Abstract
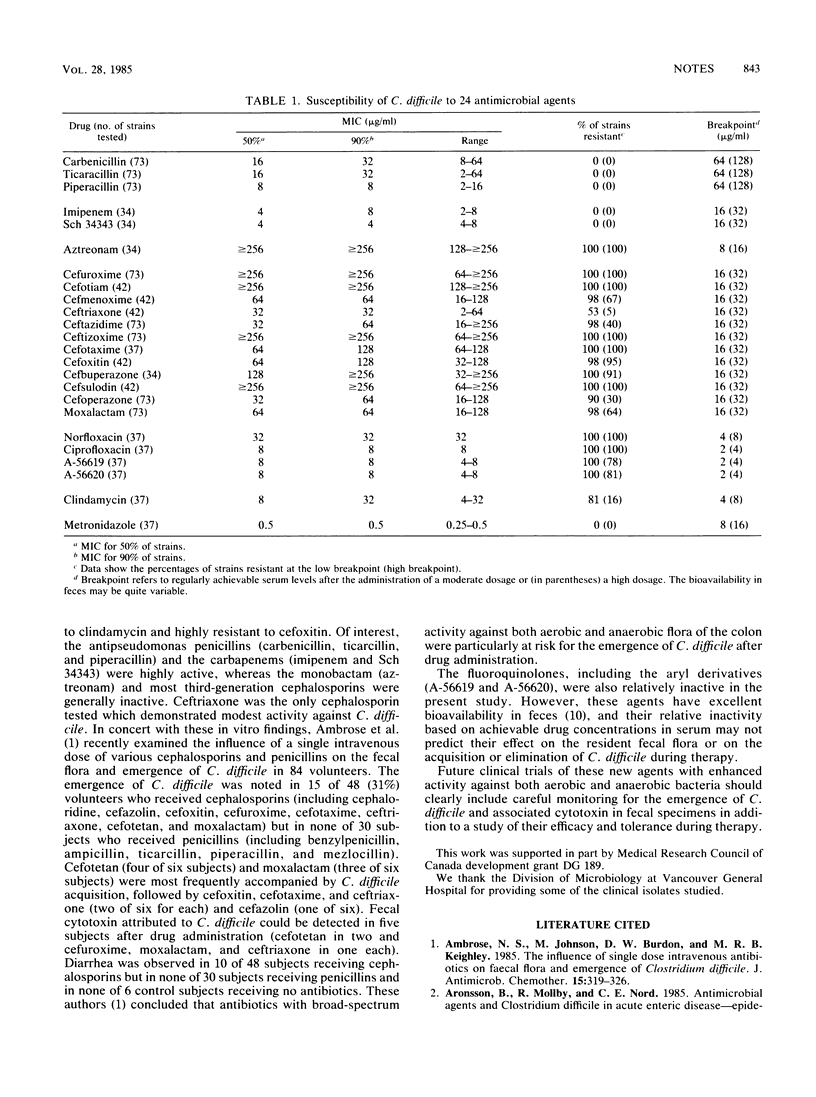
The in vitro susceptibilities of 34 to 73 clinical isolates of Clostridium difficile to 24 antimicrobial agents, including 18 beta-lactams, 4 fluoroquinolones, clindamycin, and metronidazole were examined. Metronidazole was the most active (MIC for 90% of the isolates [MIC90], 0.5 microgram/ml), followed by the carbapenems (Sch 34343, 4 micrograms/ml; imipenem, 8 micrograms/ml) and the antipseudomonas penicillins (piperacillin, 8 micrograms/ml; ticarcillin, 32 micrograms/ml; carbenicillin, 32 micrograms/ml). A monobactam (aztreonam) and most cephalosporins were either highly inactive (cefoxitin, cefuroxime, cefotiam, cefsulodin, ceftizoxime, cefbuperazone, and cefotaxime), with an MIC90 of greater than or equal to 128 micrograms/ml, or moderately inactive (ceftriaxone, cefmenoxime, cefoperazone, ceftazidime, and moxalactam), with an MIC90 of greater than or equal to 32 micrograms/ml. Clindamycin (MIC90, 32 micrograms/ml) and the fluoroquinolones (ciprofloxacin, 8 micrograms/ml; A-56619, 8 micrograms/ml; A-56620, 8 micrograms/ml; norfloxacin, 32 micrograms/ml) were only variably active. These in vitro data per se may not necessarily predict the relative risks for C. difficile-associated diarrhea or colitis during therapy with these agents. However, these data, in concert with knowledge of drug bioavailability in feces and the broad-spectrum antimicrobial activity on the resident bowel flora, may provide additional insight into the mechanisms and predictability of this complication with these agents. Careful monitoring for the emergence of C. difficile and fecal cytotoxin and for diarrhea during therapy with these agents is clearly indicated.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrose N. S., Johnson M., Burdon D. W., Keighley M. R. The influence of single dose intravenous antibiotics on faecal flora and emergence of Clostridium difficile. J Antimicrob Chemother. 1985 Mar;15(3):319–326. doi: 10.1093/jac/15.3.319. [DOI] [PubMed] [Google Scholar]
- Bartlett J. G. Antimicrobial agents implicated in Clostridium difficile toxin-associated diarrhea of colitis. Johns Hopkins Med J. 1981 Jul;149(1):6–9. [PubMed] [Google Scholar]
- Bartlett J. G., Moon N., Chang T. W., Taylor N., Onderdonk A. B. Role of Clostridium difficile in antibiotic-associated pseudomembranous colitis. Gastroenterology. 1978 Nov;75(5):778–782. [PubMed] [Google Scholar]
- Borriello S. P., Larson H. E. Antibiotic and pseudomembranous colitis. J Antimicrob Chemother. 1981 Jun;7 (Suppl A):53–65. doi: 10.1093/jac/7.suppl_a.53. [DOI] [PubMed] [Google Scholar]
- Dzink J., Bartlett J. G. In vitro susceptibility of Clostridium difficile isolates from patients with antibiotic-associated diarrhea or colitis. Antimicrob Agents Chemother. 1980 Apr;17(4):695–698. doi: 10.1128/aac.17.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George W. L., Rolfe R. D., Finegold S. M. Clostridium difficile and its cytotoxin in feces of patients with antimicrobial agent-associated diarrhea and miscellaneous conditions. J Clin Microbiol. 1982 Jun;15(6):1049–1053. doi: 10.1128/jcm.15.6.1049-1053.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens H., De Mol P., Coignau H., Levy J., Grados O., Ghysels G., Innocent H., Butzler J. P. Comparative in vitro activities of aztreonam, ciprofloxacin, norfloxacin, ofloxacin, HR 810 (a new cephalosporin), RU28965 (a new macrolide), and other agents against enteropathogens. Antimicrob Agents Chemother. 1985 Mar;27(3):388–392. doi: 10.1128/aac.27.3.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M. E., Citron D., Gabay E., Kirby B. D., George W. L., Finegold S. M. Alterations in human fecal flora, including ingrowth of Clostridium difficile, related to cefoxitin therapy. Antimicrob Agents Chemother. 1984 Sep;26(3):343–346. doi: 10.1128/aac.26.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe R. D., Finegold S. M. Comparative in vitro activity of ceftriaxone against anaerobic bacteria. Antimicrob Agents Chemother. 1982 Aug;22(2):338–341. doi: 10.1128/aac.22.2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe R. D., Finegold S. M. Comparative in vitro activity of new beta-lactam antibiotics against anaerobic bacteria. Antimicrob Agents Chemother. 1981 Nov;20(5):600–609. doi: 10.1128/aac.20.5.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafran S. D., Wong J., Chow A. W. In vitro activity of Sch 34343 and cefbuperazone against anaerobic bacteria. Antimicrob Agents Chemother. 1985 May;27(5):749–752. doi: 10.1128/aac.27.5.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor N. S., Bartlett J. G. Partial purification and characterization of a cytotoxin from Clostridium difficile. Rev Infect Dis. 1979 Mar-Apr;1(2):379–385. doi: 10.1093/clinids/1.2.379. [DOI] [PubMed] [Google Scholar]



