Abstract
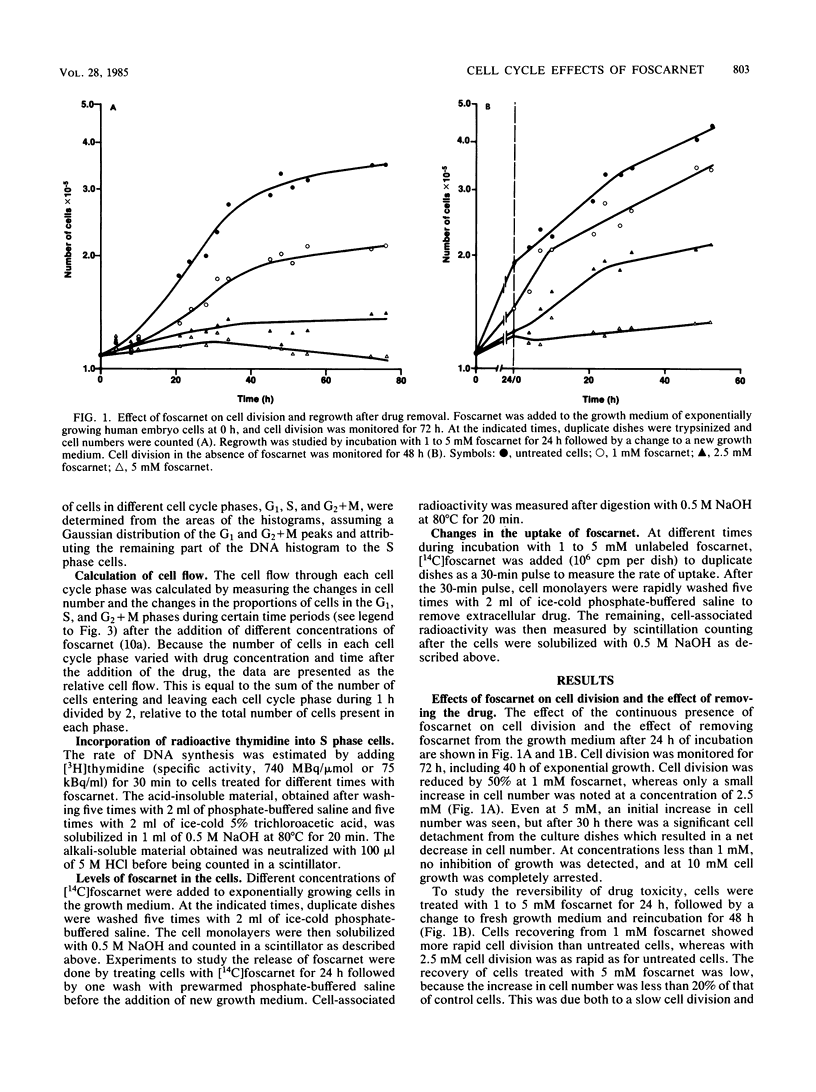
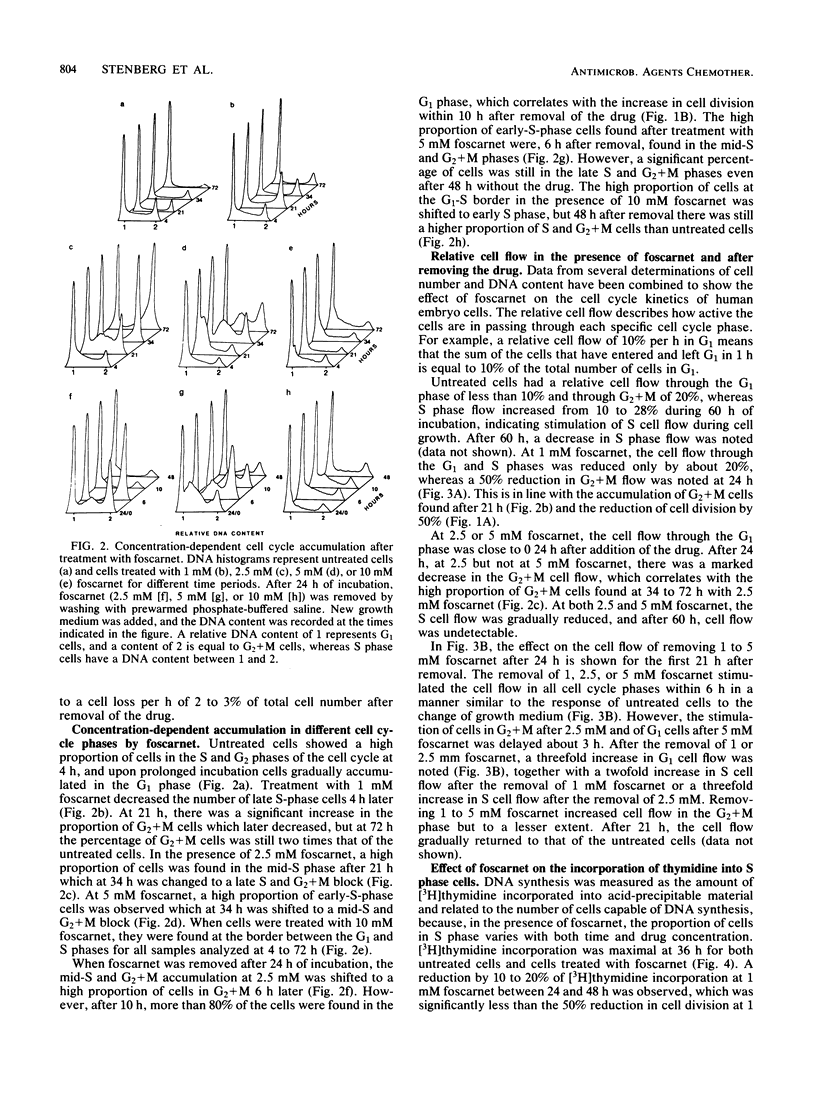
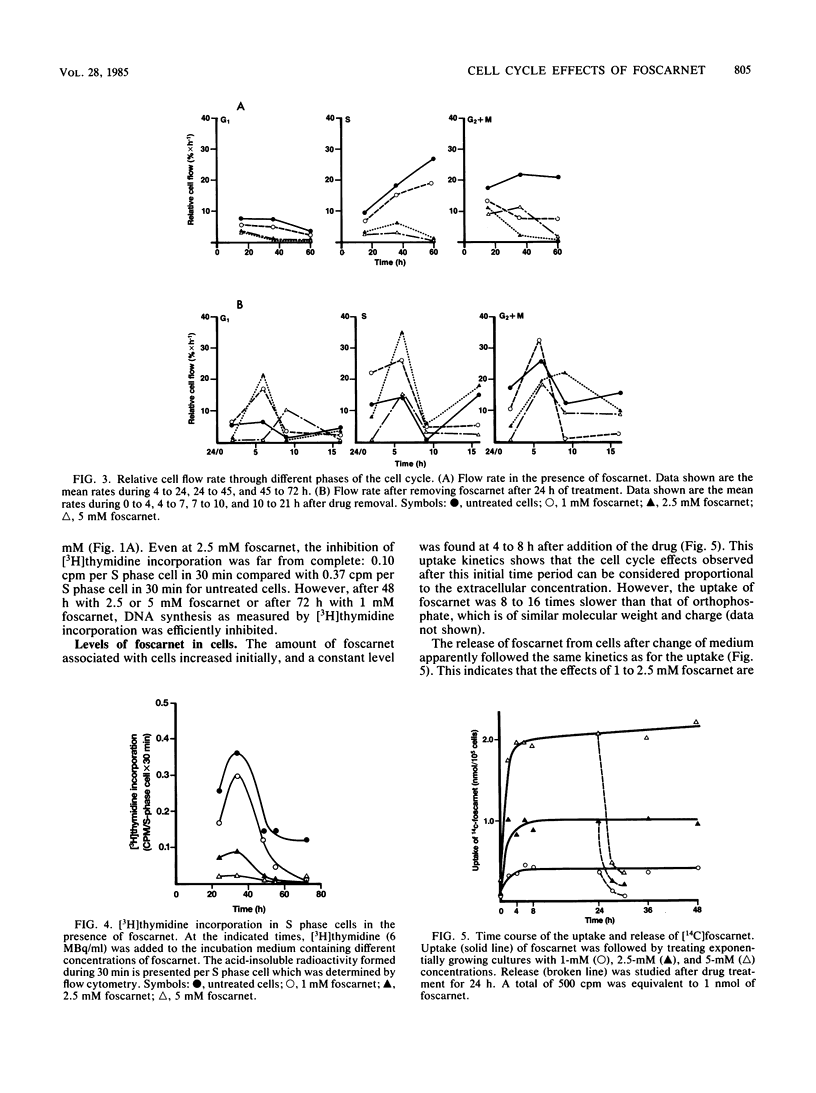
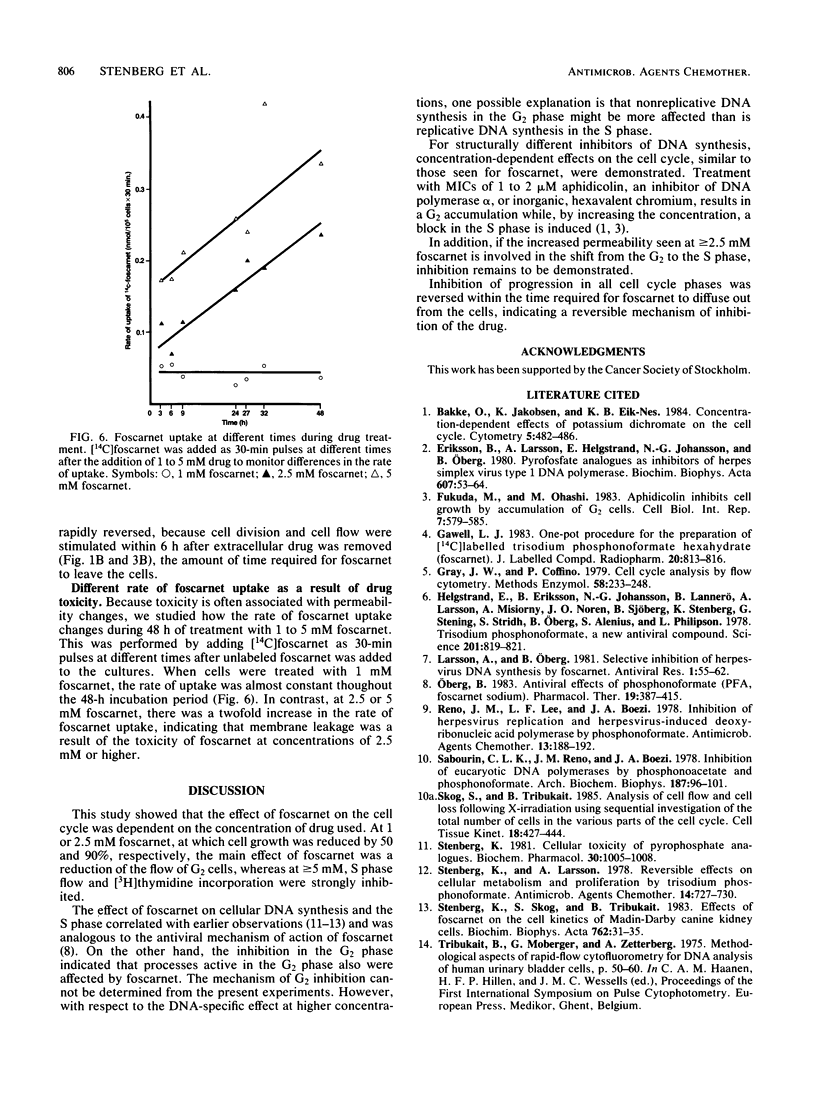
The mechanism of toxicity of foscarnet was studied by monitoring its effects on the cell cycle of exponentially growing, semisynchronous human embryo cells in culture. The effects of foscarnet on the cell cycle were dependent on the concentration of drug used. At 1 mM, cell division was reduced by 50%, whereas the cell flow was mainly reduced in the G2 phase of the cell cycle, leading to an increase in the proportion of G2+M cells. The minor reduction of thymidine incorporation in S phase cells provided additional evidence that 1 mM foscarnet did not specifically inhibit DNA synthesis. Cell division was greatly reduced at 2.5 mM foscarnet, and the G2 phase was markedly affected, whereas S cell flow was less reduced. S cell flow was 10% per h and thymidine incorporation was 25% that of control cells, while a block in the G2+M phase was evident. On the other hand, at a concentration of 5 mM foscarnet, the cell flow was greatly reduced in the G1 and S phases, with less reduction of G2 cell flow and cells accumulated in the S phase. The effects of foscarnet on the cell cycle were more pronounced with increasing times up to 72 h, which could not be explained by the slow penetration of foscarnet which required only 4 to 8 h to achieve constant levels. At 2.5 and 5 mM foscarnet, there was the additional effect of the cell membranes becoming more leaky as a result of foscarnet toxicity which might contribute to the toxic effects of the drug at high concentrations. When foscarnet was removed from the medium, the effects on the cell cycle were rapidly reversed, in the time needed for foscarnet to diffuse out from the cells, which indicates the reversible nature of the toxic effects of foscarnet.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakke O., Jakobsen K., Eik-Nes K. B. Concentration-dependent effects of potassium dichromate on the cell cycle. Cytometry. 1984 Sep;5(5):482–486. doi: 10.1002/cyto.990050508. [DOI] [PubMed] [Google Scholar]
- Eriksson B., Larsson A., Helgstrand E., Johansson N. G., Oberg B. Pyrophosphate analogues as inhibitors of herpes simplex virus type 1 DNA polymerase. Biochim Biophys Acta. 1980 Mar 28;607(1):53–64. doi: 10.1016/0005-2787(80)90220-8. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Ohashi M. Aphidicolin inhibits cell growth by accumulation of G2 cells. Cell Biol Int Rep. 1983 Aug;7(8):579–585. doi: 10.1016/0309-1651(83)90111-x. [DOI] [PubMed] [Google Scholar]
- Gray J. W., Coffino P. Cell cycle analysis by flow cytometry. Methods Enzymol. 1979;58:233–248. doi: 10.1016/s0076-6879(79)58140-3. [DOI] [PubMed] [Google Scholar]
- Helgstrand E., Eriksson B., Johansson N. G., Lannerö B., Larsson A., Misiorny A., Norén J. O., Sjöberg B., Stenberg K., Stening G. Trisodium phosphonoformate, a new antiviral compound. Science. 1978 Sep 1;201(4358):819–821. doi: 10.1126/science.210500. [DOI] [PubMed] [Google Scholar]
- Oberg B. Antiviral effects of phosphonoformate (PFA, foscarnet sodium). Pharmacol Ther. 1982;19(3):387–415. doi: 10.1016/0163-7258(82)90074-2. [DOI] [PubMed] [Google Scholar]
- Reno J. M., Lee L. F., Boezi J. A. Inhibition of herpesvirus replication and herpesvirus-induced deoxyribonucleic acid polymerase by phosphonoformate. Antimicrob Agents Chemother. 1978 Feb;13(2):188–192. doi: 10.1128/aac.13.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabourin C. L., Reno J. M., Boezi J. A. Inhibition of eucaryotic DNA polymerases by phosphonoacetate and phosphonoformate. Arch Biochem Biophys. 1978 Apr 15;187(1):96–101. doi: 10.1016/0003-9861(78)90010-3. [DOI] [PubMed] [Google Scholar]
- Skog S., Tribukait B. Analysis of cell flow and cell loss following X-irradiation using sequential investigation of the total number of cells in the various parts of the cell cycle. Cell Tissue Kinet. 1985 Jul;18(4):427–444. doi: 10.1111/j.1365-2184.1985.tb00673.x. [DOI] [PubMed] [Google Scholar]
- Stenberg K. Cellular toxicity of pyrophosphate analogues. Biochem Pharmacol. 1981 May 1;30(9):1005–1008. doi: 10.1016/0006-2952(81)90047-2. [DOI] [PubMed] [Google Scholar]
- Stenberg K., Larsson A. Reversible effects on cellular metabolism and proliferation by trisodium phosphonoformate. Antimicrob Agents Chemother. 1978 Nov;14(5):727–730. doi: 10.1128/aac.14.5.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg K., Skog S., Tribukait B. Effects of foscarnet on the cell kinetics of Madin-Darby canine kidney cells. Biochim Biophys Acta. 1983 Feb 16;762(1):31–35. doi: 10.1016/0167-4889(83)90113-1. [DOI] [PubMed] [Google Scholar]


