- 26.Panteix, G., B. Guillaumond, R. Harf, A. Desbos, V. Sapin, M. Leclercq, and M. Perrin-Fayolle. 1993. In-vitro concentration of azithromycin in human phagocytic cells. J. Antimicrob. Chemother. 31(Suppl. E):1-4. [DOI] [PubMed] [Google Scholar]
- 27.Parry, C. M. 2004. The treatment of multidrug resistant and nalidixic acid resistant typhoid fever in Vietnam. Trans. R. Soc. Trop. Med. Hyg. 98:413-422. [DOI] [PubMed] [Google Scholar]
- 28.Parry, C. M., T. T. Hien, G. Dougan, N. J. White, and J. J. Farrar. 2002. Typhoid fever. N. Engl. J. Med. 347:1770-1782. [DOI] [PubMed] [Google Scholar]
- 29.Rakita, R. M., K. Jaques-Palaz, and B. E. Murray. 1994. Intracellular activity of azithromycin against bacterial enteric pathogens. Antimicrob. Agents Chemother. 38:1915-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodrigues, C., S. Shenai, and A. Mehta. 2003. Enteric fever in Mumbai, India: the good news and the bad news. Clin. Infect. Dis. 36:535. [DOI] [PubMed] [Google Scholar]
- 31.Rupali, P., O. C. Abraham, M. V. Jesudason, T. J. John, A. Zachariah, S. Sivaram, and D. Mathai. 2004. Treatment failure in typhoid fever with ciprofloxacin susceptible Salmonella enterica serotype typhi. Diagn. Microbiol. Infect. Dis. 49:1-3. [DOI] [PubMed] [Google Scholar]
- 32.Saha, S. K., S. Y. Talukder, M. Islam, and S. Saha. 1999. A highly ceftriaxone-resistant Salmonella typhi in Bangladesh. Pediatr. Infect. Dis. J. 18:387. [DOI] [PubMed] [Google Scholar]
- 33.Smith, M. D., N. M. Duong, N. T. T. Hoa, J. Wain, H. D. Ha, T. S. Diep, N. P. J. Day, T. T Hien, and N. J. White. 1994. Comparison of ofloxacin and ceftriaxone for short-course treatment of enteric fever. Antimicrob. Agents Chemother. 38:1716-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wain, J., T. S. Diep, V. A. Ho, A. M. Walsh, N. T. T. Hoa, C. M. Parry, and N. J. White. 1998. Quantitation of bacteria in blood of typhoid fever patients and relationship between counts and clinical features, transmissibility, and antibiotic resistance. J. Clin. Microbiol. 36:1683-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wain, J., N. T. T. Hoa, N. T. Chinh, H. Vinh, M. J. Everett, T. S. Diep, N. P. Day, T. Solomon, N. J. White, L. J. Piddock, and C. M. Parry. 1997. Quinolone-resistant Salmonella typhi in Viet Nam: molecular basis of resistance and clinical response to treatment. Clin. Infect. Dis. 25:1404-1410. [DOI] [PubMed] [Google Scholar]
- 36.Wallace, M. R., A. A. Yousif, G. A. Mahroos, T. Mapes, E. J. Threlfall, B. Rowe, and K. C. Hyamms. 1993. Ciprofloxacin versus ceftriaxone in the treatment of multiresistant typhoid fever. Eur. J. Clin. Microbiol. Infect. Dis. 12:907-910. [DOI] [PubMed] [Google Scholar]
- 37.Yee, C. L., C. Duffy, P. G. Gerbino, S. Stryker, and G. J. Noel. 2002. Tendon or joint disorders in children after treatment with fluoroquinolones or azithromycin. Pediatr. Infect. Dis. J. 21:525-529. [DOI] [PubMed] [Google Scholar]