Abstract
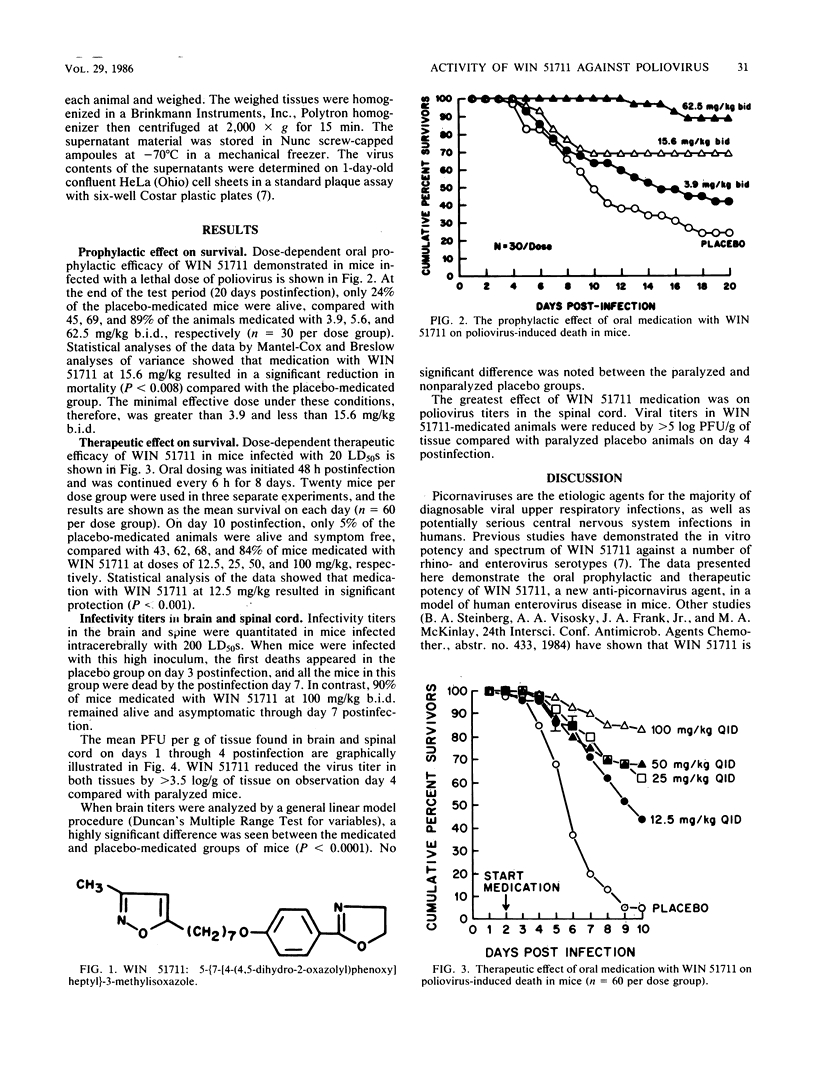
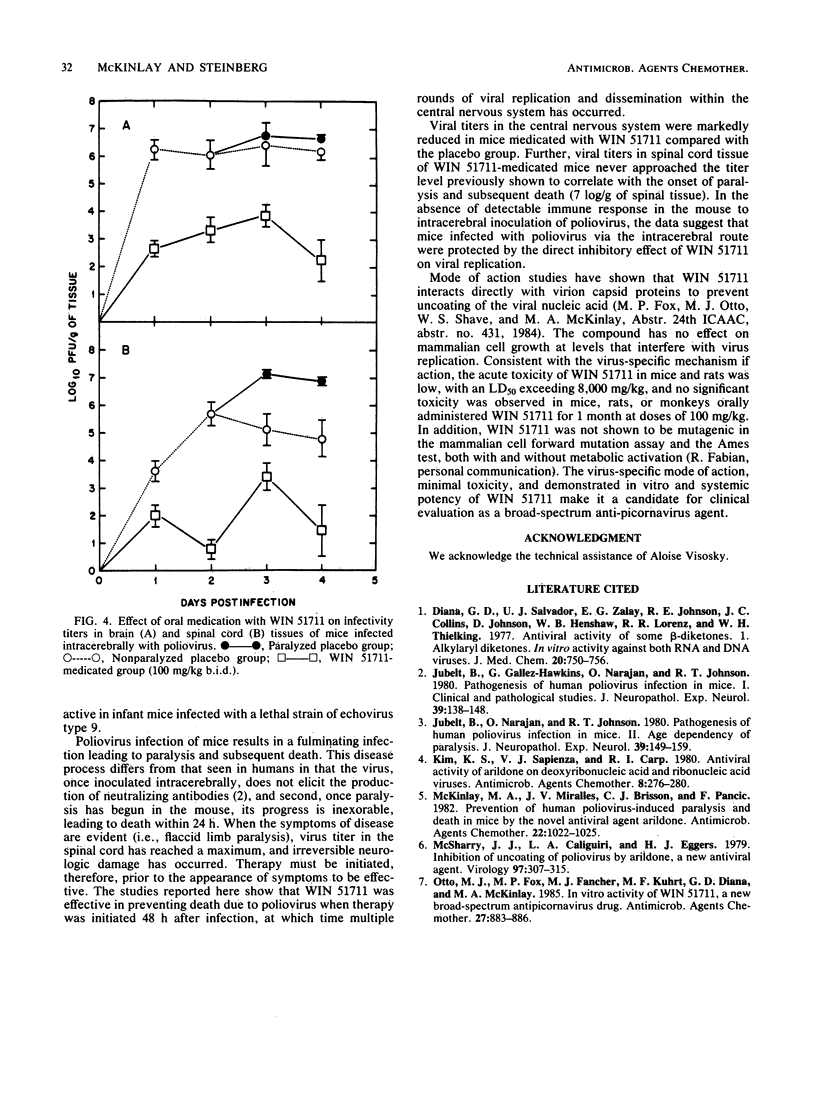
WIN 51711, a new broad-spectrum anti-picornavirus agent, prevented the development of paralysis and subsequent death in mice infected intracerebrally with a lethal dose of human poliovirus type 2 (MEF strain). The prophylactic efficacy of intragastrically administered WIN 51711 was dose dependent over the 3.9- to 62.5-mg/kg (twice daily) dose range, with a minimal significantly effective dose of less than 15.6 mg/kg per dose (twice daily) (P less than 0.008). An oral four times a day dosage regimen initiated 48 h postinfection with WIN 51711 doses as low as 12.5 mg/kg was effective in significantly reducing poliovirus-induced paralysis and death compared with a placebo. Viral titers in the brains and spines of mice infected intracerebrally with 200 50% lethal doses of poliovirus were reduced by 3 to 5 log10 PFU/g in the WIN 51711-medicated group compared with placebo-medicated animals. The potent in vitro and in vivo anti-picornavirus activity of WIN 51711 makes it a potentially useful drug for the treatment of enterovirus infections in humans.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Diana G. D., Salvador U. J., Zalay E. S., Johnson R. E., Collins J. C., Johnson D., Hinshaw W. B., Lorenz R. R., Thielking W. H., Pancic F. Antiviral activity of some beta-diketones. 1. Aryl alkyl diketones. In vitro activity against both RNA and DNA viruses. J Med Chem. 1977 Jun;20(6):750–756. doi: 10.1021/jm00216a003. [DOI] [PubMed] [Google Scholar]
- Jubelt B., Gallez-Hawkins G., Narayan O., Johnson R. T. Pathogenesis of human poliovirus infection in mice. I. Clinical and pathological studies. J Neuropathol Exp Neurol. 1980 Mar;39(2):138–148. doi: 10.1097/00005072-198003000-00003. [DOI] [PubMed] [Google Scholar]
- Jubelt B., Narayan O., Johnson R. T. Pathogenesis of human poliovirus infection in mice. II. Age-dependency of paralysis. J Neuropathol Exp Neurol. 1980 Mar;39(2):149–159. doi: 10.1097/00005072-198003000-00004. [DOI] [PubMed] [Google Scholar]
- Kim K. S., Sapienza V. J., Carp R. I. Antiviral activity of arildone on deoxyribonucleic acid and ribonucleic acid viruses. Antimicrob Agents Chemother. 1980 Aug;18(2):276–280. doi: 10.1128/aac.18.2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinlay M. A., Miralles J. V., Brisson C. J., Pancic F. Prevention of human poliovirus-induced paralysis and death in mice by the novel antiviral agent arildone. Antimicrob Agents Chemother. 1982 Dec;22(6):1022–1025. doi: 10.1128/aac.22.6.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSharry J. J., Caliguiri L. A., Eggers H. J. Inhibition of uncoating of poliovirus by arildone, a new antiviral drug. Virology. 1979 Sep;97(2):307–315. doi: 10.1016/0042-6822(79)90342-8. [DOI] [PubMed] [Google Scholar]
- Otto M. J., Fox M. P., Fancher M. J., Kuhrt M. F., Diana G. D., McKinlay M. A. In vitro activity of WIN 51711, a new broad-spectrum antipicornavirus drug. Antimicrob Agents Chemother. 1985 Jun;27(6):883–886. doi: 10.1128/aac.27.6.883. [DOI] [PMC free article] [PubMed] [Google Scholar]



