Abstract
Tubulointerstitial nephritis antigen (TIN-ag) is an extracellular matrix protein and is expressed in the renal tubular basement membranes. Its role in metanephric development was investigated. TIN-ag cDNA, isolated from the newborn mouse library, had an ORF of 1,425 nucleotides, a putative signal sequence, and an ATP/GTP-binding site. The translated sequence had ≈80% identity with rabbit TIN-ag. Among various tissues, TIN-ag mRNA was primarily expressed in the newborn kidney. In the embryonic metanephros, TIN-ag expression was confined to the distal convolution or pole of the S-shaped body, the segment of the nascent nephron that is the progenitor of renal tubules. Treatment with TIN-ag antisense oligodeoxynucleotide induced dysmorphogenesis of the embryonic metanephroi, malformation of the S-shaped body, and a decrease in the tubular population, whereas the glomeruli were unaffected. Treatment also led to a decrease of TIN-Ag mRNA, de novo synthesis of TIN-ag protein, and its antibody reactivity. The mRNA expression of glomerular epithelial protein 1 (a marker for renal podocytes), anti-heparan-sulfate-proteoglycan antibody reactivity, and wheat germ agglutinin lectin staining of the metanephros were unaffected. The anti-TIN-ag antibody treatment also caused deformation of the S-shaped body and a reduction in the tubular population, whereas the glomeruli were unchanged. The data suggest that the TIN-ag, unlike other basement membrane proteins, selectively regulates tubulogenesis, whereas glomerulogenesis is largely unaffected.
Keywords: metanephric development
Extracellular matrix (ECM) basement proteins are widely expressed in mammalian tissues and play a role in various biological processes, including the morphogenesis of various organs during embryonic development (1–4). The development of embryonic metanephros consists of two distinct processes, i.e., glomerulogenesis and tubulogenesis (5–7). It is likely that the ECM proteins are involved in both processes: they are expressed in the basal lamina of the glomerulus and of the tubule, and their formation ensues in the S-shaped body stage of the nephron. Relative to the geographic location of the ureteric bud branches, the S-shaped body has a distal and a proximal convolution or pole. The cells in the distal pole, i.e., those near the tips of ureteric bud branches, are believed to be the precursors of epithelial-lined tubules, whereas those in the proximal convolution develop into a renal glomerulus. This unique segmentation of the S-shaped body stage of the nascent nephron that dictates the divergence of glomerulogenesis and tubulogenesis is well known (5). At the S-shaped body stage, most of the basement membrane proteins are codistributed in the basal lamina of the cells lining both convolutions (4). With maturation of the S-shaped body, expression of the ECM proteins becomes accentuated, and it persists equally in the basement membranes of both the mature glomeruli and the tubules. Interestingly, tubulointerstitial nephritis antigen (TIN-ag), a recently described protein, has a restricted expression in the renal tubular basement membranes (8). It is a ≈58-kDa developmentally regulated glycoprotein, and it interacts with type IV collagen and laminin to promote cell adhesion, a process critical to organogenesis in embryonic life (9, 10). TIN-ag also serves as a ligand for integrins α3β1 and αVβ3, the latter of which seems to modulate nephrogenesis (11). These ligand-receptor interactions, i.e., integrin–ECM protein, sustain the epithelial–mesenchymal interactions that are known for their role in organogenesis (3). In view of the distinct characteristics of TIN-ag, studies were initiated to investigate its role in renal development.
MATERIALS AND METHODS
Animals.
ICR mice (Harlan–Sprague–Dawley) were used, and metanephroi (≈1,500) were harvested at embryonic day 13 (E13). In addition, kidney, lung, liver, spleen, heart, and brain of newborn mice were procured.
Isolation and Characterization of TIN-ag cDNA.
A 532-bp TIN-ag cDNA, previously isolated in our laboratory, was used for screening the mouse newborn cDNA library (10). Overlapping clones (n = 5) were isolated and subcloned into pBluescript II KS(+) phagemid by using XL1-Blue MRF′ cells (Stratagene), and single-stranded DNA preparations were made for nucleotide sequencing (12). A reticulocyte lysate in vitro translation system was used to confirm the ORF of TIN-ag cDNA and to verify the putative protein product. For the in vitro translation, two full-length TIN-ag cDNAs were selected as template. The reaction products were subjected to SDS/10% PAGE, and autoradiograms were prepared. A positive control included luciferase-encoding plasmid that yields a translated product of ≈61 kDa. A reaction mixture containing no plasmid served as a negative control.
The TIN-ag expression was assessed by Northern blot analysis. Total RNA, isolated from various tissues, was subjected to 1% agarose gel electrophoresis. A blot was prepared by transferring the RNA to a nylon membrane and hybridizing with [α-32P]dCTP-labeled mouse TIN-ag cDNA. The same blot was also hybridized with a β-actin probe (11).
In Situ Protein and Gene Expression of TIN-ag and Basement Membrane Heparan-Sulfate-Proteoglycan (HS-PG) in S-Shaped Body of E13 Metanephros.
Studies were performed to contrast the spatial distribution of TIN-ag vs. other ECM proteins, e.g., HS-PG, in the S-shaped body. For protein expression, immunofluorescence studies were carried out, and polyclonal antibodies, directed against the HS-PG and TIN-ag were used (8, 13). Cryostat sections of the E13 metanephroi were incubated with primary polyclonal antibody, followed by a wash with PBS and reincubation with rabbit anti-goat IgG or goat anti-rabbit IgG conjugated with FITC. The sections were then examined with a UV microscope.
For gene expression studies, in situ hybridization was performed (11). The RNA probes were synthesized by using an in vitro transcription system. For TIN-ag expression, full-length 1.4-kilobase cDNA was used as the template. For HS-PG, a 521-bp PCR product, generated by using sense (5′-GCTGCTAGCGGTGACGCATG-3′) and antisense (5′-CTGTGCCCAGGCGTCGGAAC-3′) primers, was used as the template. These riboprobes were used for in situ hybridization with the formaldehyde-fixed tissue sections. After hybridization, the sections were coated with a photographic emulsion, and autoradiograms were developed.
Antisense Experiments.
A 31-mer sense-phosphorothioated TIN-ag oligodeoxynucleotide (ODN) and an antisense-phosphorothioated TIN-ag ODN were prepared, and their sequence was 5′-CCACCGCAAGTGTGGCAGCTGACCGAATTGC-3′ (Fig. 1). Another nonsense 31-mer ODN was also prepared with the following sequence: 5′-TAATGATAGTAATGATAGTAATGATAGTAAT-3′. These ODNs had no significant homology with other mammalian nucleotide sequences, and their specificity was determined by an S1 nuclease protection assay (11).
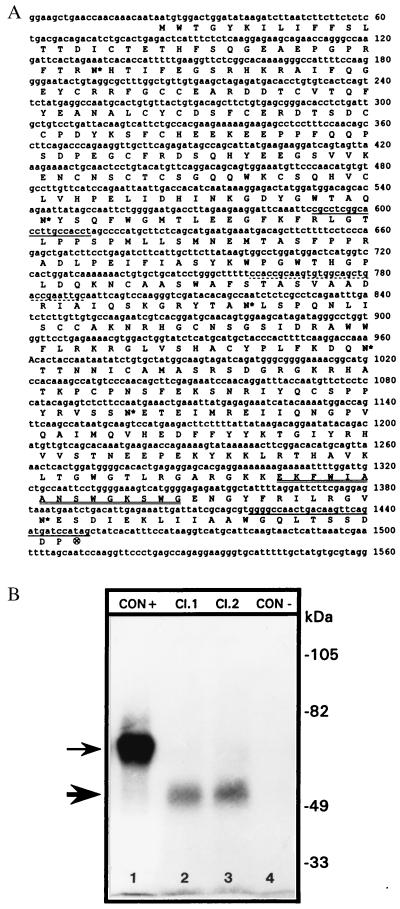
Figure 1.
(A) Nucleotide and deduced amino acid sequence of mouse TIN-ag cDNA. Dotted underline, antisense-ODN; double underlines, ATP/GTP-binding motif; single underlines, PCR primers; ⊗, termination codon; asterisks, glycosylation sites. (B) Profiles of in vitro translated products. A band of ≈52 kDa (thick arrow) is observed when two different full-length TIN-ag cDNA clones were used as the templates in the reaction mixture (lanes 2 and 3). The thin arrow indicates a ≈61-kDa product in lane 1 generated from the control plasmid. Lane 4 represents a negative control where template cDNA was omitted in the reaction mixture. CON+, translation reaction with control plasmid DNA; Cl. 1, translation reaction with TIN-ag cDNA clone 1; Cl. 2, translation reaction with TIN-ag cDNA clone 2; CON−, translation reaction without control plasmid DNA.
E13 metanephroi were maintained in an organ culture (13). The ODNs were added to the culture medium daily at concentrations ranging from 0.1 to 1.5 μM for 1–4 days. At these concentrations, the ODNs retain their translational blockade specificity with no discernible cytotoxic effects (11, 13). The treated metanephroi were processed for various studies.
Quantitative Reverse Transcription–PCR (RT-PCR) Analyses of TIN-ag mRNA of Antisense-ODN-Treated Fetal Kidneys.
Total RNA was isolated from 50 explants per variable by the acid guanidinium isothiocyanate-phenol-chloroform extraction method (14). About 50 μg of total RNAs from each variable were subjected to first-strand cDNA synthesis by using Moloney murine leukemia virus-RT and oligo(dT) as the primer. The (wild-type) cDNAs from different variables were suspended in water. Their DNA concentrations were determined, and the cDNAs were used for competitive RT-PCR analyses.
For the analyses of TIN-ag mRNA, the sense and antisense primers were 5′-CGCCTCGGCACCTTGCCACCT-3′ and 5′-CTATGGATCATCTGAACTTGTCAGTTGGCCCC-3′ (Fig. 1), with an expected PCR product of 861 bp when wild-type cDNA was used as a template. The β-actin sense and antisense primers were 5′-GACGACCATGGAGAAGATCTGG-3′ and 5′-GAGGATGCGGCAGTGCGGAT-3′), with an expected 461-bp PCR product (13). To generate competitive DNA template for TIN-ag, the primers were added into a minigene construct (13). With the use of this mutant competitive DNA template, the expected size of the PCR product is 481 bp. This minigene construct contains the primer sequences for β-actin with an expected size of 224 bp of the PCR product. Another control used in the antisense experiment included the determination of mRNA expression of glomerular epithelial protein 1 (GLEPP1), which is expressed in the renal glomerulus. For GLEPP1, the respective sense and antisense primers were 5′-CCAGAAATGACTTCTGGAAGATGGTCC-3′ and 5′-CGGTATGAGCGCATCTCTGATACCAGCCC-3′, with an expected 467-bp PCR product (15). These primers were also added into the minigene construct to generate the competitive DNA template. The expected size of the PCR product with the mutant competitive DNA template and GLEPP1 primers is 271 bp. The RT-PCR analyses were carried out as described (11, 13). Authenticity of all the PCR products was confirmed by nucleotide sequence analyses (12).
TIN-ag Protein Expression in Antisense-ODN-Treated Renal Explants.
Treated explants were maintained in the organ culture system and radiolabeled with [35S]methionine (0.25 mCi/ml) for 12 h before the termination of culture and used for protein extraction and immunoprecipitation as described (11, 13). Briefly, total incorporated radioactivity in the explants was determined by trichloroacetic acid precipitation. Then, extracts with equal amounts of radioactivity (≈5 × 106 dpm) were incubated at 4°C successively with goat anti-rabbit TIN-ag and protein A Sepharose 4B. The antigen–antibody complexes were washed, dissolved in a sample loading buffer, and subjected to SDS/10% PAGE; autoradiograms were then prepared.
In Situ Tissue Expression of TIN-ag, HS-PG, Wheat Germ Agglutinin (WGA), and GLEPP1.
The tissue expression of TIN-ag in tubules of explants treated with 1.5 μM antisense, sense, or nonsense ODN was assessed by fluorescence microscopy. Also, the expression of HS-PG was evaluated. To evaluate the population of nascent glomeruli, expression of WGA and GLEPP1 was assessed. The sections stained with anti-HS-PG antibody were also stained with rhodamine-conjugated WGA. Finally, the GLEPP1 mRNA expression was determined in the nonsense/sense- and antisense-treated explants by in situ tissue autoradiography, and the 467-bp product generated above by PCR was used to prepare the riboprobe.
TIN-ag Antibody Experiments.
The antiserum was decomplemented, followed by ammonium sulfate precipitation and purification of IgG by DEAE-cellulose chromatography (11). The IgG was reconstituted with the culture medium to a final concentration of 1.0 mg/ml and was added daily into the culture medium at a concentration range of 1–10 μg/ml for 4 days. Normal goat IgG (10 μg/ml) served as a control.
RESULTS
Characterization of Mouse TIN-ag cDNA and Its mRNA Expression.
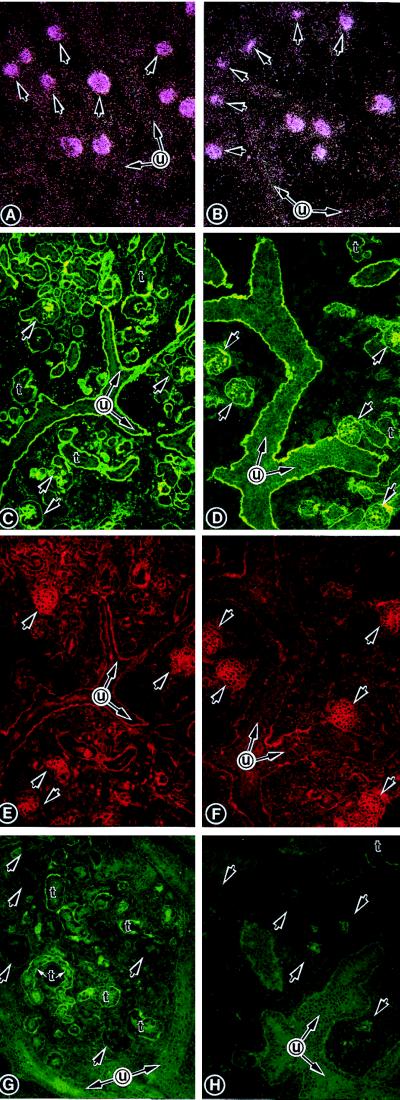
Two cDNA clones had initiation and termination codons, and the translated region was comprised of 1,425 nucleotides (Fig. 1A). The putative protein product size was confirmed by in vitro translation studies, and a band of ≈52-kDa was observed with both the clones that were used as the template (Fig. 1B). It had ≈80% identity with the rabbit TIN-ag, six N-linked glycosylation sites (NXT/NXS; N, asparagine; X, any amino acid; T, threonine; S, serine), and an ATP/GTP-binding site, i.e., EKFWIAANSWGKSWGENG. It had a hydrophobic stretch spanning up to the 19th amino acid residue, which may represent the putative signal peptide. In Northern blot analyses, a single transcript of ≈2.0 kilobases was observed in the newborn mouse kidney (Fig. 2A). A very faint band was observed in the lungs, and no message was detected in other organs.
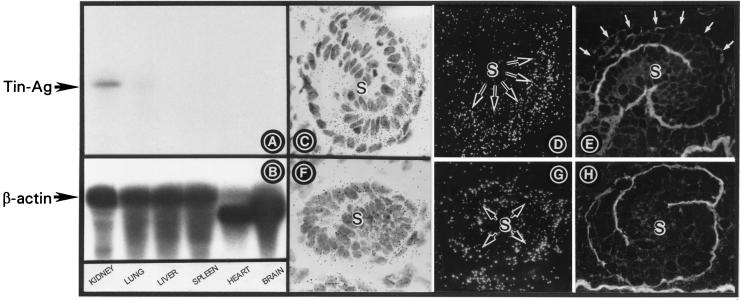
Figure 2.
(A and B) Northern blot analyses of TIN-ag and β-actin. A ≈2.0-kilobase transcript is observed in the kidney, and a very faint band is observed in the lung. The β-actin mRNA expression is similar in all the tissues. (C and D) TIN-ag mRNA expression in the S-shaped body (S). (E) TIN-ag protein expression. The expression in the basal lamina is confined to the distal convolution and is absent in the upper convolution (white arrows). (F and G) HS-PG mRNA expression. (H) HS-PG protein expression is confined to the basal lamina of both the convolutions. (C–H, ×250.)
Spatiotemporal Expression of TIN-ag and HS-PG in the S-Shaped Body Stage of Murine Renal Development.
The distribution of other ECM proteins (e.g., type IV collagen, laminin, entactin, and HS-PG) is similar in the S-shaped body (4). For instance, HS-PG protein expression is confined to the ECMs of the both the convolutions or poles, i.e., proximal and distal, of the S-shaped body (Fig. 2H). Similarly, its mRNA expression is seen in both the poles of the S-shaped body (Fig. 2 F and G). In contrast, the TIN-ag protein expression is seen only in the basal lamina of the distal convolution or pole of the S-shaped body (Fig. 2E). Furthermore, the TIN-ag mRNA expression is confined to mainly the distal pole of the S-shaped body with a slight extension into the vascular cleft (Fig. 2 C and D).
Role of TIN-ag in Tubulogenesis of the Mammalian Metanephros (Antisense Experiments).
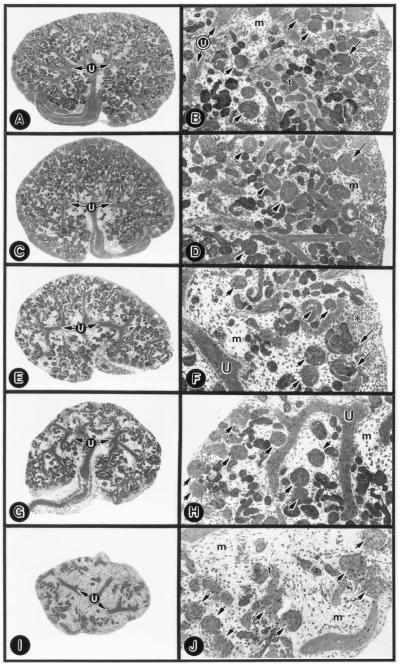
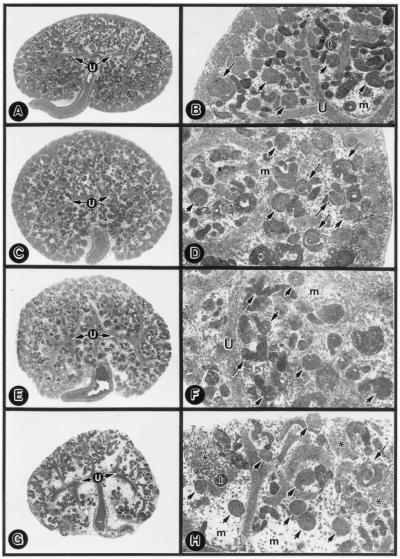
No morphologic change was observed in the ureteric bud branches, glomeruli, and tubules of the metanephroi treated with 1.5 μM nonsense or sense ODNs compared with the untreated explants (Fig. 3 C and D vs. A and B). A few S-shaped bodies were present, and they had normal S-shape configuration (Fig. 3 B and D, long arrows). The antisense treatment induced a dose-dependent reduction in the size of the explants (Fig. 3 E–J). At 0.5 μM ODN in the medium, the tubular population was reduced, and the mesenchyme was expanded (Fig. 3 E and F). The S-shaped bodies seemed to be malformed with the loss of their S-shape configuration (Fig. 3F, long arrows), and, in some, the malformation seemed to be polar, i.e., segmental loss of S-shape convolution. A few patches of compacted mesenchyme were observed (Fig. 3F, asterisk). At 1.0 μM, tubular loss was accentuated, and the tips of the ureteric bud branches were blunted (Fig. 3 G and H). At 1.5 μM, a marked decrease in the population of tubules per explant was observed (156.1 ± 10.56 → 23.7 ± 4.96; n = 10; Fig. 3 I and J). However, no significant decrease in the glomerular population was noted (20.5 ± 1.95 → 18.7 ± 2.0; n = 10; Fig. 3J, short arrows). The ureteric bud branches were rudimentary.
Figure 3.
Low- (A, C, E, G, and I) and high-magnification (B, D, F, H, and J) micrographs of untreated (A and B), nonsense-ODN-treated (C and D, 1.5 μM), and TIN-ag antisense-treated (E and F, 0.5 μM; G and H, 1.0 μM; I and J, 1.5 μM) E13 explants. The nonsense-ODN-treated explants reveal no significant alterations in the ureteric bud branches (U), in the S-shaped-body (long arrow), in the metanephric mesenchyme (m), or in the population of glomeruli (short arrows) and tubules (t) compared with the untreated explants. The antisense-treated explants reveal dose-dependent loss of tubules, whereas the glomeruli are unaffected. At a concentration of 0.5 μM (F), malformation of the S-shaped body (long arrows) and a few patches of compacted mesenchyme (asterisk) are observed. (A, C, E, G, and I, ×20; B, D, F, H, and J, ×80.)
Antisense Experiments (mRNA and Protein-Expression Studies).
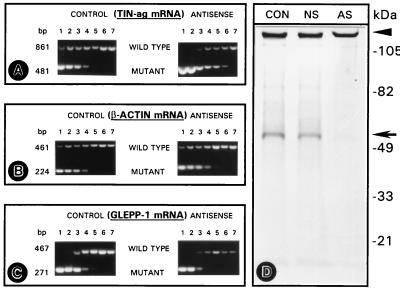
The mRNA expression was assessed by competitive RT-PCR analyses. Within the range of 10−1–10−7 serial logarithmic dilutions of the competitive (mutant) template DNA dilutions, the bands of wild-type and mutant DNA were discernible (Fig. 4 A–C, lanes 1 to 7), enabling densitometric analyses to obtain a ratio. The graphic plots are not included because of space limitations, and only the original electrophoretograms are included to indicate the intensity of the bands. In the nonsense ODN-treated (control) explants, a ratio of 1 was obtained at dilutions of 10−3–10−4 of the competitive mutant DNA (Fig. 4 A–C, lanes 3 and 4). In the antisense ODN-treated explants, the ratio of 1 for TIN-ag mRNA was obtained at dilutions of 10−5–10−6 of the competitive DNA (Fig. 4A, lanes 5 and 6), suggesting a decrease of two logs of the mRNA expression. For β-actin and GLEPP1, a ratio of 1 was obtained at dilutions of 10−3–10−4, suggesting no decrease in their mRNA expression (Fig. 4 B and C).
Figure 4.
(A–C) Competitive RT-PCR of TIN-ag (A), β-actin (B), and GLEPP1 (C) cDNAs prepared from nonsense-treated (CONTROL) and TIN-ag antisense-treated (ANTISENSE) metanephroi. A reduction in the amplification of wild-type TIN-ag DNA is observed in the antisense-treated group. This reduction is reflected by a shift in the band intensity ratio of 1 (wild-type vs. mutant) to 10−5–10−6 dilutions (lanes 5 and 6) of the competitive DNA from 10−3 to 10−4 (lanes 3 and 4) of the control group, suggesting a reduction in the TIN-ag mRNA population. No significant differences in the wild-type vs. mutant ratios are observed in the β-actin and GLEPP1 cDNAs. (D) SDS/PAGE autoradiogram of the de novo synthesized TIN-ag. A band of ≈58 kDa is seen (arrow) in the untreated control (CON) and nonsense-treated (NS) group. The band is barely discernible in the antisense-treated group (AS). The arrowhead indicates the point of sample application.
In translational blockade studies, the SDS/PAGE autoradiogram revealed a major ≈58-kDa band in the control untreated explants (Fig. 4D, CON). A similar band was noted in the sense/nonsense-treated groups (Fig. 4D, NS). However, a marked reduction in the intensity of the band was observed in the antisense-ODN-treated explants (Fig. 4D, AS), suggesting a blockade in the translation of TIN-ag.
Antisense Experiments (in Situ and Immunofluorescence Studies).
The GLEPP1 mRNA expression was localized to the glomeruli, and no significant decrease in their population was observed in the explants treated with 1.5 μM TIN-ag antisense-ODN (Fig. 5 B vs. A). The number of tubules was decreased; however, no differences in the reactivity of anti-HS-PG antibody with the ECMs of glomeruli, of ureteric bud branches, and of the remaining tubules were observed (Fig. 5 D vs. C). The staining of the same sections with WGA did not reveal any decrease in its reactivity with the glomeruli or their population (Fig. 5 F vs. E). A decrease in the intensity of immunofluorescence in the basement membranes of the remaining tubules (labeled as t) was observed in the antisense-treated explants stained with anti-TIN-ag antibody compared with the control (Fig. 5 H vs. G). The glomerular basement membrane and the mesangium had very weak immunoreactivity, but no significant differences were observed between the two groups (Fig. 5 H vs. G, short arrows).
Figure 5.
In situ autoradiograms (A and B) and fluorescence micrographs (C–H) of E13 explants treated either with 1.5 μM nonsense-ODN (A, C, E, and G) or TIN-ag antisense-ODN (B, D, F, and H). The GLEPP1 mRNA is localized to glomeruli, and no decrease in their population is observed after the antisense treatment (B vs. A, arrows). There were no changes in anti-HS-PG antibody reactivity (D vs. C), and WGA staining (F vs. E) is noted between the two groups, although a tubular (t) loss is observed in the antisense group. The TIN-ag antibody reactivity is seen in the tubular basement membranes (G), and a mild reduction is observed in the remaining tubules after antisense treatment (H). u, ureteric bud branches. (A and B, ×40; C–H, ×80.)
Anti-TIN-ag Antibody Experiments.
With 10 μg/ml of normal goat IgG in the medium, the explants did not reveal any significant alterations (Fig. 6 A and B vs. Fig. 3 A and B). However, dose-dependent alterations were noted with the inclusion of TIN-ag antibody in the medium (Fig. 6 C–H). At 2.5 μg/ml, the mesenchyme became loose, and the tubular population was decreased (Fig. 6 C and D). The S-shaped bodies were also deformed and had lost their normal configuration (Fig. 6D, long arrows). The deformation seemed to be segmental and polar in some of the S-shaped bodies. At 5 μg/ml, there was a further decrease in the population of tubules (Fig. 6F, long arrows). At 7.5 μg/ml, deformed S-shaped bodies were seen (Fig. 6H, long arrows), and the tubular population per explant was notably decreased (160.7 ± 10.5 → 30.9 ± 5.36; n = 10). A few patches of compacted mesenchyme were discernible, although most of it was loose and expanded (Fig. 6H, asterisks). The glomerular morphology and population were minimally affected (20.6 ± 1.84 → 18.8 ± 1.55; n = 10; Fig. 6H, short arrows).
Figure 6.
Low- (A, C, E, and G) and high-magnification (B, D, F, and H) micrographs of the goat-IgG-treated (A and B, 10 μg/ml) and anti-TIN-ag antibody-treated (C and D, 2.5 μg/ml; E and F, 5.0 μg/ml; G and H, 7.5 μg/ml) E13 explants. Normal goat-IgG induced no alterations in metanephroi. Anti-TIN-ag antibody treatment induced a dose-dependent deformation of the S-shaped bodies (long arrows) and reduction of the tubules (t), whereas glomeruli (short arrows) were unaffected. A few patches of compacted mesenchyme (asterisks) are discernible. u, ureteric bud branches; m, metanephric mesenchyme. (A, C, E and G, ×20; B, D, F, and H, ×80.)
DISCUSSION
TIN-ag is an ECM protein with restricted renal expression, and it is believed to interact with type IV collagen, laminin, and integrins α3β1 and αvβ3 (9, 10). Interestingly, unlike other ECM proteins, TIN-ag lacks RGD sequence, which is essential to cell-matrix interactions that are prevalent during organogenesis in embryonic life (16). Conceivably, its interactions with integrin receptor do not involve the RGD sequence. Thus, the lack of RGD sequence may suggest that other motifs, e.g., the ATP/GTP-binding motif, may regulate the functions of TIN-ag. As to how this ATP/GTP-binding motif modulates the TIN-ag functions remains to be investigated. Besides the ATP/GTP-binding motif, the other interesting feature of the TIN-ag is its selective expression in the renal tubules, and the fact that TIN-ag is developmentally regulated in the metanephros would suggest its potential role in renal development. Moreover, the findings that TIN-ag has a segment-specific spatial expression in the S-shaped body (i.e., in its distal convolution or pole), and the assumption that it may selectively influence renal tubulogenesis and not glomerulogenesis led us to investigate its role in renal development by using antisense techniques.
The antisense technology has been employed in studying various developmental processes (17), and it is a reliable method, provided that rigorous controls are included in a given study as previously reported (11, 13). The TIN-ag antisense-ODN induced dose-dependent alterations with reduction in the size of the explants, decreased population of the nephrons, and a loss in the acuteness of the tips of the ureteric bud branches. The blunting of the tips of the ureteric bud branches has been shown to perturb epithelial–mesenchymal interactions with an arrest of nephrogenesis (13). Interestingly, the loss of nephrons was confined selectively to the tubular population, whereas the glomeruli were unaffected, suggesting that the effects of TIN-ag antisense-ODN are specifically targeted at the segment of the S-shaped body that is the progenitor of the renal tubules. The fact that one could observe segmental dysmorphogenesis of the S-shaped bodies with the treatment of low concentration of the antisense-ODN would be in line with such an assertion. In the past, where various types of specific antisense-ODNs—whether targeted against the growth factors, protooncogenes, or matrix proteins—were used, none yielded a selective effect (11, 13); the reason may be that their respective genes have no segment-specific spatiotemporal expression. The specificity of the antisense effect is also supported by the competitive RT-PCR analyses, in which a ≈100-fold selective reduction in the TIN-ag mRNA expression was observed. Also, the fact that GLEPP1 expression was unaltered suggests that the TIN-ag antisense mainly affected the tubular epithelial mRNA population. The specificity of the antisense effect was also reflected in the translational blockade studies, in which a decrease in the intensity of the 58-kDa band in the SDS/PAGE autoradiogram and a decreased anti-TIN-ag immunoreactivity in the basal lamina of the remaining tubules were observed. Interestingly, anti-HS-PG reactivity in the basement membranes of the metanephros remained intact, suggesting that the other ECM proteins were unaffected. Because anti-HS-PG and WGA reactivity and the GLEPP1 mRNA expression in the glomeruli were not altered, it is reasonable to propose that the selective hypogenesis of the tubules is associated with the specific gene disruption and translational blockade of the TIN-ag.
Further evidence for this proposal was obtained by experiments in which the formation of tubules from the S-shaped body was arrested specifically by the anti-TIN-ag antibody. The utility of the antibodies to elucidate the role of various basement membrane proteins (e.g., laminin) in organ development has been documented (4, 18). It is interesting to note here that, like the antisense-ODN, the anti-TIN-ag antibody also selectively affected tubular morphogenesis that presumably ensued after the malformation of the S-shaped body. The mechanism or mechanisms by which anti-TIN-ag antibody or antisense-ODN induced tubular dysmorphogenesis remain elusive. There may be perturbation in ligand–receptor interactions—in this case between TIN-ag and α3β1 or αvβ3, the latter of which certainly influences nephrogenesis (11). The disruption of such interactions leading to renal dysmorphogenesis has been reported in the α8β1 knockout experiments (19). Finally, it is hoped that the results of this investigation would give impetus for future in vivo genetic manipulation studies to elucidate the role of TIN-ag in renal development.
Acknowledgments
The TIN-ag antibody was a generous gift from Drs. Charonis and Butkowski. This work was supported by National Institutes of Health Grant DK28492.
ABBREVIATIONS
- TIN-ag
tubulointerstitial nephritis antigen
- ECM
extracellular matrix
- En
embryonic day n
- GLEPP1
glomerular epithelial protein 1
- HS-PG
heparan-sulfate-proteoglycan
- ODN
oligodeoxynucleotide
- RT-PCR
reverse transcription–PCR
- WGA
wheat germ agglutinin
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF153366).
References
- 1.Farquhar M G. In: Cell Biology of Extracellular Matrix. Hay E D, editor. New York: Plenum; 1991. pp. 365–418. [Google Scholar]
- 2.Stetler-Stevenson W G. Am J Pathol. 1996;148:1345–1350. [PMC free article] [PubMed] [Google Scholar]
- 3.Hay E D, editor. Cell Biology of Extracellular Matrix. New York: Plenum; 1991. pp. 419–462. [Google Scholar]
- 4.Ekblom P. In: Molecular and Cellular Aspects of Basement Membranes. Rohrbach D H, Timpl R, editors. New York: Academic; 1993. pp. 359–383. [Google Scholar]
- 5.Saxen L. Organogenesis of the Kidney. New York: Cambridge Univ. Press; 1987. [Google Scholar]
- 6.Clapp W L, Abrahamson D R. Curr Opin Nephrol Hypertens. 1993;2:419–429. [PubMed] [Google Scholar]
- 7.Nigam S, Aperia A C, Brenner B M. In: The Kidney. Brenner B M, editor. Philadelphia: Saunders; 1996. pp. 72–98. [Google Scholar]
- 8.Nelson T R, Charonis A S, Mcivor R S, Butkowski R J. J Biol Chem. 1995;270:16265–16270. doi: 10.1074/jbc.270.27.16265. [DOI] [PubMed] [Google Scholar]
- 9.Kalfa T A, Thull J D, Butkowski R J, Charonis A S. J Biol Chem. 1994;269:1654–1659. [PubMed] [Google Scholar]
- 10.Kumar A, Ota K, Wada J, Wallner E I, Charonis A S, Kanwar Y S. Kidney Int. 1997;52:620–627. doi: 10.1038/ki.1997.375. [DOI] [PubMed] [Google Scholar]
- 11.Wada J, Kumar A, Liu Z, Ruoslahti E, Reichardt L, Marvaldi J, Kanwar Y S. J Cell Biol. 1996;132:1161–1176. doi: 10.1083/jcb.132.6.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanger F, Nicklen S, Coulson A R. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Z, Wada J, Kumar A, Ota K, Carone F A, Kanwar Y S. Dev Biol. 1996;178:133–148. doi: 10.1006/dbio.1996.0204. [DOI] [PubMed] [Google Scholar]
- 14.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 15.Thomas P E, Wharram B, Goyal M, Wiggins J, Holzman L, Wiggins R. J Biol Chem. 1994;269:19953–19961. [PubMed] [Google Scholar]
- 16.Humphries M J. In: Molecular and Cellular Aspects of Basement Membranes. Rohrbach D H, Timpl R, editors. New York: Academic; 1993. pp. 289–308. [Google Scholar]
- 17.Bronner-Fraser M. FASEB J. 1994;8:699–706. doi: 10.1096/fasebj.8.10.8050668. [DOI] [PubMed] [Google Scholar]
- 18.Schuger L, Skubitz A N, O’Shea K S, Chang J F, Varani J. Dev Biol. 1991;146:531–541. doi: 10.1016/0012-1606(91)90254-z. [DOI] [PubMed] [Google Scholar]
- 19.Muller U, Wang D, Denda S, Meneses J J, Pederson R A, Reichardt L F. Cell. 1997;88:603–613. doi: 10.1016/s0092-8674(00)81903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]