Abstract
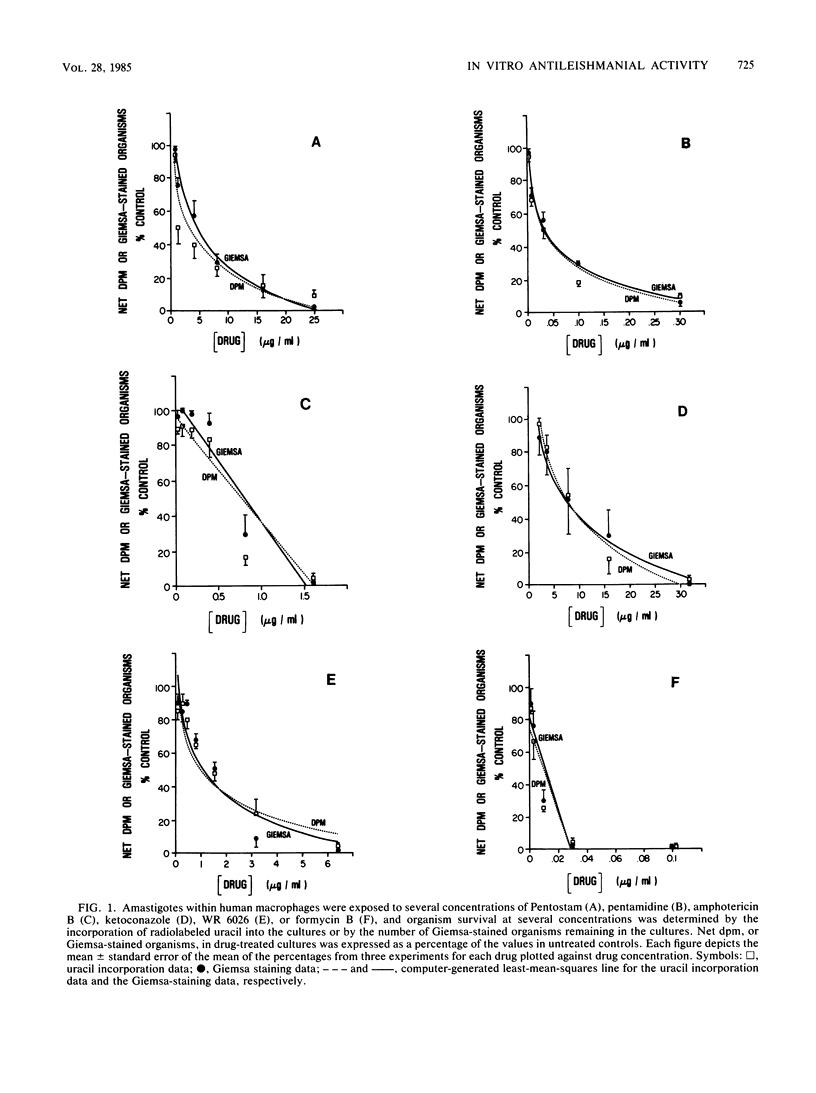
We have compared the in vitro activity of six agents against macrophage-contained Leishmania tropica amastigotes determined by the conventional Giemsa staining procedure, with the activity determined by the semiautomated assessment of incorporation of radiolabeled uracil into the nucleic acid of the organisms. Although the mean 50% effective dose of Pentostam by Giemsa staining (4.1 micrograms/ml) was somewhat higher than that by uracil incorporation (2.8 micrograms/ml), the ED50S for the other two clinical agents (pentamidine, 0.035 versus 0.037 micrograms/ml; amphotericin B, 0.67 versus 0.70 micrograms/ml) and for three promising experimental agents (ketoconazole, 11.3 versus 11.3 micrograms/ml; the 8-aminoquinoline WR 6026, 1.6 versus 1.5 micrograms/ml formycin B, 0.018 versus 0.017 micrograms/ml) were virtually identical. The radiolabeling technique has several advantages over the Giemsa staining procedure. These include the need for relatively few macrophages, rapid and objective data generation, and viability of the test organism being measured. The successful application of the radiolabeling technique to at least six different chemical classes of compounds suggests that it would be useful for the routine assessment of antileishmanial activity in vitro.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berman J. D., Dwyer D. M., Wyler D. J. Multiplication of Leishmania in human macrophages in vitro. Infect Immun. 1979 Oct;26(1):375–379. doi: 10.1128/iai.26.1.375-379.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman J. D., Lee L. S. Activity of antileishmanial agents against amastigotes in human monocyte-derived macrophages and in mouse peritoneal macrophages. J Parasitol. 1984 Apr;70(2):220–225. [PubMed] [Google Scholar]
- Berman J. D. Leishmania tropica: quantitation of in vitro activity of antileishmanial agents by Giemsa staining, viability, and 3H-formycin B incorporation. J Parasitol. 1984 Aug;70(4):561–562. [PubMed] [Google Scholar]
- Berman J. D., Wyler D. J. An in vitro model for investigation of chemotherapeutic agents in leishmaniasis. J Infect Dis. 1980 Jul;142(1):83–86. doi: 10.1093/infdis/142.1.83. [DOI] [PubMed] [Google Scholar]
- Desjardins R. E., Canfield C. J., Haynes J. D., Chulay J. D. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother. 1979 Dec;16(6):710–718. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidaris C. G., Bonventre P. F. Efficacy of combined immunostimulation and chemotherapy in experimental visceral Leishmaniasis. Am J Trop Med Hyg. 1983 Mar;32(2):286–295. doi: 10.4269/ajtmh.1983.32.286. [DOI] [PubMed] [Google Scholar]
- McLeod R., Remington J. S. A method to evaluate the capacity of monocytes and macrophages to inhibit multiplication of an intracellular pathogen. J Immunol Methods. 1979 May 10;27(1):19–29. doi: 10.1016/0022-1759(79)90235-7. [DOI] [PubMed] [Google Scholar]
- Nacy C. A., Diggs C. L. Intracellular replication of Leishmania tropica in mouse peritoneal macrophages: comparison of amastigote replication in adherent and nonadherent macrophages. Infect Immun. 1981 Oct;34(1):310–313. doi: 10.1128/iai.34.1.310-313.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacy C. A., Meltzer M. S., Leonard E. J., Wyler D. J. Intracellular replication and lymphokine-induced destruction of Leishmania tropica in C3H/HeN mouse macrophages. J Immunol. 1981 Dec;127(6):2381–2386. [PubMed] [Google Scholar]
- Neal R. A., Matthews P. J. In vivo antileishmanial properties of pentavalent antimonial compounds. Trans R Soc Trop Med Hyg. 1982;76(2):284–284. doi: 10.1016/0035-9203(82)90305-4. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn E. R., Pfefferkorn L. C. Specific labeling of intracellular Toxoplasma gondii with uracil. J Protozool. 1977 Aug;24(3):449–453. doi: 10.1111/j.1550-7408.1977.tb04774.x. [DOI] [PubMed] [Google Scholar]


